Abstract
Current immunisation programmes against hepatitis B virus (HBV) increasingly often involve novel tri-component vaccines containing—together with the small (S-HBsAg)—also medium and large surface antigens of HBV (M- and L-HBsAg). Plants producing all HBsAg proteins can be a source of components for a potential oral ‘triple’ anti-HBV vaccine. The objective of the presented research was to study the potential of M/L-HBsAg expression in leaf tissue and conditions of its processing for a prototype oral vaccine. Tobacco and lettuce carrying M- or L-HBsAg genes and resistant to the herbicide glufosinate were engineered and integration of the transgenes was verified by PCR and Southern hybridizations. M- and L-HBsAg expression was confirmed by Western blot and assayed by ELISA at the level of micrograms per g of fresh weight. The antigens displayed a common S domain and characteristic domains preS2 and preS1 and were assembled into virus-like particles (VLPs). Leaf tissues containing M- and L-HBsAg were lyophilised to produce a starting material of an orally administered vaccine formula. The antigens were distinctly sensitive to freeze-drying conditions and storage temperature, in the aspect of stability of S and preS domains and formation of multimeric particles. Efficiency of lyophilisation and storage depended also on the initial antigen content in plant tissue, yet M-HBsAg appeared to be approximately 1.5–2 times more stable than L-HBsAg. The results of the study provide indications concerning the preparation of two other constituents, next to S-HBsAg, for a plant-derived prototype oral tri-component vaccine against hepatitis B.
Electronic supplementary material
The online version of this article (doi:10.1007/s00299-011-1223-7) contains supplementary material, which is available to authorized users.
Keywords: Anti-HBV vaccine, Lyophilised plant tissue, L-HBsAg, M-HBsAg, Plant-derived oral vaccines, Transgenic lettuce
Introduction
Recombinant vaccines against the hepatitis B virus (HBV), based on the HBV small surface antigen (S-HBsAg) produced in yeasts, were introduced into hepatitis B (HepB) vaccination programmes in the early 1980s. Although safe and effective vaccines substantially provided prophylaxis and control of HepB (Michel and Tiollais 2010), the number of HBV chronic carriers and patients suffering from HepB or hepatocellular carcinoma is still high (Kew 2010; Romano et al. 2011). Insufficient medical infrastructure and growing needs in many countries cause delays in mass-scale HepB vaccination programmes. The situation is deteriorating, also in developed countries, due to mutated HBV strains, which can appear in response to selection pressure caused by vaccination (Huang et al. 2004). Furthermore, a number of patients, mainly with immunodeficiency syndromes and other ailments fail to mount a sufficient immune response following the application of conventional vaccines, despite their 90–95% effectiveness.
In the light of the above, research efforts have been conducted to produce a novel, 3rd generation, tri-component vaccine, containing S-HBsAg and the other envelope proteins of HBV, i.e. medium (M-HBsAg) and large (L-HBsAg) surface antigens. All HBsAg proteins are encoded within a single open reading frame and contain a common S domain. While S-HBsAg contains only the S domain, M-HBsAg includes an additional preS2 fragment and L-HBsAg consists of S, preS2 and preS1 domains. S-HBsAg assembles itself into stable and immunogenic virus-like particles (VLPs) and is the basic constituent of the virus envelope. The S fragment also anchors M and L antigens in the lipid membrane and displays preS1 and preS2 domains. These protruding epitopes form strongly immunogenic determinants, apart from infectivity functions (Blanchet and Sureau 2007). Consequently, preparations containing all HBV surface antigens exhibit enhanced immunogenicity by eliciting a larger spectrum of antiviral antibodies. Therefore, tri-component vaccines are more effective than vaccines based solely on S-HBsAg and they are gradually introduced into immunisation programmes (Madaliński et al. 2002; Rendi-Wagner et al. 2006; Young et al. 2001).
However, it is impossible to produce M- and L-HBsAg in bacteria and even in yeasts they need specific conditions for expression (Brocke et al. 2005). Therefore, anti-HBV novel vaccines require costly production in Chinese hamster ovary (CHO) or other mammalian cell expression systems and are too expensive for common use and remain restricted to special cases. Yet, plant-based oral vaccines are considered as alternatives or supplements for standard injection vaccines, since they have the potential for cheaper production and simplified vaccination procedures (Aziz et al. 2007). So far, research on oral plant-based vaccines against the HBV has focused mainly on the S-HBsAg. The small HBV surface antigen was expressed in tobacco, potato, lettuce and other species at a level of several up to tens of μg/g fresh weight (FW), due to the formation of stable VLPs (Huang et al. 2005; Kong et al. 2001; Pniewski et al. 2011; Richter et al. 2000; Shulga et al. 2004). Anti-HBs immune response, both in mice and humans, was induced exclusively by oral administration of plant material containing S-HBsAg (Kapusta et al. 1999, 2001; Pniewski et al. 2011), although in most cases oral immunisation was combined with the parenteral priming or boosting using purified HBsAg (Joung et al. 2004; Kong et al. 2001; Mason et al. 2003; Richter et al. 2000; Thanavala et al. 2005). In comparison, M- and L-HBsAg were expressed in plant systems, such as potato tubers or tomato fruits, only in a few experiments and at levels of tens or hundreds of ng/g FW (Ehsani et al. 1997; Lou et al. 2007; Salyaev et al. 2010). Plant-associated M-HBsAg was also able to induce some immune response after immunisation conducted in oral priming and injection boosting patterns (Joung et al. 2004; Youm et al. 2007). However, despite some successes in laboratory experiments exploiting the raw material, a potential mass-scale plant-derived oral vaccine against HBV and other pathogens requires a formulation based on lyophilised plant tissue (Huang et al. 2005; Pniewski et al. 2011; Webster et al. 2006) to facilitate and standardize specimen administration, improve stability and immunisation effectiveness, and to some extent control mucosal tolerance induction (Kirk et al. 2005; Mestecky et al. 2007).
We present here studies on M/L-HBsAg expression in leaf tissue and their stability during processing and storage of a prototype oral vaccine formula, as indispensable steps prior to immunisation trials. Herbicide-resistant tobacco and lettuce were engineered, stably expressing micrograms of M- or L-HBsAg per g FW, which displayed domain S and crucial domains preS2 and preS1 and were assembled into VLPs or analogous aggregates. The influence of freeze-drying together with storage temperature on lyophilised plant tissue was investigated in the aspect of S and preS domain stability and assembly into multimeric aggregates of M- and L-HBsAg. The results of the study provide indications on the preparation of semi-products containing M/L-HBsAg, which together with previously obtained plant-associated S-HBsAg, can be used as constituents of a plant-derived oral tri-component vaccine against hepatitis B.
