Abstract
The spatial scale of disturbance is a factor potentially influencing the relationship between disturbance and diversity. There has been discussion on whether disturbances that affect local communities and create a mosaic of patches in different successional stages have the same effect on diversity as regional disturbances that affect the whole landscape. In a microcosm experiment with metacommunities of aquatic protists, we compared the effect of local and regional disturbances on the disturbance–diversity relationship. Local disturbances destroyed entire local communities of the metacommunity and required reimmigration from neighboring communities, while regional disturbances affected the whole metacommunity but left part of each local community intact. Both disturbance types led to a negative relationship between disturbance intensity and Shannon diversity. With strong local disturbance, this decrease in diversity was due to species loss, while strong regional disturbance had no effect on species richness but reduced the evenness of the community. Growth rate appeared to be the most important trait for survival after strong local disturbance and dominance after strong regional disturbance. The pattern of the disturbance–diversity relationship was similar for both local and regional diversity. Although local disturbances at least temporally increased beta diversity by creating a mosaic of differently disturbed patches, this high dissimilarity did not result in regional diversity being increased relative to local diversity. The disturbance–diversity relationship was negative for both scales of diversity. The flat competitive hierarchy and absence of a trade-off between competition and colonization ability are a likely explanation for this pattern.
Electronic supplementary material
The online version of this article (doi:10.1007/s00442-011-2140-8) contains supplementary material, which is available to authorized users.
Keywords: Disturbance scale, Local versus regional, Metacommunity, Microcosms, Protists
Introduction
Disturbance has long been proposed as a mechanism enabling the coexistence of species, with a unimodal relationship between diversity and the level of disturbance predicted by the intermediate disturbance hypothesis (IDH; Connell 1978). Diversity is expected to be low in both weakly and strongly disturbed systems through competitive exclusion and extinction of disturbance-intolerant species, respectively. Diversity should be maximized by intermediate disturbances that disrupt competitive displacement before elimination of weak competitors but do not drive disturbance-intolerant species to extinction. However, only a minority of experimental and observational studies on the disturbance–diversity relationship reports the expected unimodal pattern (reviewed in Mackey and Currie 2001).
One factor possibly influencing the disturbance–diversity relationship is the spatial scale of the disturbance. There has been discussion on whether the IDH is a between-patch mechanism relying on the presence of a mosaic of patches in different successional stages (Wilson 1994), or works both within a single patch and between patches (Collins and Glenn 1997). In a modeling approach, Roxburgh et al. (2004) compared between-patch and within-patch disturbances. Between-patch disturbances completely destroyed patches within a landscape and required recolonization by reimmigration from outside the disturbed patch, while within-patch disturbances affected the whole landscape but left residuals behind. This study demonstrated that coexistence via disturbance of intermediate frequency is possible for both disturbance types, as long as the species show a trade-off between the ability to recolonize the disturbed patch and competitive ability. However, the two disturbance types require different traits for recolonization. Spatially patchy disturbances enable species coexistence when the inferior competitor is better at dispersing to and colonizing a recently disturbed patch. Theoretically, such a trade-off between competition and colonization ability can enable coexistence of an unlimited number of species, assuming that inferior competitors have sufficiently higher colonization abilities than superior competitors (Tilman 1994). Global within-patch disturbances require other traits for recolonization. Such disturbances favor species that either have higher maximal growth rates enabling them to profit from the resource-rich conditions after the disturbance (Pacala and Rees 1998; Rees et al. 2001), or that leave more residuals behind due to production of resting eggs or cysts, vegetative resprouting, higher survival in the seed bank, protective structures or less susceptible growth forms (Sousa 1984b; Pake and Venable 1996; Cáceres 1997; Turner et al. 1998).
