Abstract
In many bird populations, variation in the timing of reproduction exists but it is not obvious how this variation is maintained as timing has substantial fitness consequences. Daily energy expenditure (DEE) during the egg laying period increases with decreasing temperatures and thus perhaps only females that can produce eggs at low energetic cost will lay early in the season, at low temperatures. We tested whether late laying females have a higher daily energy expenditure during egg laying than early laying females in 43 great tits (Parus major), by comparing on the same day the DEE of early females late in their laying sequence with DEE of late females early in their egg laying sequence. We also validated the assumption that there are no within female differences in DEE within the egg laying sequence. We found a negative effect of temperature and a positive effect of female body mass on DEE but no evidence for differences in DEE between early and late laying females. However, costs incurred during egg laying may have carry-over effects later in the breeding cycle and if such carry-over effects differ for early and late laying females this could contribute to the maintenance of phenotypic variation in laying dates.
Keywords: Daily energy expenditure, Timing of breeding, Cost of reproduction, Cost of egg laying, Match–mismatch
Introduction
Strong linear or nonlinear relationships between the timing of breeding and offspring fitness are commonly observed in birds reproducing in highly seasonal environments. In many insectivorous species, for example, reproductive success declines over the course of the breeding season (e.g. Perrins 1970; Verhulst and Tinbergen 1991; Verhulst et al. 1995). Earlier broods have more fledglings with a higher recruitment rate due to their higher fledging mass and an effect of date per se (Tinbergen and Boerlijst 1990; Verboven and Visser 1998; Visser and Verboven 1999). In some cases (e.g. great tits, Parus major, in The Netherlands), selection for early breeding has intensified over the past few decades because of an increasing mismatch between different trophic levels as a result of climate change (Visser et al. 1998). However, considerable among-individual variation appears to be maintained within populations, despite consistent patterns of directional and/or stabilizing selection, highlighting the need to understand proximate and ultimate factors responsible for generating and maintaining these differences among individuals.
The classical explanation for differences in timing of breeding is that variation in the timing of local food availability during the nestling phase exists and as a consequence birds adapt their timing of reproduction to this local timing of food availability (Rowan 1926; Lack 1968; Daan et al. 1989). Differences in the mean breeding date of populations might thus reflect adaptive evolutionary divergence, where early breeding occurs in places where food peaks in the nestling phase are earliest, while variation within populations could reflect a combination of genetic differences and adaptive plasticity (e.g. where individuals occupying different territories adjust their breeding time to match local food peaks).
An alternative explanation for adaptive variation in timing of breeding is that laying dates (date when the first egg of a clutch is laid) are affected by food conditions in the period before egg laying. Egg laying is energetically costly as shown by increased metabolic rates in egg laying females (Walsberg 1983; Nilsson and Raberg 2001; Vezina and Williams 2002). In order to match the timing of maximum food requirements (late in the nestling period; Moreno et al. 1995; Verhulst and Tinbergen 1997) with the timing of maximum food availability (caterpillar food peak; Visser et al. 2006), eggs have to be laid up to 5 weeks in advance of the food peak. Thus, eggs are laid in cold weather conditions under which foraging efficiency is low (Avery and Krebs 1984) and energetic costs are high (Stevenson and Bryant 2000). For a long time, it was thought that limitation of resources set the earliest possible laying date within a year, and that birds did not lay earlier simply because they were unable to obtain enough resources (Perrins 1970). However, this resource limitation hypothesis could also be seen as a trade-off between costs and benefits. Early laying potentially allows for a better match with the food peak for the nestlings (resulting in nestlings of good condition which are more likely to recruit), but producing eggs under harsh conditions early in the season can result in an increase in energetic costs (direct costs) which can have negative effects on current and future reproductive success (indirect costs). Fitness costs of increased work load have been studied by Monaghan et al. (1998), who showed that female lesser black-backed gulls (Larus fuscus) which had experimentally increased costs during egg laying (by producing more eggs) had reduced offspring rearing capacity in the nestling stage, resulting in reduced fledgling body mass. Visser and Lessells (2001) showed that experimentally increasing costs of reproduction in 1 year affected the timing of breeding the following year—female great tits with increased costs started egg laying later in the year after manipulation. Survival of females also decreased with increasing costs sustained during the breeding cycle (Visser and Lessells 2001), at the expense of future reproductive success. Therefore, it may be adaptive for some females to lay her eggs later than what would be best for the match between chicks’ needs and food conditions during chick feeding in order to increase chances of future reproductive success (Visser et al., submitted). As income breeders, the energetic expenditure of gathering enough food for egg production by a female great tit can vary depending on local food availability before and during egg laying. So, under this hypothesis, differences in laying dates can result from differences in the timing or abundance of food availability on the territory level in the period before egg laying. Alternatively, differences in laying date may arise if there are among female differences in the energetic expenditure necessary to produce an egg, perhaps due to intrinsic differences in female quality.