Materials and methods
Construction of plant transformation vectors
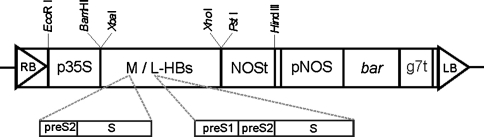
The coding sequences of M-HBsAg of 846 bp or L-HBsAg of 1,170 bp in length were amplified on a template of the plasmid pHB321 containing the complete genome of HBV subtype ayw4 (GenBank: Z35716), using the following primers: 5′-GGATCCATGCAGTGGAACTCCACAAC-3′ (forward for M-HBs sequence), 5′-GGATCCATGATGGGGCAGAATCTTTCC-3′ (forward for L-HBs) and 5′-CTGCAGTTAAATGTATACCCAAAGACAAAAGA-3′ (reverse for both). The amplified fragments were cloned using introduced BamHI and PstI restriction sites into the pMG2A plasmid, a derivative of pUC18, containing the 35S RNA CaMV promoter (p35S) and the nopaline synthase terminator (NOSt). The expression cassettes p35S-M/LHBs-NOSt were cloned into the EcoRI and HindIII sites of the pGPTV-BAR plasmid (Becker et al. 1992), replacing the GUS gene. Constructed vectors pKHBMBAR or pKHBLBAR (Fig. 1), carrying the bar gene conferring resistance to glufosinate, were moved into the Agrobacterium tumefaciens EHA105 strain via electroporation.
Fig. 1.
Organization of T-DNA of binary plasmids pKHBMBAR and pKHBLBAR. M/L-HBs coding sequence of surface antigen of HBV: medium (preS2 and S domains) or large (preS1, preS2 and S domains), in pKHBMBAR or pKHBLBAR, respectively; bar coding sequence of phosphinothricin acetyltransferase; p35S CaMV 35S promoter, pNOS nopaline synthase promoter, NOSt nopaline synthase terminator, g7t g7 terminator; LB and RB left and right T-DNA border sequences
Plant transformation, regeneration and cultivation
Glufosinate-resistant lettuce plants were obtained according to the previously described Agrobacterium-mediated transformation procedure (Pniewski et al. 2011). Tobacco leaf fragments were transformed using Agrobacterium tumefaciens, according to a slightly modified routine procedure (Horsch et al. 1985). All stages of transformed tobacco in vitro regeneration were conducted on selection media, i.e. supplemented with 10 mg l−1 of glufosinate ammonium (Sigma, USA) and 300 mg l−1 of timentin (GlaxoSmithKline, Poland). Directly after co-culture with Agrobacterium, explants were cultured on MST medium consisting of MS salts and vitamins (Murashige and Skoog 1962), 3% (w/v) sucrose, 1 mg l−1 BAP (6-benzylamminopurine) and 0.1 mg l−1 NAA (naphtaleneacetic acid). Calli with adventitious buds were transferred onto the MST1 medium consisting of MS salts and vitamins, 3% (w/v) sucrose, 1 mg l−1 KIN (kinetin) and 0.1 mg l−1 IAA (indole-3-acetic acid). Developed plantlets were rooted on a half-strength MS medium.
In vitro cultures were conducted under 4,000 lx of light intensity, 16/8 photoperiod and at a 25/18°C temperature regime. Rooted plantlets of tobacco and lettuce were transferred into soil and grown in a greenhouse under 15–20 klx light intensity, 16/8 photoperiod and at a 22/16°C temperature regime. Harvested seeds of lettuce were germinated on the half-strength MS medium with 2.5 mg l−1 glufosinate ammonium and seedlings of progeny transformants were cultivated in soil as described above.
PCR and Southern blot analysis
Genomic DNA was extracted from leaves in a small-scale procedure according to McGarvey and Kaper (1991). Polymerase chain reactions were performed in a volume of 25 μl, containing approx. 100 ng of plant genomic DNA, 10 pM of each primer used for vector construction (see above), 0.2 mM of each dNTP, 3.5 mM MgCl2 and 1 unit of Taq DNA polymerase (Invitrogen, USA). HBsAg sequences were amplified under the following temperature profile: initial denaturation at 94°C for 4 min, next 35 cycles of denaturation at 94°C for 1 min, annealing at 68°C for 45 s, elongation at 72°C for 1 min, and final extension at 72°C for 5 min. Plants were also screened by PCR for the chvA gene to exclude false positive results caused by residual contamination of Agrobacterium DNA, as described previously (Pniewski and Kapusta 2005). Molecular weight of PCR products was estimated by agarose gel electrophoresis using the 200 bp DNA Ladder (MBI Fermentas, EU).
DNA for Southern hybridization was extracted according to Doyle and Doyle (1990), cut with the EcoRI, run on 0.75% agarose gel and then blotted onto a positively charged membrane (Roche, USA). The probe specific to the M- or L-HBs sequence was prepared by PCR using a DIG labelling kit (Roche), hybridized according to the manufacturer’s protocol and signals were detected on an X-ray film using CSPD (Roche) as a chemiluminescent substrate.
Enzyme-linked immunosorbent assay (ELISA) of M- and L-HBsAg
Contents of M- and L-HBsAg, both VLPs and total antigens regarding the common S domain and characteristic preS2 and preS1 domains, were assayed in tobacco and lettuce leaves and in lyophilised tissue derived from lettuce (see below). Tobacco individual plants were analysed at the age of 6 months, i.e. shortly after adaptation to ex vitro conditions past regeneration and after long-term (here 1 year) cultivation, i.e. at the age of 18 months. Lettuce plants were analysed at generations T0 (primary transformants) and T1 (progeny transformants).
Samples were ground in a mortar on ice in a gradually added extraction buffer consisting of PBS pH 7.4 (phosphate-buffered saline), 10 mM Na2SO3, 2% (w/v) PVP40000 (polyvinylpyrrolidone MW 40,000), 0.2% (w/v) BSA (bovine serum albumin) and supplemented with an appropriate detergent. The detergents were respectively 0.05% (v/v) Triton X-100 or 1% (v/v) Tween®20, for M- or L-HBsAg samples, and were experimentally selected from among 0.02–2% (v/v) of Tween®20, SDS (sodium dodecyl sulphate) and Triton X-100. Crude extracts were centrifuged for 10 min at 10,000 rpm at 4°C. The ratio of the extraction buffer volume to the sample weight was 25:1 for leaves and 200:1 for lyophilised tissue.
Total M- or L-HBsAg contents were assayed by quantitative sandwich ELISA assays using mono- and polyclonal antibodies, and performed according to typical guidelines with details specific to a particular antigen. A Microtiter MaxiSorp (NUNC, Denmark) microplate was coated overnight with 0.5 μg ml−1 of a monoclonal antibody (mAb) at 4°C in carbonate buffer pH 9.6. Domains of M- or L-HBsAg proteins were assayed using the following mAbs: mAb C86123 M (Meridian Life Science, USA) for the common S domain, mAb C8A031 M (Meridian Life Science) for the preS2 domain characteristic of M-HBsAg and mAb 5a-19 (kindly provided by Prof. Agata Budkowska, Institut Pasteur, Paris, France) for the preS1 domain of L-HBsAg. The coated plate was saturated with 5% (w/v) PBS-fat-free milk at 25°C for 1 h. Plant extracts were added to PBS-filled wells and serially diluted. All the following incubations with extracts or antibodies were conducted at room temperature for 45 min. After sample incubation, wells were incubated with 1:4,000 anti-HBsAg domain S-specific polyclonal rabbit antibody (B65811R, Meridian Life Science), then with 1:20,000 anti-rabbit IgG γ-specific biotin-conjugated mAb (Sigma), followed by 1:4,000 AP-conjugated ExtrAvidin® (Sigma). The reaction with pNPP substrate (Sigma) was developed at 25°C for 1 h and absorbance was measured at 405 nm using a Microplate Reader, Model 680 (Bio-Rad, USA). The M- or L-HBsAg concentration was calculated in micrograms per gram of fresh weight (FW) or lyophilised mass, respectively, as the arithmetic mean with standard deviation (SD) from 3 to 6 repeated assays, using Microplate Manager 5.2.1 software (Bio-Rad) according to the standard curve for CHO-derived M/L-HBsAg (kindly provided by Prof. Reinhold Schirmbeck, University of Ulm, Germany) diluted in the crude extract of a negative control plant tissue.