Both types of disturbance are common in nature. Examples for spatially patchy disturbances are wave disturbances in intertidal boulder fields or mussel beds (Sousa 1979, 1984a; Paine and Levin 1981), mound building by burrowing animals in grasslands (Platt 1975; Guo 1996; Questad and Foster 2007), and small floods creating a mosaic of differently disturbed patches for river invertebrates (Matthaei et al. 1999, 2000). Examples for within-patch disturbances are droughts in marshes and large chaparral fires with subsequent colonization from the seed bank or by vegetative resprouting (Keeley et al. 1981; Bonis et al. 1995). However, differences between these two disturbance types are not always unequivocal and different groups of organisms may experience the same disturbance as patchy and as global, respectively. For example, some species survive the drying of temporary ponds as resting stages, while others have to reimmigrate from permanent ponds after refilling (Wellborn et al. 1996; Chase 2007). It is therefore a within-patch disturbance for the former, but a spatially patchy disturbance for the latter species.
Microcosm experiments with aquatic protists have often been used to study the effect of disturbance on diversity. However, studies have examined either disturbances within single patches (e.g., Scholes et al. 2005; Haddad et al. 2008; Jiang and Patel 2008) or disturbances affecting local communities within a metacommunity, thereby creating a mosaic of patches in different successional stages (Warren 1996; Östman et al. 2006; Cadotte 2007). To our knowledge, the present experiment is the first that directly compares the effect of these two spatial scales of disturbance on diversity. In a microcosm experiment, we assembled metacommunities consisting of four local communities connected by active dispersal of organisms. Disturbances that reduced abundances of all species in a non-selective way and introduced fresh resources were applied at three intensities and at two spatial scales. Disturbances at the local scale resulted in a complete destruction of selected local communities and required reimmigration from neighboring communities for recolonization. Regional disturbances, however, affected the whole metacommunity but left part of each local community intact. We hypothesized that regional disturbances would have a less severe effect on diversity, as they allowed recolonization not only through reimmigration but also through growth within a patch. Moreover, we predicted that local disturbances would have a positive effect on beta diversity by creating a mosaic of patches in different successional stages and would thus increase regional diversity relative to local diversity. We further predicted that species with high growth rates would be favored by intense regional disturbances, while both high growth and high dispersal rates would be important for survival under strong local disturbances.
Materials and methods
Microcosms were small plexiglass basins (12 × 12 × 8 cm) filled with 300 ml of 0.2 μm-filtered pond water. As resources, we used a benthic diatom (Navicula pelliculosa; obtained from the culture collection SAG at Göttingen) and bacteria associated with algal and ciliate cultures. To simulate a benthic system, we placed 25 tiles (2.27 × 2.27 × 0.5 cm) covered with a biofilm of algae and bacteria into each basin. A metacommunity consisted of four microcosms arranged in a square, with each basin connected to its two neighboring basins with silicon tubing of 10 cm length and 0.5 cm inner diameter. For a more detailed description of microcosms, see Limberger and Wickham (2011).
At the beginning of the experiment, we added five benthic ciliate species (Tachysoma pellionellum, Stylonychia pustulata, Onychodromopsis flexilis, Frontonia angusta and Paramecium caudatum) to each basin. These species were true competitors for the resources and did not show any predatory effects on other community members. The ciliates had been isolated from freshwater habitats around the city of Salzburg, Austria. Depending on the species’ biovolume, 80–1,000 individuals were introduced into each basin. One of the ciliate species (Onychodromopsis flexilis) did not grow in any of the basins irrespective of treatment. As this species had been the strongest competitor in an earlier experiment, the rapid extinction of this species was probably the result of senescence of the original culture and not due to competitive exclusion.
Disturbance treatments
We simulated disturbances that reduce species’ abundances but return resources into the system. A disturbance was replacement of part of the metacommunity with filtered pond water and with tiles covered by a biofilm of algae and bacteria, but free of ciliates. The experiment was conducted in a two-way factorial design with disturbance intensity and disturbance scale as main factors. We compared three disturbance intensities: undisturbed, low disturbance intensity (a quarter of the metacommunity disturbed every other week) and high disturbance intensity (half of the metacommunity disturbed once a week). Thus, disturbance intensity was in fact a combination of intensity and frequency, but for simplicity we call this factor disturbance intensity.