We investigated whether there is variation among females in their daily energetic expenditure (DEE; measured using the doubly labelled water technique; Speakman 1997) during egg production. A difference in DEE between females indicates that something in their environment (ability to obtain resources at lower energetic costs) or their physiology (ability to produce eggs at lower energetic cost) differs. As environmental conditions improve over the season (and assuming that the environmental conditions are equal for all females), those females who are able to produce eggs at low costs will be able to lay already under poorer environmental conditions, and thus will breed earlier. Therefore, when comparing early and late breeding females on the same day, we predicted that early laying females have a lower DEE than later laying females.
Comparing DEE of early and late breeding females is difficult, since factors affecting DEE (e.g. weather conditions and food availability) change over the course of the season and from day to day. We compared DEE of females with different first egg laying dates on the same day, and thus avoid day to day variation in conditions, by making use of the overlap in their egg laying period (the great tit’s laying period is around 8 days depending on the clutch size; 1 egg per day, average clutch size ~9 eggs). We compared DEE late in the laying sequence of early females with DEE of late females early in their laying sequence during their overlapping laying period. By comparing within single days, we did not need to correct for the effects of temperature and food availability. We also tested the assumption that there are no systematic differences in DEE at different times during the egg laying period by measuring DEE of a number of females twice during egg laying.
Materials and methods
In 2008 and 2009, DEE of 78 female great tits in two nearby study areas (20 km apart) was measured during egg laying (Table 1); 19 females (2008) from the Oosterhout population (51°52′22″N, 5°50′22″E) and 59 females (2008, n = 50; 2009, n = 9) from the Hoge Veluwe population (52°02′07″N, 5°51′32″E). Of these females, DEE of 13 females [Hoge Veluwe, n = 9 (2009); Oosterhout, n = 4 (2008)] was measured twice during their laying sequence for a within-female comparison of DEE as a function of egg sequence, resulting in a total of 91 measurements of DEE. Of the 59 females measured in the Hoge Veluwe study area, 43 females were randomly assigned to be measured early or late in their laying sequence for the comparison of DEE between early and late females.
Table 1.
Overview of the number of daily energy expenditure (DEE) measurements of great tits (Parus major) taken for this study
| Egg 4 | Egg 8 | Other | Measured twice | Total number of females | Total number of DEE samples | |
|---|---|---|---|---|---|---|
| Hoge Veluwe | ||||||
| 2008 | 25 | 18 | 7 | 0 | 50 | 50 |
| 2009 | 9 | 9 | 9 | 18 | ||
| Oosterhout | ||||||
| 2008 | 19 | 4 | 19 | 23 | ||
| Totals | 25 | 18 | 35 | 13 | 78 | 91 |
Egg 4, Egg 8 and Other refer to the periods over which DEE is measured (i.e. for females measured on egg 4 we injected doubly labelled water in the morning the second egg was laid, we collected egg 3 and egg 4 and measured isotope elimination rates between egg 3 and 4 from which DEE was calculated. One egg was laid per day). Only measurements under Egg 4 and Egg 8 were used in the analyses for comparison of early and late females. Measured twice refers to the number of females of which DEE was measured twice during the same laying period
The Hoge Veluwe study area is part of a large forest area and consists of 171 ha of mixed woodland on poor sandy soils, dominated by oak (Quercus robur), American oak (Quercus rubra), larch (Larix decidua) and pine (Pinus sylvestris) with about 400 nest boxes. The Oosterhout study site is an isolated deciduous forest dominated by oak trees (11 ha) along a residential area on rich clay soils near the river Waal with 150 nest boxes. Average first egg laying dates of the Oosterhout population are 3.4 days earlier compared to the Hoge Veluwe population (range −4–15.4; 5.8 days earlier in 2008; M.E.V., unpublished data).