For measurements of M- and L-HBsAg assembled into VLPs or analogous aggregates, samples were assayed using a monoclonal kit HBsAg Reagent v. 2.0 for IMx® system, module 3A3011 (Cat. No. 2228-22, Abbott, USA), specific for the ‘a’ epitope displayed by all HBsAg proteins assembled into VLPs. The samples were extracted as above and assayed according to the provided protocol.
Western blot of M- and L-HBsAg
Leaf pieces, approx. 50–60 mg in weight, were ground in 300 μl of PBS with 0.5% (v/v) Tween®20, then Laemmli buffer with DTT (dithiothreitol) was added and samples were incubated at 65°C for 15 min. Samples were run on 12% polyacrylamide gel with 0.1% (w/v) SDS and then blotted onto a nitrocellulose membrane (Roche). The blot was blocked with 3% (w/v) BSA in TBS (Tris-buffered saline), then incubated with TBS-diluted 1 μg ml−1 anti-preS2 mAb C8A031 M (Meridian Life Science) or anti-preS1 mAb 5a-19 (Institut Pasteur). The 1:10,000 TBS-diluted anti-mouse whole-molecule polyclonal AP-conjugated antibody (Sigma) was used as a secondary antibody. The molecular weight of protein bands, visualized after a reaction with the BCIP/NBT substrate (Sigma), was estimated using a protein marker (MBI Fermentas).
Preparation, storage and analysis of lyophilised plant material
Leaves from T1 generation lettuce plants, attributed to particular HBsAg expression groups, were lyophilised under experimentally selected conditions (see “Results” for details). Leaf tissue was frozen at −35°C in a freezer chamber and freeze-dried (BETA 1-16, CHRIST®, Germany) in vacuum at 0.2 mbar, at a shelf temperature of 20°C for 20 h in primary drying and 22°C for 2 h in secondary drying. Lyophilised tissue was powdered in a coffee mill chilled on ice and stored in the dark at 4, 22 (room temperature) or 37°C in tightly sealed bottles. Residual moisture in the tissue was determined using the gravimetric method (May et al. 1989). Contents of M- and L-HBsAg were assayed (see above for details) directly after freeze-drying had been completed (day 0) and after 30 and 90 days of storage.
Mean values of M/L-HBsAg content in the stored lyophilised tissue, obtained for different temperatures and diverse antigen content in source plants, were compared statistically by analysis of variance, separately for three antigen constituents (S and preS domains and VLP form) and two times of assay (30 and 90 days). Homogeneous groups of category combinations (HBsAg initial content in source plants × temperature) for each antigen domain/form with the largest mean values were identified using the Fisher’s protected least significant difference test (Genstat 14, VSN Int.).
Results
Expression of M- and L-HBsAg in tobacco and lettuce plants
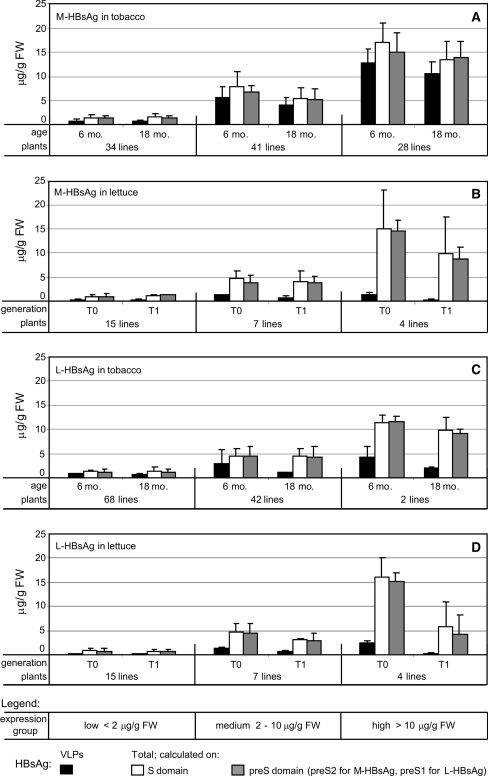
More than 100 regenerated glufosinate-resistant tobacco plants were PCR-positive (Supplementary Fig. 1a, b) for each transgene carrying M-HBs or L-HBs sequence (Fig. 1), and T-DNA integration was confirmed by Southern hybridizations (Supplementary Fig. 2a, b). Verified plants initiated transgenic lines, where HBsAg expression was analysed. Total contents of the antigens, both M- or L-HBsAg, calculated in regard to the S domain, were highly diverse among the transformants. Hence, they were divided into three expression groups, with gradually decreasing numbers of lines, i.e. of a ‘low’ antigen level <2 μg/g FW, ‘medium’ 2–10 μg/g FW and ‘high’>10 μg/g FW (Fig. 2a, c), in which some tendencies of HBsAg expression were more visible. The total content of M-HBsAg was 1.3–1.4 times higher than for L-HBsAg. This trend was even more distinct for the antigens assembled in VLPs-analogous structures (for simplification further described as VLPs). The mean level of M-HBsAg VLPs amounted to 51, 70 and 76% of the total antigen content for the ‘low’, ‘medium’ and ‘high’ group, respectively, and these ratios were maintained or slightly decreased within the groups after long-term, here 18-month cultivation period. However, the mean level of L-HBsAg VLPs showed an opposite pattern—approx. 60% of the total antigen content for the ‘low’ and the ‘medium’, in contrast to 36% for the ‘high’ expressors, and it decreased even twofold after a long-cultivation period.
Fig. 2.