Disturbances were implemented at two spatial scales: local and regional. Local disturbances were complete removals of basins and replacement with microcosms containing water and resource-covered substrate but no ciliates. With low local disturbance, one basin was replaced every other week starting with day 7, and at each following disturbance date, a basin successive to the most recently disturbed basin was selected for disturbance. With high local disturbance, two neighboring basins were replaced once a week, so that each local community was disturbed every other week.
Regional disturbances were, depending on disturbance intensity, removals of half or a quarter of tiles, water and ciliates from each of the four local communities of the metacommunity. Using a plexiglass sampler that allowed removal of single tiles and the water column above them, we removed 6–7 or 12–13 adjacent tiles from each basin. However, complete removals of basins in the local disturbance treatments removed more water volume than the removal of the respective number of tiles in the regional disturbance treatments. We measured this difference and removed it by pipette from the regionally disturbed treatments. The removed water and tiles were replaced by filtered pond water and resource-covered tiles. Disturbance treatments were replicated three times and implemented after sampling. All connections were closed with tube clamps during sampling and disturbances.
Sampling
The experiment lasted for 8 weeks and was sampled weekly by removing three tiles from each basin using the tile sampler before implementation of the disturbance treatments. The removed water was replaced by filtered pond water enriched with nutrients. For quantification of ciliates and resources, the biofilm on the tiles was scraped off with a razor blade and merged with the withdrawn water volume. Ciliates were live-counted under a dissecting microscope, while algal abundance was measured fluorometrically. For enumeration of bacteria, a subsample was fixed with glutardialdehyde (2% final concentration), and then sonicated to disaggregate clumps of algae and bacteria. A subsample was stained with DAPI (2.5 μg ml−1 final concentration) and filtered onto a black polycarbonate membrane filter (0.2 μm pore size; Nuclepore). Bacteria were classified into different size categories (<1 μm, 1–5 μm, 5–10 μm, >10 μm) and counted by epifluorescence microscopy in 30 randomly selected fields at ×1,000 magnification. Dimensions of at least ten individuals per size category were measured to transform counts into biovolume.
Data analysis
To measure diversity, we calculated the Shannon-Wiener index, species richness and evenness. These three measures of diversity were computed at two spatial scales: local diversity was measured at the scale of local communities and then averaged over the four basins of a metacommunity, while regional diversity was measured at the scale of the metacommunity. Calculation of regional diversity was based on the mean abundances in the whole metacommunity and the species number in the metacommunity, respectively. As a measure of dissimilarity of species composition, we calculated the Bray–Curtis distance between the four basins of a metacommunity, based on the log10-transformed species’ abundances.
We used two-way ANOVAs with repeated measures on both factors (henceforth two-way rm-ANOVA) to test whether disturbance intensity and scale significantly affected diversity, species richness, evenness, and species abundances across time. When the assumption of sphericity was violated, a Greenhouse–Geisser correction was used. The undisturbed control was not part of the factorial design, and was therefore excluded from the analysis. One-way rm-ANOVAs with Dunnett’s post-hoc test showed that the control did not differ significantly from the low intensity treatments, therefore justifying its exclusion from the analysis. However, when testing for disturbance effects on dissimilarity, Dunnett’s post-hoc test revealed a significant difference between the undisturbed treatment and the treatment of low local disturbance. Therefore, we used a one-way rm-ANOVA in this case. Dissimilarity values were arcsine square-root transformed prior to analysis to account for the fact that the Bray–Curtis distance is confined to the range from 0 to 1.
To test whether resources differed between the treatments, we conducted one-way rm-ANOVAs with bacterial biovolumina as dependent variable and an rm-ANCOVA with algal biovolume as dependent variable and light as covariable. Light intensity was not completely homogeneous in the laboratory, and was thus measured at the position of each basin to partial out a possible effect of light during data analysis. Abundances and biovolumina of ciliates, algae and bacteria were log10-transformed and averaged over the four local communities prior to analyses. Statistical analyses were conducted with PASW 18.0 for Windows.