Measuring daily energy expenditure
Doubly labelled water technique using eggs as samples
The double labelled water technique enables us to measure DEE in free-living animals by measuring elimination rates of 2H and 18O which are enriched in the body water of the animal through injection (Speakman 1997; see below). Instead of measuring elimination rates of 2H and 18O isotopes in blood samples (as is usually done), we measured elimination rates in the albumen of the eggs produced (as was done by Ward 1996; Stevenson and Bryant 2000). This avoided recapture and handling stress in a period during which disturbance leads to high desertion rates (Kania 1989). The period over which DEE is measured using this method depends on the exact timing after which water molecules can not freely move between the females’ body water pool and the water in the egg (i.e. the timing of which the egg shell is produced, separating the female body water pool from the water in the egg). Ward (1996) studied the relationship between isotope concentrations in the albumen and blood for barn swallows (Hirundo rustica) and found that the average time of the day at which the water pools of female and egg separate was around 2245 hours. Although this timing is not known in great tits, it is the timing of separation within a female over successive eggs which is important. No evidence exist that this timing differs between successive eggs within a female’s laying sequence. Values of DEE found in this study are comparable to DEE values during egg laying found by Stevenson and Bryant (2000) and are about 2.5 times BMR, which was calculated with equation 5.1 in Kendeigh et al. (1977) for the average body mass of a female great tit of our dataset (19.6 g), which falls well within the range of DEE/BMR-ratios found for this and other species (King 1974; Daan et al. 1990; Peterson et al. 1990) in other parts of the breeding season.
Field methods
Nest boxes were checked twice a week from the beginning of April to monitor nest building. Once the bottom of the box was covered with nest material, nests were checked daily to determine exact laying dates. Eggs were numbered on the day they were laid. Female great tits were caught when leaving the nest box early in the morning, soon after egg laying using temporarily placed ‘box nets’ (te Marvelde et al. 2011a). Immediately after catching, each female was injected intraperitoneally with 200 μl DLW using a 0.3-ml syringe with a 0.33 × 12 mm needle. After injecting, the female was weighed to the nearest 0.1 g and then released (usually within 10 min after catching). Early females were caught and injected with DLW on the morning they laid their sixth egg (n = 25), whereas late females were injected in the morning they laid their second egg (n = 18). The day after the injection, the third egg of a late and the seventh egg of an earlier female were collected. The next day, we collected the fourth and the eighth egg of those females. Because we can only capture the female the morning after we detect the first egg, the earliest measurement of DEE is the 24 h between the production of the third and the fourth egg. For the early females, we chose the eighth egg as a last sample since not all females produce more than eight eggs. All eggs taken as samples were replaced immediately by unbrooded eggs from a nearby study site to prevent the production of replacement eggs.
About 12 h after collection, the length, width and mass of the eggs were measured and the yolk and the albumen were separated and weighed to the nearest mg in the laboratory. The albumen was homogenised by hand and three samples of ~15 μl were transferred to non-heparinised 25-μl capillaries which were flame-sealed immediately.
Measuring isotope ratios in the samples
Isotopes were analysed at the Centre for Isotope Research (Groningen, The Netherlands) using methods described in detail elsewhere (Visser and Schekkerman 1999; Visser et al. 2000; Van Trigt et al. 2002). The albumen in the capillary tubes was distilled in a vacuum line and brought into a standard vial for automatic injection into the isotope ratio mass spectrometer system. Local water standards (gravimetrically prepared from pure 2H- and 18O-water), that cover the entire enrichment range of the albumen samples, were applied for calibration purposes. The actual 18O and 2H measurements were performed in automatic batches using a High Temperature pyrolysis unit (Hekatech) coupled to a GVI Isoprime Isotope Ratio Mass Spectrometer for the actual isotope analysis.
Calculating daily energy expenditure
The rate of CO2 production was calculated according to formula 7.17 of Speakman (1997):
 |
where N is the total body water (TBW) and k 18O and k 2H are the 18O and 2H decay rates per hour (decay on a log scale divided by time interval between the two samples; 24 h for 81 measurements, 48 h for 10 measurements in case of a laying pause of 1 day after the first sample). The rightmost term is a correction to the simple proportionality between TBW, decay rates difference and rCO2, which is caused by isotope fractionation effects. The coefficient of this term depends on the assumptions regarding the fraction of water loss through breath. Here, we follow Speakman’s assumption that this fraction is 25%. This assumption has been found to be the most appropriate (Visser and Schekkerman 1999; Van Trigt et al. 2002). TBW depends on TBW% and body mass. TBW% can be estimated when a sample is taken 1 h after injection of the DLW. We were not able to estimate TBW% since our first sample is taken about 20 h after injection. We assume a constant TBW percentage of 66% based on dried great tits (Mertens 1987). We weighed the female immediately after injection of the DLW and do not have body mass at the sampling times represented by each egg. Since the TBW changes during egg laying (the production of the egg increases body mass, which drops again when the egg is laid), we used the average pool size as suggested by Lifson and McClintock (1966) by averaging the weight of the female with and without the egg of the final sample. An energy equivalent of 27.8 kJ per litre CO2 produced was used to transform CO2 production into energy expenditure per hour after which it is multiplied by 24 to get daily energy expenditure.