Expression of VLPs and total HBsAg in plants. a Expression of M-HBsAg in tobacco plants of 6- and 18-months of age, b expression of M-HBsAg in lettuce plants of T0 and T1 generation, c expression of L-HBsAg in tobacco plants of 6- and 18-months of age, d expression of L-HBsAg in lettuce plants of T0 and T1 generation. Plants were divided into three expression groups, depending on total HBsAg content calculated in μg/g of fresh weight (FW): low < 2 μg/g FW, medium 2–10 μg/g FW and high > 10 μg/g FW. Level of both VLP-assembled antigens was determined by monoclonal IMx® diagnostic kit (Abbott). Total contents of the antigens were determined by sandwich ELISA using anti-HBsAg rabbit polyclonal antibody (B65811R, Meridian Life Science) and monoclonal antibodies specific for S, preS2 or preS1 domains (respectively, C86123 M and C8A031 M, Meridian Life Science, and 5a-19, Institut Pasteur). Data show values calculated as the arithmetic mean and standard deviation (SD) from three assays
Twenty-six PCR-positive, both for the M- and L-HBs sequences, glufosinate-resistant transgenic lettuce lines (T0 generation) were obtained. The transgenes with HBs sequences were transmitted to ≥75% of the progeny plants (Supplementary Fig. 1c, d), which corresponded to the inheritance of 1 or more T-DNA loci (Supplementary Fig. 2c, d). Tendencies of HBsAg expression, revealed in tobacco, were more evident in lettuce lines, also divided into three expression groups (Fig. 2b, d). M-HBsAg was expressed approx. 2 times more efficiently than L-HBsAg, mainly in progeny plants (T1 generation), yet within groups, especially in the ‘medium’ and ‘high’ ones, mean contents of the antigens were lower than in tobacco. The level of VLPs in T0 plants reached only 39, 26 and 10% (M-HBsAg), or 27, 26 and 14% (L-HBsAg) of total antigen contents, respectively, for the ‘low’, ‘medium’ and ‘high’ group. The contents of the antigens, particularly VLPs and for L-HBsAg, dropped in T1 plants and the extent of decrease was larger for ‘high’ expressors (Fig. 2b, d).
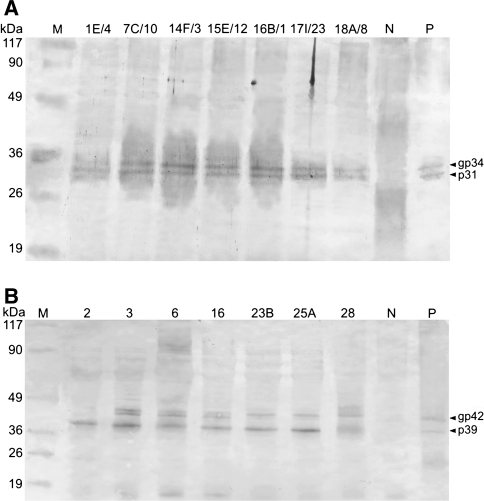
Despite a gradual deterioration of expression intensity, the immunogenic VLP form of M- and L-HBsAg amounted to 0.4–2.2 μg/g FW in lettuce and 0.4–12.8 μg/g FW in tobacco (Fig. 2). Analyses using preS-specific mAbs revealed characteristic preS2 or preS1 domains displayed by the antigens. The total antigen contents calculated in relation to preS2 or preS1 were consistent in ≥90% with the basic S domain (Fig. 2). Antibodies specific to preS fragments were also used in Western blot analyses of M- and L-HBsAg in leaf extracts (Fig. 3). Despite some background and weak non-specific signals, protein bands of M- and L-HBsAg were clearly visible. Detected antigens were of predicted size, both unmodified and putative glycosylated forms, i.e. of 31 and 34 kDa for M-HBsAg and 39 and 42 kDa for L-HBsAg. Plant materials characterised in the way described above were used for lyophilisation experiments.
Fig. 3.
Western blot analysis of M-HBsAg in lettuce (a) and L-HBsAg in tobacco (b) using monoclonal antibodies specific for preS2 or preS1 domains. Lanes: M protein molecular weight marker (MBI Fermentas), 1E/4–18A/8 or 2–28 analyzed lettuce or tobacco plants, respectively, N non-transgenic plant (negative control), P M-HBsAg or L-HBsAg, respectively (positive control, University of Ulm, Germany). Arrows indicate non-glycosylated (p31 M-HBsAg, p39 L-HBsAg) and putative glycosylated (gp34 M-HBsAg, gp42 L-HBsAg) proteins of HBsAg antigens. M- and L-HBsAg proteins were detected using anti-preS2 (C8A031 M, Meridian Life Science) or anti-preS1 (5a-19, Institut Pasteur) monoclonal antibodies
Lyophilisation of plant material containing M- and L-HBsAg
Freeze-drying conditions of lettuce leaves expressing M- or L-HBsAg were selected experimentally from among combinations of the following parameters: freezing temperature (liquid nitrogen, −35 and −20°C), vacuum pressure (0.1–0.4 mbar), temperature of primary and secondary drying (5–22°C) and duration (20–72 h). Differences in the process yield were not large and reached max. 10% (data not shown). However, the highest (Table 1), thus potentially optimal, lyophilisation efficiency was observed for freezing at −35°C, drying at 0.2 mbar and at 20–22°C for 22 h (see “Materials and methods” for details).
Table 1.
Lyophilisation efficiency—antigens preserved directly after freeze-drying had been completed (day 0 of storage), in materials coming from plants attributed to particular expression groups: low <2 μg/g FW, medium 2–10 μg/g FW and high >10 μg/g FW
| Antigen | Content in initial fresh tissue (μg/g) | Antigen preservation in lyophilised tissue | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute content (μg/g) | Relative content (%) | ||||||||
| Low | Medium | High | Low | Medium | High | Low | Medium | High | |
| M-HBsAg | |||||||||
| S domain | 1.8 | 4.2 | 9.8 | 24.5 | 26.1 | 34.2 | 94.3 | 43.6 | 24.5 |
| preS2 domain | 1.3 | 3.8 | 9.0 | 7.2 | 8.1 | 9.1 | 40.1 | 14.8 | 7.1 |
| VLPs | 0.4 | 0.8 | 1.1 | 0.8 | 5.5 | 14.6 | 13.2 | 48.2 | 93.8 |
| L-HBsAg | |||||||||
| S domain | 1.0 | 3.1 | 5.7 | 13.3 | 16.2 | 15.9 | 94.2 | 37.2 | 19.6 |
| preS1 domain | 0.6 | 2.8 | 4.1 | 7.9 | 8.0 | 7.7 | 91.8 | 19.8 | 13.1 |
| VLPs | 0.3 | 0.5 | 0.2 | 3.8 | 2.3 | 1.2 | 95.9 | 33.9 | 38.2 |
Antigen content in fresh tissue is a mean from 6 to 8 plants of T1 generation used for lyophilisation; the antigen content in T1 generation decreased in comparison to T0 generation, when expression groups were distinguished
Relative content calculated as a ratio of obtained value and theoretical value, if whole antigen would be preserved and assuming that dry mass accounts for 7% of leaf fresh weight; dry mass determined using the gravimetric method (May et al. 1989)
Efficiency of lyophilisation, considered as a relative degree of HBsAg retention (assuming that dry mass is ca. 7% of fresh plant tissue as determined by gravimetric method, detailed data not shown) was different for M- or L-HBsAg and depended on the initial HBsAg content in plant material (Table 1). The storage temperature of lyophilised tissue combined with the initial antigen content in source plants also exerted a marked and distinct effect on M- or L-HBsAg preservation (Table 2).