Species’ traits
The test organisms’ competitive abilities, growth and dispersal rates were examined in detail in a previous study (Limberger and Wickham 2011). Briefly, each species’ growth rate was measured in single species trials started with 500 individuals per basin. Abundances were counted regularly until carrying capacities had been reached. Dispersal rate was measured as the proportion of individuals dispersing from a donor basin to an uncolonized recipient basin connected by tubing of 10 cm. Competitive ability was quantified by comparing performance of each species in all pairwise species combinations to performance in single species trials. Competitive response was calculated as the decrease in a species’ abundance when grown with a competitor relative to its equilibrium abundance when grown alone. Competitive effect was calculated as a species’ ability to reduce the abundance of a competitor relative to the competitor’s equilibrium abundance in the single species trial. A species’ competitive ability was computed as the difference between its mean competitive effect and response and served as the basis for calculation of competitive ranks. A bootstrap procedure was used to estimate confidence intervals. Since fewer species were used in the present than in the cited experiment, we recalculated competitive ranks by using only those competition trials that contained the four species of the present experiment.
Results
Diversity
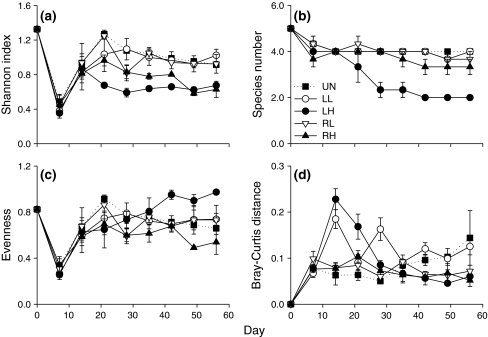
Local and regional diversity were strongly influenced by both the intensity and scale of disturbance. Since responses of local and regional diversity were similar (apart from a faster initial decline of local than regional species richness in the treatment with high local disturbance), we present here only results for regional diversity [see Electronic Supplemental Material (ESM) Fig. S1 for results of local diversity].
Averaged over time, high intensity disturbances reduced regional Shannon diversity compared to low intensity disturbances, but neither disturbance scale nor the interaction between scale and intensity influenced Shannon diversity (Table 1; Fig. 1a). However, effects of disturbance intensity and scale were highly time dependent, with the negative effect of high disturbance intensity becoming apparent earlier when disturbances were applied at the local scale than when applied at the regional scale (Fig. 1a). While species richness was strongly reduced by local scale disturbances of high intensity (Table 1; Fig. 1b), evenness was unaffected by disturbance treatments when averaged over time (Table 1). However, the effect of disturbance scale on evenness strongly interacted with time and differences between treatments became apparent during the final phase of the experiment. Regional disturbances led to lower evenness than local disturbance, but only when disturbances were of high intensity (Fig. 1c).
Table 1.
Significance values of two-way rm-ANOVAs testing for effects of time, disturbance intensity, disturbance scale, and possible interactions on measures of regional diversity, on total ciliate abundance and biovolume, and on abundances of the four ciliate species
| Time | Time × intensity | Time × scale | Time × scale × intensity | Intensity | Scale | Intensity × scale | |
|---|---|---|---|---|---|---|---|
| Shannon index | <0.001 | 0.001 | 0.032 | 0.034 | 0.005 | 0.420 | 0.468 |
| Species richness | 0.001 | 0.044 | 0.126 | 0.074 | <0.001 | 0.001 | 0.001 |
| Evenness | <0.001 | 0.331 | 0.006 | 0.081 | 0.820 | 0.140 | 0.118 |
| Total abundance | <0.001 | 0.346 | 0.014 | 0.472 | 0.195 | 0.003 | 0.068 |
| Total biovolume | <0.001 | 0.041 | 0.017 | 0.218 | 0.001 | 0.029 | 0.085 |
| Tachysoma | <0.001 | 0.107 | 0.037 | 0.131 | 0.561 | 0.009 | 0.478 |
| Stylonychia | 0.017 | 0.081 | 0.511 | 0.030 | 0.595 | 0.455 | 0.971 |
| Frontonia | <0.001 | 0.355 | 0.202 | 0.223 | 0.005 | 0.022 | 0.111 |
| Paramecium | <0.001 | <0.001 | 0.280 | 0.004 | 0.002 | 0.050 | 0.262 |
When the assumption of sphericity was violated, a Greenhouse–Geisser correction was used
P values ≤0.05 in bold
Fig. 1.