An important assumption in the comparison between early and late females is that no consistent differences in DEE exist within females during the egg laying sequence. To test this, DEE of 13 females [Hoge Veluwe, n = 9 (2009); Oosterhout, n = 4 (2008)] were measured twice in their egg laying sequence.
Temperature data
Temperatures (measured every hour) were retrieved from the Royal Netherlands Meteorological Institute location Deelen (~2 km from the Hoge Veluwe and ~21 km from the Oosterhout study site). Both study locations are inland and we have no reason to believe that differences in temperature exist for the two locations. Since the timing of separation of the egg from the female TBW is largely unknown (but falls between ~1900 and 0600 hours), we tested average temperatures over three different 24-h periods (1900–1900, 0000–0000 and 0600–0600 hours). Our results do not change depending on the period over which we average temperature, but since midnight–midnight temperature explained most of the variation, we present the results of average temperatures during this period.
Statistics
Effect of egg number on DEE
To test the assumption that no consistent differences exist in DEE within a females’ laying sequence, we used the ‘within-subject centering’ procedure to separate within and between effects of egg number (e.g. egg number 4 is the 4th egg laid) on DEE in a linear mixed model (van de Pol and Wright 2009). We tested the significance of within female egg number on DEE in a mixed model with DEE as response variable (and female identity as a random effect) and average temperature (midnight–midnight), female body mass, mean egg number (showing the between female effect of egg number on DEE), deviation of the mean egg number for each individual DEE measurement (showing the within female effect of egg number on DEE) as explanatory variables. The latter variable is of most interest. We also included temperature and female body mass since these variables appeared to be of importance in the analyses of the full data set.
Full data set
We explored which factors affected DEE using the whole dataset (both populations; n = 78). A linear mixed model (with date as random effect) was used to account for the fact that multiple females were measured on a single day. Our starting model with DEE as response variable contained average temperature (midnight–midnight), study area, female body mass, number of eggs laid after the final sampled egg, yolk and albumen mass of the final sample, date, final clutch size and the interaction between egg number and temperature. Of those females that were measured twice (n = 13), one randomly chosen measurement was included in the analyses. Least significant terms were removed from the model, starting with the interaction, resulting in a final model with only significant variables (Table 2A).
Table 2.
Effects of ambient temperature (averaged from midnight–midnight), study area, female body mass, the number of eggs laid after the date DEE was measured (eggs left), various egg characteristics, final clutch size and date on daily energy expenditure (DEE) during egg laying analysed using the full dataset (A); to test the difference between earlier and later laying females we used data from the Hoge Veluwe females for which DEE (kJ day−1) was measured on egg 4 or 8 (egg number; late vs. early breeders); first, we ran a 2-way ANOVA (B) followed by linear mixed model correcting for temperature and female body mass (C)
| Variable | Estimate | SE | df | F | P | |
|---|---|---|---|---|---|---|
| A | Linear mixed model: full data set (n = 78 females, 78 measurements) | |||||
| Temperature | 1 | 42.9 | <0.0001 | |||
| Area | ||||||
| Hoge Veluwe | 31.02 | 12.46 | 1 | 5.01 | 0.029 | |
| Oosterhout | 33.52 | 12.59 | ||||
| Female body mass | 2.93 | 0.64 | 1 | 20.92 | <0.0001 | |
| Temperature × area | ||||||
| Hoge Veluwe | −1.14 | 0.34 | 1 | 0.047 | 0.83 | |
| Oosterhout | −1.03 | 0.53 | ||||
| Year (2009) | 2.96 | 1.93 | 1 | 3.60 | 0.06 | |
| Eggs left | 0.32 | 0.30 | 1 | 1.13 | 0.29 | |
| Mass yolk | −6.20 | 17.06 | 1 | 0.13 | 0.72 | |
| Mass albumen | 3.32 | 6.19 | 1 | 0.29 | 0.59 | |
| Final clutch size | 0.21 | 0.33 | 1 | 0.41 | 0.53 | |
| Date | −0.06 | 0.15 | 1 | 0.14 | 0.72 | |
| B | 2-way ANOVA: early versus late breeders (n = 43, only Hoge Veluwe area) | |||||
| Female body mass | 2.16 | 0.98 | 1 | 4.87 | 0.03 | |
| Date | 8 | 1.37 | 0.25 | |||
| Egg number | −2.22 | 1.76 | 1 | 1.58 | 0.22 | |
| Date × egg number | 8 | 0.58 | 0.78 | |||
| C | Linear mixed model: early versus late breeders (n = 43, only Hoge Veluwe area) | |||||
| Temperature | −1.19 | 0.39 | 1 | 8.99 | 0.005 | |
| Female body mass | 2.18 | 0.89 | 1 | 5.98 | 0.020 | |
| Egg number | −1.37 | 1.65 | 1 | 0.69 | 0.41 | |
| Egg number × temperature | −0.31 | 0.84 | 1 | 0.14 | 0.72 | |
Significant values, p < 0.05, in bold
Comparison between early and late females (Hoge Veluwe data only)
We used two separate tests (two alternative ways) to test the difference in DEE between early and late laying females. First, we ran a 2-way ANOVA with DEE as the response variable, date as factor (with 9 levels) and egg number (egg 4 vs. egg 8 as 2 levels; Table 2B). We used date as a factor to account for all possible differences that occurred between days.