Table 2.
Mean values of M-HBsAg and L-HBsAg content in lyophilised tissue, grouped according to day of storage (30, 90) and antigen domain (S domain, preS) or VLP form, with least significant differences calculated for categories: combination of antigen content in source plants × storage temperature
| Day | Antigen domain/form | Temperature (°C) | M-HBsAg content (μg/g) in tissue derived from plant expression groups | LSD (p = 0.05) | L-HBsAg content (μg/g) in tissue derived from plant expression groups | LSD (p = 0.05) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | |||||
| 30 | S | 4 | 9.49 | 12.81 | 21.09 | n.s. | 6.68 | 6.42 | 9.15 | n.s. |
| 22 | 11.43 | 22.82 | 27.70 | 12.91 | 15.34 | 12.91 | ||||
| 37 | 11.60 | 17.41 | 17.07 | 13.07 | 15.90 | 12.77 | ||||
| preS | 4 | 0.04 | 0.15 | 0.08 | 1.71 | 2.78 | 4.00 | 2.66 | n.s. | |
| 22 | 1.19 | 1.36 | 3.53a | 2.34 | 3.73 | 3.04 | ||||
| 37 | 2.00a | 3.20a | 3.70a | 2.88 | 3.66 | 2.93 | ||||
| VLP | 4 | 0.41 | 0.98 | 1.69 | 0.97 | 1.33 | 1.34 | 1.00 | n.s. | |
| 22 | 0.70 | 1.35 | 5.79b | 1.35 | 1.16 | 1.14 | ||||
| 37 | 0.60 | 1.53 | 3.80 | 1.40 | 1.32 | 1.12 | ||||
| 90 | S | 4 | 0.80 | 2.39 | 3.41 | 7.05 | 2.39 | 2.88 | 1.00 | 3.54 |
| 22 | 10.40c | 14.98c | 14.98c | 7.32a | 5.13 | 2.56 | ||||
| 37 | 6.35 | 7.47 | 10.09c | 9.43a | 10.11a | 9.43a | ||||
| preS | 4 | 0.04 | 0.14 | 0.08 | 0.58 | 0.37 | 1.15 | 0.22 | 1.68 | |
| 22 | 0.05 | 0.40 | 1.53d | 2.34b | 3.73b | 1.40 | ||||
| 37 | 0.25 | 1.45d | 1.95d | 0.72 | 2.88b | 0.90 | ||||
| VLP | 4 | 0.04 | 0.41 | 1.17 | 0.21 | 0.76c | 0.78c | 0.44 | 0.31 | |
| 22 | 0.18 | 0.63 | 1.95e | 0.33 | 0.13 | 0.11 | ||||
| 37 | 0.20 | 0.36 | 0.83 | 0.23 | 0.12 | 0.14 | ||||
Categories with the largest means, determined according to the Fisher’s protected least significant difference test, are marked by letter indexes, separately for M- and L-HBsAg
LSD least significant difference at p = 0.05; n.s. not significant differences, here differences among nine combinations; preS preS2 for M-HBsAg, preS1 for L-HBsAg
Antigen content in source plants attributed to expression groups: low < 2 μg/g FW, medium 2–10 μg/g FW, high > 10 μg/g FW
The absolute content of freshly prepared freeze-dried M-HBsAg was the highest in the material derived from plants of the ‘high’ expression group, in regard to the S and preS2 domains and VLPs. In contrast, the relative freeze-drying efficiency of the S and preS2 domains decreased in rank from ‘low’ to ‘high’ expressing plants; however, VLPs were preserved in another manner (Table 1).
The content of long-term stored M-HBsAg, gradually declined over time at all temperatures, in materials derived from all plant expression groups (Table 2). The concentrations of M-HBsAg were not statistically different only for S domain after 30-day storage, but other trends were revealed and maintained after 90-day storage. The preservation of the antigen was the lowest at 4°C, while 37°C, besides 22°C, appeared to be advantageous for preS2 domain (Table 2). However, for all other category combinations, i.e. antigen content in source plants and temperature (see “Materials and methods”), both after 30 and 90 days of storage, the combination of high antigen initial content and 22°C, appeared in the group with the largest mean values (Table 2). Therefore, this combination can be chosen as optimal for processing and storage of plant-associated M-HBsAg, both for S and preS2 domains and VLPs.
Lyophilisation and storage of plant tissue containing the large surface antigen of HBV appeared different from M-HBsAg in several aspects (Tables 1, 2). The absolute content of total L-HBsAg directly after freeze-drying was similar in all preparations derived from consecutive plant expression groups. Therefore, the process efficiency for the S and preS1 domains was the highest for material coming from the ‘low’ group, and the lowest for the ‘high’ one. Stability of VLPs corresponded generally with retaining of the total antigen (Table 1).
During long-term storage of plant-associated L-HBsAg, its particular domains and forms were preserved differently, depending on temperature and the initial antigen content in source plants (Table 2). However, concentrations of the antigen were not statistically different on day 30 for S and preS1 domains as well as VLPs, so any choice of a category is equally good on this date. The temperature of long-term storage of plant-associated antigen exerted a distinct influence regarding the S and preS1 domains and VLPs after 90-day storage period, although there was no combination which was always in the group with the largest mean values. L-HBsAg, particularly regarding preS1 domain and VLPs, was the most stable in preparations obtained from plants of the ‘low’ or ‘medium’ expression groups. Optimal temperature for the preservation of the S domain of the antigen was 37°C, while 22°C for preS1 and 4°C for VLPs (Table 2). However, considering the fact that immunogenic form of the antigen is VLP displaying preS1 domain, the combination of medium L-HBsAg initial content and 4°C seems to be a good choice. In that variant, VLPs concentration is statistically higher in comparison to others as well as absolute values of S and preS1 domains remain at reasonable level (Table 2).
Although contents of both antigens in stored materials gradually decreased, considering overall preservation of the S and preS domains and VLPs, in the optimal variants (see above) the antigens were maintained at the level of several μg/g of lyophilised tissue (Table 2).
The storage temperature also distinctly affected a reduction of residual moisture content of the lyophilised plant material, as observed after a 90-day shelf life, from the initial 4.1 to 3.8% at 4°C, 2.3% at 22°C and 0.2% at 37°C, respectively.
Discussion
Tobacco was investigated to study the potential of M/L-HBsAg stable expression in leaf tissue for its possible exploitation as a bio-reactor producing antigens for a long time, here 18 months, which would be purified and applied as classical injection vaccines. At the same time, our research was focused on lettuce, as the target species for preparation of a prototype oral prophylactic vaccine. Lettuce is edible in the raw state, naturally free of harmful and anti-nutritional substances and for an orally administered vaccine the antigen concentration can be increased by lyophilisation (Pniewski et al. 2011). Biomass of lettuce leaves is relatively low, but they are easily freeze-dried in comparison to tomatoes, tubers, carrot or bananas. Additionally, lettuce cultivation can be adapted to various conditions and local needs.