Regional diversity and dissimilarity in the undisturbed control (UN), and in the treatments of local low (LL), local high (LH), regional low (RL) and regional high (RH) disturbance. Low disturbance is shown with open symbols, high disturbance with closed symbols; local disturbance treatments are the circles, regional disturbance treatments the triangles. The undisturbed control is shown with dotted line and filled squares. The measures of diversity used were a Shannon-Wiener index, b species number, c evenness, and dissimilarity is expressed as d Bray–Curtis distance, means ± SE, n = 3
The type of disturbance significantly affected the Bray–Curtis dissimilarity of the local communities of a metacommunity, but the disturbance effect strongly interacted with time (Fig. 1d; one-way rm-ANOVA: time: P < 0.001; time × disturbance: P < 0.001; disturbance: P = 0.008; Tukey’s post-hoc test: RH, RL, UN < LL; see Fig. 1 for treatment abbreviations). Dissimilarity was comparatively high when disturbances were applied at the local scale. With high local disturbance, dissimilarity reached especially high values during the first half of the experiment, while low local disturbance resulted in strong fluctuations, with high dissimilarity 1 week after a disturbance and lower dissimilarity 2 weeks after a disturbance.
Species abundances
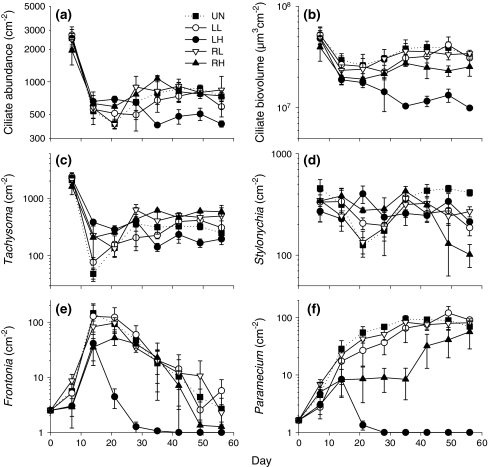
Total ciliate abundance was significantly affected by disturbance scale. Local disturbances reduced ciliate abundance to a stronger extent than regional disturbances, with high local disturbances having the most severe effect (Table 1; Fig. 2a). When abundance was converted to biovolume, the negative effect of high local disturbance became even more pronounced (Table 1; Fig. 2b).
Fig. 2.
a Total ciliate abundance, b total ciliate biovolume; abundances of c Tachysoma, d Stylonychia, e Frontonia and f Paramecium in the five disturbance treatments, means ± SE, n = 3. Note that untransformed data (+1) are presented on a log scale, while the statistical analyses presented in the text were calculated with log-transformed data. For better visibility of treatment differences, initial abundances and biovolume on day 0 have been omitted from panels with high maximum abundances or biovolume (a–d). Treatment abbreviations and symbols as in Fig. 1
The four ciliate species responded differently to disturbance treatments. Tachysoma reached significantly higher abundances in metacommunities that were affected by regional disturbances than in locally disturbed or undisturbed treatments (Table 1; Fig. 2c). Stylonychia abundances were unaffected by disturbance treatments when averaged across time but showed strong temporal dynamics (Table 1; Fig. 2d). During the intermediate period of the experiment, Stylonychia density temporally decreased in the undisturbed and weakly disturbed treatments. In the final phase of the experiment, however, Stylonychia reached highest abundances in the undisturbed control, while sharply declining with high regional disturbance. Frontonia and Paramecium were both affected by disturbance scale and intensity, and quickly approached extinction in the treatments with high local disturbance (Table 1; Fig. 2e, f). In contrast to Frontonia, abundances of Paramecium also showed differences between other treatments, with a strong interaction between time and disturbance intensity. With high regional disturbance, the increase in Paramecium abundance was slower than in the weakly disturbed and undisturbed treatments.