As an alternative way to answer the same question, we ran a linear mixed model with DEE as response variable, date as random variable and temperature, female body mass, egg number (egg 4 vs. egg 8 as 2 levels) and the interaction between temperature and egg number (Table 2C). Least significant terms were removed from the model, starting with the interaction terms, resulting in a final model with only significant variables.
All statistics were carried out using R version 2.9.2 (R Development Core Team 2009). All tests were two-tailed and an alpha level of 0.05 was applied throughout.
Results
Effect of egg number on DEE
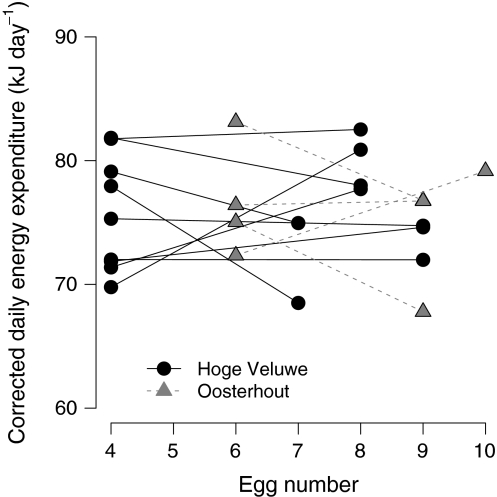
When corrected for temperature, female body mass and the among female effect of egg number on DEE, we found no significant within female effect for egg number on DEE (estimate ± SE = 0.080 (±0.45), df = 1, error df = 10, t value = 0.18, P = 0.86; Fig. 1), validating the assumption that costs during egg laying do not depend on where in the sequence of egg laying DEE is measured, and thus justifying the between female comparison below.
Fig. 1.
Within female effect of egg number on daily energy expenditure (kJ day−1) of female great tits (Parus major) of the Hoge Veluwe (n = 9, collected in 2009) and the Oosterhout population (n = 4, collected in 2008). The DEE values are plotted after correcting for female body mass, temperature and area for graphing purposes only. Lines connect the two measurements of individual females
Full dataset
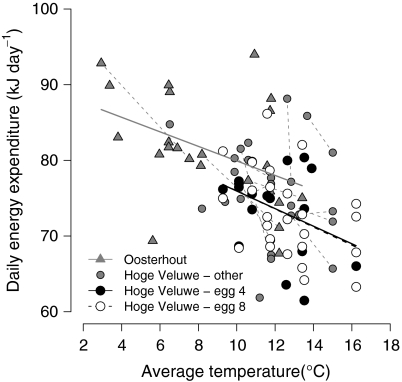
Daily energy expenditure of female great tits during egg laying decreased with ambient temperature (using all 78 DEE measurements; Fig. 2; see Table 2A for statistics). The slope of the temperature effect on DEE did not differ between the two study areas, but Oosterhout females expended more energy than the Hoge Veluwe females at a given temperature. Furthermore, DEE increased with body mass. There was no effect of various egg characteristics, clutch size, date or the number of eggs left to lay after the DEE measurement date on DEE.
Fig. 2.