Medium and large surface antigens of HBV were expressed in leaf cells in the native and immunogenic forms. The antigens displayed domain S, common for all HBsAg proteins, and characteristic preS1 and preS2 domains and were assembled into VLPs or analogous aggregates, as confirmed by ELISA and Western blot analyses using specific anti-preS monoclonal antibodies and VLP-specific diagnostic kits (Figs. 2, 3).
Observation of M/L-HBsAg expression and its fluctuations in particular transgenic lines may suggest that the antigens are not indifferent to plant cells. Accumulation of M/L-HBsAg, especially their VLP or aggregate forms, corresponded with the plant host, but the actual reasons of lower expression efficiency in lettuce remain unknown. However, both in lettuce and tobacco M-HBsAg was synthesised more intensively than L-HBsAg. Probably, the medium surface antigen is more durable in plant cells, similarly to natural or recombinant expression systems (Bruss 2007; Imamura et al. 1987). Both antigens contain the S domain (226 aa), identical with the small surface antigen (S-HBsAg), which anchors protein molecules in the membrane and assembles them into dimers and following VLPs of 22 nm in diameter. However, preS fragments, connected to the N-terminus of the S domain, are of a different size. M-HBsAg displays only the preS2 domain of 55 aa, while L-HBsAg exhibits preS2 together with the preS1 domain consisted of 108–124 aa (here 108 aa). Although the structure of the preS1 epitope is still not precisely known, joined preS domains of L-HBsAg are larger and can make a spatial obstacle for quaternary structure formation (Bruss 2007; Chi et al. 2006, 2007). Thus, the medium antigen assembles into relatively stable multimeric particles analogous to VLPs, while the large antigen can probably form oligomeric aggregates, whereas unstable VLPs are formed only under specific conditions (Bruss 2007; Imamura et al. 1987; Yamada et al. 2001). However, VLP-analogues (VLPs for simplification) formed by M- and L-HBsAg are significantly less stable than the actual VLPs of S-HBsAg. It could explain the several times lower level of M/L-HBsAg VLPs, observed in this study as well as previously (Ehsani et al. 1997; Joung et al. 2004; Lou et al. 2007; Salyaev et al. 2010) in comparison to tens of micrograms of S-HBsAg VLPs (Pniewski et al. 2011; Richter et al. 2000). In accordance to lower stability of VLPs assembled by M/L-HBsAg, the antigens exist mainly in small-size forms, probably monomers or dimers, visualised as the total antigen content excluding VLPs (Fig. 2). Moreover, the percentage content of the antigens in VLP structures, especially formed by L-HBsAg, adversely correlated with plant expression groups, as well as the total antigen and VLP content reduced along with plant age or generation (Fig. 2). Hence, it may be presumed that plant cells can produce M- and especially L-HBsAg in their VLP form, to a limited extent, ‘tolerable’ for cell integrity and functionality, resembling the situation in HBV infected cells (Bruss 2007). This supposition could also explain the decreasing number of plant lines along with the increasing expression level of the antigens. On the other hand, formation of multi-particle structures inert for host cells and still displaying whole or truncated preS1 regions, was enhanced, but so far only in microorganisms. Mixed VLPs were obtained in yeasts by co-expression of L-HBsAg and S-HBsAg (Brocke et al. 2005). Another approach represents expression in E. coli of preS1N-terminal fragments, crucial for infectivity and containing from 7 to 50 aa, fused to carrier protein HBcAg (Chen et al. 2004). However, specimens based on HBcAg can be used strictly for therapy, not as prophylactic vaccines.
Despite fluctuations of the expression, observed contents of M- and L-HBsAg at the level of several μg/g FW were essentially higher than the previously noted hundreds of ng/g FW (Ehsani et al. 1997; Joung et al. 2004, Lou et al. 2007, Salyaev et al. 2010). A possible explanation for the higher accumulation of M/L-HBsAg in lettuce or tobacco, apart from the undefined species effect, is that the antigens were synthesized in leaves instead of potato tubers or tomato fruits. It may be presumed that cell type and metabolism could affect the M/L-HBsAg expression. Regardless of reasons, the obtained values of M/L-HBsAg concentrations were as high as those usually recorded for S-HBsAg (Huang et al. 2005; Kong et al. 2001; Pniewski et al. 2011; Richter et al. 2000; Shulga et al. 2004) and were generally maintained after 18-month cultivation (tobacco) or in the consecutive generation (lettuce). Consequently, the resulting plant materials carrying M/L-HBsAg seemed sufficient for the next processing steps.
Tobacco could be utilized as a bio-reactor for long-term and mass-scale production of the antigens. Yet, due to the main goal of the project, only lettuce was directly used for lyophilisation experiments as the first step of conversion of plant tissue into a prototype oral vaccine (Huang et al. 2005; Pniewski et al. 2011; Webster et al. 2006). We decided to determine conditions of freeze-drying and storage prior to immunisation trials, since antigen stability is a fundamental factor of an efficient immunisation regime, including parameters such as defined dosage and administration timing.
Physiochemical transformations occurring during freeze-drying and storage of lyophilised tissue differently affected the S or preS domains of M- or L-HBsAg, as well as their ability to maintain quaternary structures, i.e. VLPs or aggregates. In general, hydrophilic preS domains were more sensitive than the hydrophobic S domain, which assembles into multimeric particles together with membrane lipids (Table 1). Thus, it could be assumed that, similarly as in fresh tissue, higher preservation of M than L antigen resulted from the formation and stability of VLPs versus oligomeric aggregates and a more durable structure of the minor preS2 domain than the preS1 one (Bruss 2007; Chi et al. 2006, 2007; Imamura et al. 1987; Yamada et al. 2001). The retention ratio of VLPs/aggregates during freeze-drying was uppermost (M-HBsAg) or reasonably high (L-HBsAg) for materials with an initial high content of the antigens, while the relative preservation of the antigens with preS domains was the most efficient for materials derived from plants classified to the ‘low’ expression group (Table 1). This observation may be explained by dissimilar requirements of individual antigen domains and molecules versus multimeric particles for membrane lipids and water content affecting the hydration shell.
The same phenomenon, but with a different effect, turned out to be essential during storage of lyophilised tissues. M-HBsAg, both small-size particles (mono- and dimers) and VLPs, as well as the minor preS2 domain were mainly preserved at 22°C and more efficiently in materials coming from ‘high’ expressors (see “Results” and Table 2). The temperature of 37°C could cause a loss of a major part of indispensable structural water, while most of residual water was retained at low storage temperature (see “Results”), which was then probably conducive to gradual degradation (Table 2). In the case of L-HBsAg, the most likely requirement for a larger hydration shell of the joined preS1-preS2 domains differently affected antigen monomers/dimers and aggregates. The ratio of the stored antigen preservation was significantly the highest for materials of initial low and medium antigen contents (Table 2), where residual water ‘supply’ was probably sufficient. The small-size L-HBsAg particles displaying the preS fragment were stored at a satisfactory rate at higher temperatures, whereas extended water retention at 4°C seemed to be indispensable for stabilization of aggregates. Probably residual water formed not only a relatively thin hydration shell around separate preS epitopes but also penetrated between them, facilitating proper interactions and consequently maintaining the structure of oligomeric aggregates with protruding large preS epitopes (Table 2). Different temperatures required for the preservation of preS domains and aggregates of L-HBsAg pose an evident problem. However, at this moment the resultant optimal storage temperature for plant-associated L-HBsAg was found to be 4°C, preferably for material derived from plants of the ‘medium’ expression group (see “Results”, Table 2).