Community composition and succession
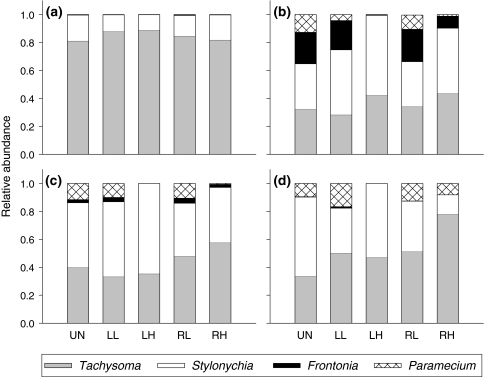
All treatments were initially dominated by Tachysoma and Stylonychia (Fig. 3a), the species with the fastest growth rates (Table 2). After this initial phase, successional trajectories differed between treatments (Fig. 3). In the weakly disturbed and undisturbed treatments, Frontonia reached mean relative metacommunity abundances of 20–30% during weeks 2 and 3 (Fig. 3b), before becoming increasingly unimportant towards the end of the experiment (Fig. 3c, d). Paramecium increased in relative abundance towards the end of the experiment, though not exceeding a mean relative metacommunity abundance of 17% (Fig. 3d). Tachysoma and Stylonychia were the dominant species in the end, with Stylonychia reaching comparatively high values (57%) in the undisturbed control (Fig. 3d).
Fig. 3.
Relative metacommunity abundances of Tachysoma (gray), Stylonychia (white), Frontonia (black) and Paramecium (hatched) on a day 7, b day 21, c day 35, and d day 56. Treatment abbreviations as in Fig. 1
Table 2.
Biovolume (n = 15), growth rate (n = 3), dispersal rate (n = 5), and bootstrap estimates of competitive rank (high rank means high competitive ability) of the four species
| Species | Biovolume (103 μm3) | Growth rate (ind ind−1 day−1) | Dispersal rate (ind ind−1 day−1) | Competitive rank (2.5, 97.5 percentiles) |
|---|---|---|---|---|
| Tachysoma | 17 | 1.75 | 0.001 | 2.87 (2, 4) |
| Stylonychia | 31 | 1.47 | 0.014 | 3.66 (2, 4) |
| Frontonia | 89 | 0.35 | 0.003 | 1.29 (1, 3) |
| Paramecium | 216 | 0.62 | 0.083 | 2.18 (1, 4) |
Competitive ability was measured by comparing growth in single species treatments with performance in all pairwise species combinations (n = 3). Data are from Limberger and Wickham (2011). Competitive ranks have been recalculated, as fewer species were used in the present experiment. Competitive ranks are therefore based only on competition trials containing pairs of those four species
In the highly disturbed treatments, succession was less pronounced or completely stopped, depending on spatial scale of disturbance. With high local disturbance, succession remained in an early stage and the community was dominated by Stylonychia and Tachysoma (Fig. 3). With high regional disturbance, relative abundances of the intermediate and late successional species (Frontonia and Paramecium) were reduced compared to the weakly and undisturbed treatments. In the final phase of the experiment, Tachysoma reached extremely high relative abundance (78%) in the treatment with high regional disturbance (Fig. 3d).