Effect of ambient temperature (°C) on daily energy expenditure (kJ day−1) of female great tits during egg laying in two study areas (Oosterhout and Hoge Veluwe). Comparison of DEE between early and late females was done for those females measured on the day their fourth or their eighth egg was laid. Lines represent the regression lines through the individual sets of points. Grey dashed lines connect two DEE measurements of individual females measured twice in their laying period. Note that the regression lines of early and late females of the Hoge Veluwe (Hoge Veluwe egg 8 and egg 4, respectively) fall on top of each other and are not statistically different (see Table 2C)
Comparison between early and late females (Hoge Veluwe data only)
In a 2-way ANOVA with date (factor with 9 levels) and egg number (factor with 2 levels; egg 4 vs. egg 8), we found no difference in DEE between early and late breeding female great tits (Table 2B), but DEE was related to female body mass. However, since females measured on egg 8 (early females) tended to be heavier than females measured on egg 4 (late females; Welch two-sample t test, P = 0.059), differences in body mass could not mask differences in DEE between early and late breeding females. In fact, the difference in DEE between early and later breeders increased when correcting for female body mass (from 0.89 to 2.22 kJ day−1) but remained non-significant. Although no clear differences in DEE existed between the females tested on different dates (Table 2B), in a linear mixed model where we substituted temperature for date, we found a temperature effect on DEE, similar to that in the full dataset. This effect of temperature on DEE did not differ between early and late laying females (interaction egg number × temperature) (Fig. 2; Table 2C).
Discussion
Female great tits expended more energy during egg laying under colder conditions and when their body mass was high. On average, earlier females will therefore spend more energy during egg laying due to the seasonal trend of increasing temperatures in spring. However, we found no difference in energy expenditure over a 24-h period during egg laying between early and late females measured on the same day, under the same weather conditions. The hypothesis that variation in laying date could be explained by differences in the relationship of temperature with energetic expenditure during egg laying between early and late birds was thus not confirmed.
To compare early with late females, we made use of the overlapping nature of egg laying periods. In our case, the difference in laying date between early and late laying females was 4 days. Although 4 days is a relatively short period compared to the total duration on which birds start egg laying (difference in laying date between the earliest bird vs. latest bird is ~30 days), the standard deviation of laying dates in 2008 was only 4.2 days. This means that 68.2% of all birds start egg laying in a period of 8.4 days only and thus that an interval of 4 days is biologically relevant.
Even though we found no intrinsic differences in energy expenditure between early and late breeding females, this does not mean that there are no differences in the fitness consequences of laying under cold conditions between early and late breeders. It is possible that variation in the fitness costs of energetic expenditure exists among females, potentially explaining variation in laying dates. Many examples exist in which an increased workload (for example by increasing the number of nestlings in a brood) negatively affects the survival of the parents (e.g. Dijkstra et al. 1990; Deerenberg and Overkamp 1999). However, to explain the maintenance in variation in laying dates from an optimality perspective, there have to be individual differences in fitness costs due to increased energetic expenditure. To our knowledge, this has never been demonstrated.
Our results show that female great tits in Oosterhout expended more energy during egg laying than the Hoge Veluwe females. This difference could be caused by genetic differences between the populations [none of the 3,376 known breeding birds in Oosterhout were born in Hoge Veluwe and only two known breeding birds in Hoge Veluwe (out of 13,979 birds) were born in Oosterhout]. Alternatively, the between-site difference in energy expenditure could be due to differences in the timing of the caterpillar food peak. The Oosterhout population is situated on rich river clay soil compared to the sandy soil of the Hoge Veluwe. The difference in soil type is most likely why tree leafing is earlier in Oosterhout compared to the Hoge Veluwe, resulting in an earlier caterpillar food peak. This means that Oosterhout females have to lay eggs earlier (and under colder conditions; Fig. 2) and thus expend more energy. Since great tits do not store large amounts of fat, maximum energy expenditure will directly depend on energy intake. From radio tracking data in both populations in the period before egg laying (L. te Marvelde and M.E. Visser, unpublished data), we know that females in Oosterhout often make use of the vast amount of supplementary bird food in the nearby village, whereas a food source like this is not available to the Hoge Veluwe population. The supplementary food in Oosterhout consists mainly of fat and peanuts; food full of energy but which does not contain the proteins needed for egg production. We have seen that females often visit the supplementary food for ~15 min after which they fly back to their territory to forage by hopping in the crown of the tree, most likely looking for (protein-rich) insects. The supplementary food then only serves as fuel that facilitates foraging for protein rich foods. As a consequence, they are able to work harder and therefore expend more energy compared to the Hoge Veluwe females.
A better understanding of the causes and consequences of variation in laying date among females is important as, due to climate warming, there is increased selection on laying date (Visser et al. 2006). Selection is influenced by, and potentially alters, the costs and benefits of the entire reproductive cycle, so we need to understand how females differ in their energetic costs (and in fitness consequences of these costs) not only during egg laying but also during other periods of the breeding cycle, such as chick feeding (te Marvelde et al. 2011b). Understanding the proximate and ultimate factors maintaining variation among females will provide insight into patterns of phenotypic selection in natural populations.