Despite the gradual degradation of lyophilised plant-associated M/L-HBsAg during storage, antigenicity of both proteins was preserved, including VLP forms and preS domains, and in the optimal variants the antigen contents were maintained at the level of several μg/g of tissue. Presented results are 2–3 times lower in comparison to S-HBsAg (Pniewski et al. 2011), but essentially higher than previously observed for M-HBsAg (Salyaev et al. 2010), and in the case of plant-expressed L-HBsAg this report is the first successful attempt at freeze-drying. We presume that a potential plant-derived tri-component anti-HBV vaccine could be prepared by a combination of separate semi-products containing M- and L-HBsAg and previously obtained S-HBsAg (Pniewski et al. 2011).
However, although at this moment the maintained antigen contents in stored tissues seem sufficient, oral immunisation approaches using lyophilised tissue could be performed only directly after the processing had been completed, due to the subsequent gradual deterioration of the stored material. Yet, a potential oral plant-derived vaccine should be durable, according to the immunisation procedures, which usually require 2–3 or more constant antigen doses together with the associated plant tissue, administered within several months. It was shown that plant tissue is conducive to oral tolerance acquisition (Kostrzak et al. 2009), hence an antigen should be stable to avoid the administration of an increased dose of ‘ballast’ tissue. Therefore, we suspended animal immunisation trials till sufficient antigen stability (acceptable 10% fluctuation) would be achieved. Improved processing of plant tissue bearing M/L-HBsAg can be accomplished using appropriate cryoprotectants, buffers, etc. We assume that not only freeze-drying efficiency but also all antigen stability during long-term storage at room temperature would be enhanced, which is particularly important in the case of L-HBsAg.
In conclusion, we showed a high accumulation of M/L-HBsAg in plants, which consequently can be converted into an oral vaccine. At the same time, presented data indicate the importance of initial plant material for antigen stability during lyophilisation and storage and problems revealing concurrently with the processing. In this sense, the results can be considered as a good starting point for optimization experiments and advanced research on physiochemical properties and immunogenecity of lyophilised plant-associated M- and L-HBsAg. Nevertheless, the presented data show that the preparation of an oral plant-derived tri-component anti-HBV vaccine is a realistic goal.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by grants No. 2 P04B 001 27 and No. NN302 157837 from the Polish State Committee for Scientific Research and under the auspices of Medana Pharma Ltd, Sieradz, Poland. We are grateful to Prof. Reinhold Schirmbeck (University of Ulm, Germany) for his kind gift of the CHO-expressed M/L-HBsAg and Prof. Agata Budkowska (Institut Pasteur, Paris, France) for providing anti-preS1 monoclonal antibody 5a-19 as well as Mrs. Teresa Szcześniak for her plant care.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
A contribution to the Special Issue: Plant Molecular Pharming in 2012 and Beyond.
References
- Aziz MA, Midcha S, Waheed SM, Bhatnagar R. Oral vaccines: new needs, new possibilities. BioEssays. 2007;29:591–604. doi: 10.1002/bies.20580. [DOI] [PubMed] [Google Scholar]
- Becker D, Kemper E, Schell J, Masterson R. New plant binary vectors with selectable markers located proximal to the left T-DNA border. Plant Mol Biol. 1992;20:1195–1197. doi: 10.1007/BF00028908. [DOI] [PubMed] [Google Scholar]
- Blanchet M, Sureau C. Infectivity determinants of the hepatitis b virus pre-s domain are confined to the N-terminal 75 amino acid residues. J Virol. 2007;81:5841–5849. doi: 10.1128/JVI.00096-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocke P, Schaefer S, Melber K, Jenzelewski V, Müller F, Dahlems U, Bartelsen O, Park K-N, Janowicz ZA, Gellissen G. Recombinant hepatitis B vaccines: disease characterization and vaccine production. In: Gellissen G, editor. Production of recombinant proteins. Novel microbial and eukaryotic systems. Weinheim: Wiley-VCH Verlag GmbH & Co KGaA; 2005. pp. 319–359. [Google Scholar]
- Bruss V. Hepatitis B virus morphogenesis. World J Gastroenterol. 2007;13:65–73. doi: 10.3748/wjg.v13.i1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li M, Le X, Ma W, Zhou B. Recombinant hepatitis B core antigen carrying preS1 epitopes induce immune response against chronic HBV infection. Vaccine. 2004;22:439–446. doi: 10.1016/j.vaccine.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Chi SW, Kim DH, Kim JS, Lee MK, Han KH. Solution conformation of an immunodominant epitope in the hepatitis B virus preS2 surface antigen. Antiviral Res. 2006;72:207–215. doi: 10.1016/j.antiviral.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Chi SW, Kim DH, Lee SH, Chang I, Han KH. Pre-structured motifs in natively unstructured preS1 surface antigen of hepatitis B virus. Protein Sci. 2007;16:2108–2117. doi: 10.1110/ps.072983507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Ehsani P, Khabiri A, Domansky NN. Polypeptides of hepatitis B surface antigen produced in transgenic potato. Gene. 1997;190:107–111. doi: 10.1016/S0378-1119(96)00647-6. [DOI] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Wallroth M, Eichholtz D, Rogers SG, Fraley RT. Simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Huang X, Lu D, Ji G, Sun Y, Ma L, Chen Z, Zhang L, Huang J, Yu L. Hepatitis B virus (HBV) vaccine-induced escape mutants of HBV S gene among children from Qidong area, China. Virus Res. 2004;99:63–68. doi: 10.1016/j.virusres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Huang Z, Elkin G, Maloney BJ, Buehner N, Arntzen CJ, Thanavala Y, Mason HS. Virus-like particles expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine. 2005;23:1851–1858. doi: 10.1016/j.vaccine.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Imamura T, Araki M, Miyanohara A, Nakao J, Yonemura H, Ohtomo N, Matsubara K. Expression of hepatitis B virus middle and large surface antigen genes in Saccharomyces cerevisiae. J Virol. 1987;61:3543–3549. doi: 10.1128/jvi.61.11.3543-3549.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung YH, Youm JW, Jeon JH, Lee BC, Ryu CJ, Hong HJ, Kim HC, Joung H, Kim HS. Expression of the hepatitis B surface S and preS2 antigens in tubers of Solanum tuberosum. Plant Cell Rep. 2004;22:925–930. doi: 10.1007/s00299-004-0775-1. [DOI] [PubMed] [Google Scholar]