Resources
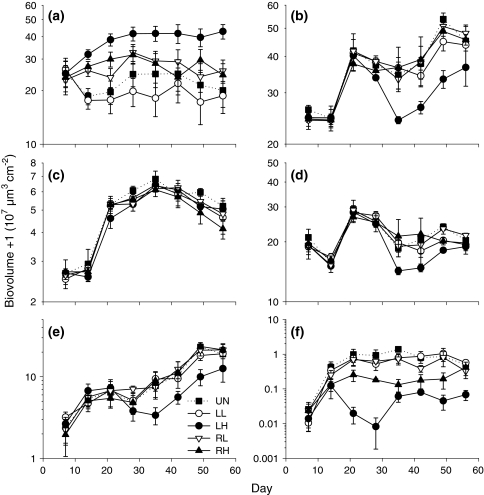
Algal biovolume differed between disturbance treatments, with comparatively high algal biovolume in the treatment with high local disturbance (Fig. 4a; rm-ANCOVA: time: P = 0.2; time × light: P = 0.197; time × disturbance: 0.191; light: P = 0.001; disturbance: P = 0.017; Sidak’s post-hoc test: LL < LH). Disturbance also affected total bacterial biovolume (Fig. 4b; rm-ANOVA: time: P < 0.001; time × disturbance: P = 0.366; disturbance: P = 0.001; Tukey’s post-hoc test: LH < all other treatments). However, when split into different size classes (Fig. 4c–f), an effect of disturbance treatment on bacterial biovolume was only found for the size class of >10 μm (rm-ANOVA: time: P < 0.001; time × disturbance: P = 0.004; disturbance: P = 0.002; Tukey’s post-hoc test: LH < all other treatments).
Fig. 4.
a Algal biovolume with values adjusted using ANCOVA to account for the confounding effect of light, b total bacterial biovolume, c bacteria <1 μm, d bacteria 1–5 μm, e bacteria 5–10 μm, f bacteria >10 μm, means ± SE, n = 3. Note that untransformed data are presented on a log scale, while the statistical analyses presented in the text were calculated with log-transformed data. Treatment abbreviations and symbols as in Fig. 1
Discussion
High intensity disturbances reduced diversity, irrespective of the spatial scale of disturbance. However, the mechanism behind this decrease in diversity was clearly dependent on the scale of disturbance. Strong local disturbance decreased diversity by reducing species richness, while strong regional disturbance decreased diversity by reducing evenness (Fig. 1). Apart from different effects on the two components of diversity, spatial scale of disturbance also affected the speed of diversity decline. Species extinctions due to strong local disturbances occurred even early in the experiment, while the decrease in evenness with high regional disturbance occurred only at the end of the experiment.
We hypothesized that local disturbances would have a stronger effect on diversity than regional disturbances. The confirmation of this hypothesis depends on whether one gives more weight to species richness, evenness, or the combination of both. Species extinctions in the regional species pool may indeed have a more severe effect on the community since there is no chance for recovery. Conversely, with reduced evenness, all species are still present in the species pool and recovery to a community of higher diversity is possible. By reducing species richness, local disturbances can thus be regarded as having a stronger effect on the community.
Disturbance scale and species richness
The reasons for species loss with strong local disturbance can be elucidated when looking at the species’ traits (Table 2). While a regression analysis between species’ traits and persistence in disturbed treatments was not possible due to the low number of data points, some qualitative comparisons between species with different responses to disturbance can be made. With strong local disturbance, only the two species with the highest growth rates survived (Stylonychia, Tachysoma), while the slow-growing species rapidly went extinct. A microcosm experiment analyzing the importance of several species’ traits in predicting the response to within-patch disturbances also found growth rate to be the best predictor for persistence under disturbance (Haddad et al. 2008). Interestingly, a high growth rate was also the most important trait for survival in our between-patch disturbance treatments, while dispersal rate was unimportant. Paramecium, one of the species that went extinct, had by far the highest dispersal rate in our model community, while one of the survivors (Tachysoma) had the lowest dispersal rate. Within our experimental set-up, the ability to rapidly build up large populations seems to have been more important than fast dispersal to undisturbed patches. However, the importance of dispersal rate for survival with between-patch disturbances will probably depend on the distance between disturbed patches. With greater distance, a high dispersal rate will become increasingly important.