Acknowledgments
‘Stichting Het Nationale Park De Hoge Veluwe’ and family Van Boetzelaer in Oosterhout kindly allowed us to conduct our research on their property. We thank Berthe Verstappen and Henk Jansen for their careful sample preparations and measurements, Benoît Sandjian, Henri Bouwmeester and Louis Vernooij for help during the field season and Esa Lehikoinen, Kate Lessells, Thomas E. Reed and an anonymous reviewer for comments on an earlier version of the manuscript. M.E.V. is supported by a NWO-VICI Grant. The experiments reported here comply with the current law in The Netherlands and were carried out under licenses of the Animal Experimental Committee of the KNAW (DEC protocol no. CTE 08.01).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Avery MI, Krebs JR. Temperature and foraging success of great tits (Parus major) hunting for spiders. Ibis. 1984;126:33–38. doi: 10.1111/j.1474-919X.1984.tb03661.x. [DOI] [Google Scholar]
- Daan S, Dijkstra C, Drent RH, Meijer T. Food supply and the annual timing of avian reproduction. Acta Int Ornithol Congr. 1989;19:392–407. [Google Scholar]
- Daan S, Masman D, Groenewold A. Avian basal metabolic rates—their association with body-composition and energy-expenditure in nature. Am J Physiol. 1990;259:R333–R340. doi: 10.1152/ajpregu.1990.259.2.R333. [DOI] [PubMed] [Google Scholar]
- Deerenberg C, Overkamp GJF. Hard work impinges on fitness: an experimental study with zebra finches. Anim Behav. 1999;58:173–179. doi: 10.1006/anbe.1999.1123. [DOI] [PubMed] [Google Scholar]
- Dijkstra C, Bult A, Bijlsma S, Daan S, Meijer T, Zijlstra M. Brood size manipulations in the kestrel (Falco tinnunculus)—effects on offspring and parent survival. J Anim Ecol. 1990;59:269–285. doi: 10.2307/5172. [DOI] [Google Scholar]
- Kania W. Safety of catching adult European birds at the nest. Ringers’ opinions. Ring. 1989;14:5–50. [Google Scholar]
- Kendeigh SC, Dolnik VR, Gavrilov VM. Avian energetics. In: Pinowski J, Kendeigh SC, editors. Granivorous birds in ecosystems. Cambridge: Cambridge University Press; 1977. pp. 127–204. [Google Scholar]
- King JR. Seasonal allocation of time and energy resources in birds. In: Paynter RA Jr, editor. Avian energetics. Cambridge: Nuttall Orn Club; 1974. pp. 4–70. [Google Scholar]
- Lack DL. Ecological adaptations for breeding in birds. London: Methuen; 1968. [Google Scholar]
- Lifson N, McClintock R. Theory of use of turnover rates of body water for measuring energy and material balance. J Theor Biol. 1966;12:46–74. doi: 10.1016/0022-5193(66)90185-8. [DOI] [PubMed] [Google Scholar]
- Mertens JAL. The influence of temperature on the energy reserves of female great tits during the breeding-season. Ardea. 1987;75:73–80. [Google Scholar]
- Monaghan P, Nager RG, Houston DC. The price of eggs: increased investment in egg production reduces the offspring rearing capacity of parents. Proc R Soc Lond B. 1998;265:1731–1735. doi: 10.1098/rspb.1998.0495. [DOI] [Google Scholar]
- Moreno J, Cowie RJ, Sanz JJ, Williams RSR. Differential response by males and females to brood manipulations in the pied flycatcher—energy-expenditure and nestling diet. J Anim Ecol. 1995;64:721–732. doi: 10.2307/5851. [DOI] [Google Scholar]
- Nilsson JA, Raberg L. The resting metabolic cost of egg laying and nestling feeding in great tits. Oecologia. 2001;128:187–192. doi: 10.1007/s004420100653. [DOI] [PubMed] [Google Scholar]
- Perrins CM. Timing of birds breeding seasons. Ibis. 1970;112:242–255. doi: 10.1111/j.1474-919X.1970.tb00096.x. [DOI] [Google Scholar]
- Peterson CC, Nagy KA, Diamond J. Sustained metabolic scope. Proc Natl Acad Sci USA. 1990;87:2324–2328. doi: 10.1073/pnas.87.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan W. On photoperiodism, reproductive periodicity, and the annual migration of birds and certain fishes. Proc Boston Soc Nat Hist. 1926;38:147–189. [Google Scholar]