- Kapusta J, Modelska A, Figlerowicz M, Pniewski T, Letellier M, Lisowa O, Yusibov V, Koprowski H, Plucienniczak A, Legocki AB. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 1999;13:1796–1799. doi: 10.1096/fasebj.13.13.1796. [DOI] [PubMed] [Google Scholar]
- Kapusta J, Modelska A, Pniewski T, Figlerowicz M, Jankowski K, Lisowa O, Plucienniczak A, Koprowski H, Legocki AB. Oral immunization of human with transgenic lettuce expressing hepatitis B surface antigen. Adv Exp Med Biol. 2001;495:299–303. doi: 10.1007/978-1-4615-0685-0_41. [DOI] [PubMed] [Google Scholar]
- Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol. 2010;58:273–277. doi: 10.1016/j.patbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Kirk DD, McIntosh K, Walmsley AM, Peterson RKD. Risk analysis for plant-made vaccines. Transgenic Res. 2005;14:449–462. doi: 10.1007/s11248-005-5697-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q, Richter L, Yang YF, Arntzen CJ, Mason HS, Thanavala Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc Natl Acad Sci USA. 2001;98:11539–11544. doi: 10.1073/pnas.191617598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrzak A, Cervantes Gonzales M, Guetard D, Nagaraju DB, Wain-Hobson S, Tepfer D, Pniewski T, Sala M. Oral administration of low doses of plant-based HBsAg induced antigen-specific IgAs and IgGs in mice, without increasing levels of regulatory T cells. Vaccine. 2009;27:4798–4807. doi: 10.1016/j.vaccine.2009.05.092. [DOI] [PubMed] [Google Scholar]
- Lou X-M, Yao Q-H, Zhang Z, Peng R-H, Xiong A-S, Wang H-K. Expression of the human hepatitis B virus large surface antigen gene in transgenic tomato plants. Clin Vaccine Immunol. 2007;14:464–469. doi: 10.1128/CVI.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaliński K, Sylvan SP, Hellstrom U, Mikołajewicz J, Zembrzuska-Sadkowska E, Piontek Es. Antibody responses to preS components after immunization of children with low doses of BioHepB. Vaccine. 2002;20:92–97. doi: 10.1016/S0264-410X(01)00312-7. [DOI] [PubMed] [Google Scholar]
- Mason HS, Thanavala Y, Arntzen CJ, Richter E (2003) Expression of immunogenic hepatitis B surface antigen in transgenic plants. US Patent 6551 820 B1
- May JC, Wheeler RM, Grim E. The gravimetric method for the determination of residual moisture in freeze-dried biological products. Cryobiology. 1989;26:277–284. doi: 10.1016/0011-2240(89)90023-0. [DOI] [PubMed] [Google Scholar]
- McGarvey P, Kaper JM. A simple and rapid method for screening transgenic plants using PCR. Biotechniques. 1991;4:428–432. [PubMed] [Google Scholar]
- Mestecky J, Russell MW, Elson CO. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J Immunol. 2007;179:5633–5638. doi: 10.4049/jimmunol.179.9.5633. [DOI] [PubMed] [Google Scholar]
- Michel M-L, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol. 2010;58:288–295. doi: 10.1016/j.patbio.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Pniewski T, Kapusta J. Efficiency of transformation of Polish cultivars of pea (Pisum sativum L.) with various regeneration capacity by using hypervirulent Agrobacterium tumefaciens strains. J Appl Genetics. 2005;46:139–147. [PubMed] [Google Scholar]
- Pniewski T, Kapusta J, Bociąg P, Wojciechowicz J, Kostrzak A, Gdula M, Fedorowicz-Strońska O, Wójcik P, Otta H, Samardakiewicz S, Wolko B, Płucienniczak A. Low-dose oral immunization with lyophilized tissue of herbicide-resistant lettuce expressing hepatitis B surface antigen for prototype plant-derived vaccine tablet formulation. J Appl Genetics. 2011;52:125–136. doi: 10.1007/s13353-010-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendi-Wagner P, Shouval D, Genton B, Lurie Y, Rümke H, Boland G, Cerny A, Heim M, Bach D, Schroeder M, Kollaritsch H. Comparative immunogenicity of a PreS/S hepatitis B vaccine in non- and low responders to conventional vaccine. Vaccine. 2006;24:2781–2789. doi: 10.1016/j.vaccine.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Richter LJ, Thanavala Y, Arntzen CJ, Mason HS. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol. 2000;18:1167–1171. doi: 10.1038/81153. [DOI] [PubMed] [Google Scholar]
- Romano L, Paladini S, Van Damme P, Zanetti AR. The worldwide impact on the control and protection of viral hepatitis B. Dig Liver Dis. 2011;43S:S2–S7. doi: 10.1016/S1590-8658(10)60685-8. [DOI] [PubMed] [Google Scholar]
- Salyaev RK, Stolbikov AS, Rekoslavskaya NI, Shchelkunov SN, Pozdnyakov SG, Chepinoga AV, Hammond RV. Obtaining tomato plants transgenic for the preS2-S-HDEL gene, which synthesize the major hepatitis B surface antigen. Doklady Biochem Biophys. 2010;433:187–190. doi: 10.1134/S1607672910040113. [DOI] [PubMed] [Google Scholar]
- Shulga NYa, Rukavtsova EB, Krymsky MA, Borisova VN, Melnikov VA, Bykov VA, YaI Buryanov. Expression and characterization of hepatitis B surface antigen in transgenic potato plants. Biochemistry (Moscow) 2004;69:1158–1164. doi: 10.1023/B:BIRY.0000046891.46282.c8. [DOI] [PubMed] [Google Scholar]
- Thanavala Y, Mahoney M, Pal S, Scott A, Richter L, Natarajan N, Goodwin P, Arntzen CJ, Mason HS. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc Natl Acad Sci USA. 2005;102:3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DE, Smith SD, Pickering RJ, Strugnell RA, Dry IB, Wesselingh SL. Measles virus hemagglutinin protein expressed in transgenic lettuce induces neutralising antibodies in mice following mucosal vaccination. Vaccine. 2006;24:3538–3544. doi: 10.1016/j.vaccine.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Yamada T, Iwabuki H, Kanno T, Tanaka H, Tomoji K, Fukuda H, Kondo A, Seno M, Tanizawa K, Kuroda S. Physiochemical and immunological characterization of hepatitis B virus envelope particles exclusively consisting of the entire L (pre-S1 + pre-S2 + S) protein. Vaccine. 2001;19:3154–3163. doi: 10.1016/S0264-410X(01)00017-2. [DOI] [PubMed] [Google Scholar]
- Youm J-W, Won Y-S, Jeon JH, Ryu CJ, Choi Y-K, Kim H-C, Kim B-D, Joung H, Kim HS. Oral immunogenicity of potato-derived HBsAg middle protein in BALB/c mice. Vaccine. 2007;25:577–584. doi: 10.1016/j.vaccine.2006.05.131. [DOI] [PubMed] [Google Scholar]
- Young MD, Rosenthal MH, Dickson B, Du W, Maddrey WC. A multi-center controlled study of rapid hepatitis B vaccination using a novel triple antigen recombinant vaccine. Vaccine. 2001;19:3437–3443. doi: 10.1016/S0264-410X(01)00054-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.