- Speakman JR. Doubly labelled water: theory and practice. London: Chapman & Hall; 1997. [Google Scholar]
- Stevenson IR, Bryant DM. Avian phenology—climate change and constraints on breeding. Nature. 2000;406:366–367. doi: 10.1038/35019151. [DOI] [PubMed] [Google Scholar]
- te Marvelde L, Webber SL, van den Burg AB, Visser ME (2011) A new method for catching cavity-nesting birds during egg laying and incubation. J Field Ornithol (doi:10.1111/j.1557-9263.2011.00335.x)
- te Marvelde L, Webber SL, Meijer HAJ & Visser ME (2011) Mismatched reproduction is energetically costly for chick feeding female great tits. Funct Ecol (doi:10.1111/j.1365-2435.2011.01889.x)
- R Development Core Team (2009) R: a language and environment for statistical computing., Vienna (Austria), R Foundation for Statistical Computing
- Tinbergen JM, Boerlijst MC. Nestling weight and survival in individual great tits (Parus major) J Anim Ecol. 1990;59:1113–1127. doi: 10.2307/5035. [DOI] [Google Scholar]
- van de Pol MV, Wright J. A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav. 2009;77:753–758. doi: 10.1016/j.anbehav.2008.11.006. [DOI] [Google Scholar]
- Van Trigt R, Kerstel ERT, Neubert REM, Meijer HAJ, McLean M, Visser GH. Validation of the DLW method in Japanese quail at different water fluxes using laser and IRMS. J Appl Physiol. 2002;93:2147–2154. doi: 10.1152/japplphysiol.01134.2001. [DOI] [PubMed] [Google Scholar]
- Verboven N, Visser ME. Seasonal variation in local recruitment of great tits: the importance of being early. Oikos. 1998;81:511–524. doi: 10.2307/3546771. [DOI] [Google Scholar]
- Verhulst S, Tinbergen JM. Experimental evidence for a causal relationship between timing and success of reproduction in the great tit Parus major. J Anim Ecol. 1991;60:269–282. doi: 10.2307/5459. [DOI] [Google Scholar]
- Verhulst S, Tinbergen JM. Clutch size and parental effort in the great tit Parus major. Ardea. 1997;85:111–126. [Google Scholar]
- Verhulst S, Van Balen JH, Tinbergen JM. Seasonal decline in reproductive success of the great tit—variation in time or quality. Ecology. 1995;76:2392–2403. doi: 10.2307/2265815. [DOI] [Google Scholar]
- Vezina F, Williams TD. Metabolic costs of egg production in the European starling (Sturnus vulgaris) Physiol Biochem Zool. 2002;75:377–385. doi: 10.1086/343137. [DOI] [PubMed] [Google Scholar]
- Visser ME, Lessells CM. The costs of egg production and incubation in great tits (Parus major) Proc R Soc Lond B. 2001;268:1271–1277. doi: 10.1098/rspb.2001.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser GH, Schekkerman H. Validation of the doubly labeled water method in growing precocial birds: the importance of assumptions concerning evaporative water loss. Physiol Biochem Zool. 1999;72:740–749. doi: 10.1086/316713. [DOI] [PubMed] [Google Scholar]
- Visser ME, Verboven N. Long-term fitness effects of fledging date in great tits. Oikos. 1999;85:445–450. doi: 10.2307/3546694. [DOI] [Google Scholar]
- Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM. Warmer springs lead to mistimed reproduction in great tits (Parus major) Proc R Soc Lond B. 1998;265:1867–1870. doi: 10.1098/rspb.1998.0514. [DOI] [Google Scholar]
- Visser GH, Boon PE, Meijer HAJ. Validation of the doubly labeled water method in Japanese Quail Coturnix c. japonica chicks: is there an effect of growth rate? J Comp Physiol B Biochemical Syst Env Physiol. 2000;170:365–372. doi: 10.1007/s003600000112. [DOI] [PubMed] [Google Scholar]
- Visser ME, Holleman LJM, Gienapp P. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia. 2006;147:164–172. doi: 10.1007/s00442-005-0299-6. [DOI] [PubMed] [Google Scholar]
- Walsberg GE. Avian ecological energetics. In: Farner DS, King JR, Parkes KC, editors. Avian Biology . New York: Academic; 1983. pp. 161–220. [Google Scholar]
- Ward S. Energy expenditure of female barn swallows Hirundo rustica during egg formation. Physiol Zool. 1996;69:930–951. [Google Scholar]