With strong regional disturbance, all four species survived, demonstrating that this type of disturbance is a less strong environmental filter than local disturbance. Presence or absence of surviving organisms and propagules has also been found to strongly influence post-disturbance succession in many natural systems (Turner et al. 1998; Turner 2010). Succession in forests and other plant communities is more predictable and re-establishment of the pre-disturbance community is faster when residuals are present, as more species are able to recolonize a patch where they still have propagules or individuals present than when they have to recolonize a distant patch by reimmigration. Despite the simplicity of our model system, the results of our experiment are thus comparable to observations in natural communities.
Disturbance scale and evenness
While regional disturbances had no effect on species richness, they led to a decrease in evenness due to strong dominance of the fastest-growing species. It has been suggested that inferior competitors with high maximal growth rates are able to temporally dominate after disturbances of small spatial extent, as they are best at exploiting the resource-rich conditions after the disturbance (Pacala and Rees 1998; Rees et al. 2001). Accordingly, Tachysoma, the species with the highest growth rate in our community, reached high abundances with strong regional disturbance (Figs. 2c and 3d). In our experiment, disturbances increased mortality but introduced fresh resources, thereby creating conditions that were best exploited by species with high growth rates. In contrast, when disturbances reduce nutrients, it is particularly the fast-growing species that are negatively affected since their growth rates are more severely reduced than those of slower-growing species (Haddad et al. 2008). The effect of disturbance on the dominance structure of the community will thus depend on whether disturbances increase or decrease productivity. We only tested disturbances increasing resource concentrations, a type of disturbance common in nature. Examples are the input of nutrient-rich river water into floodplain lakes during flood events (Van den Brink et al. 1993), increased light availability within treefall gaps (Denslow 1987), and higher palatability of early-successional plant species that establish after disturbances (Cates and Orians 1975).
While Tachysoma reached high absolute abundance with strong regional disturbance early in the experiment, its relative abundance increased and thus evenness decreased only during the final 2 weeks, concurrent with a sharp decline in Stylonychia abundance (Fig. 2d). Stylonychia gained high abundances with strong local disturbance due to high growth and dispersal rates, its low abundance with strong regional disturbance in the end of the experiment is thus surprising. Even more, since it was also the strongest competitor in the community and dominated in the undisturbed control. However, the competitive hierarchy was rather flat (Table 2) and an earlier experiment has shown temporal variability of competitive effects. We found Tachysoma to have negative effects on Stylonychia during the early phase of succession (Limberger and Wickham 2011). With strong regional disturbance, succession was regularly set back to early-successional and resource-rich conditions, and competitive interactions might thus be the reason for the reduction of Stylonychia abundance. Also, the decline of Stylonychia abundance in the weakly and undisturbed treatments during the intermediate period of the experiment probably is a result of competition. The mid-successional species Frontonia was found to have strong negative effects on Stylonychia (Limberger and Wickham 2011).
Our results show that regional disturbances that increase productivity of the system lead to high dominance of the fastest-growing species. Despite a similar input of resources with strong local disturbance, the double requirement of dispersal and growth with high local disturbance prevented the slow disperser Tachysoma from reaching similar dominance as with high regional disturbance. The two survivors attained nearly equal abundances, leading to high evenness. Thus, while the requirement of dispersal seemed to be unimportant for survival with strong local disturbance, it did affect the abundances of the surviving species.
Results on species’ responses to disturbance treatments could have been influenced by trait evolution. There is increasing empirical evidence that rapid evolution affects ecological dynamics (Hairston et al. 2005; Fussmann et al. 2007). Accordingly, treatments with high disturbance intensity could have selected for individuals with high growth rates. With our data, however, we cannot assess whether evolutionary processes influenced species’ responses to disturbance. Measuring species’ growth rates at the beginning and the end of disturbance experiments would be an interesting avenue for future studies.
Scale of disturbance not only influenced species richness, evenness, and species’ abundances, it also affected resource biomass (Fig. 4). Although strong local and regional disturbances both introduced the same amount of resources, algal biomass was higher with local disturbance. The probable reason was a negative effect of strong local disturbance on ciliate abundance, and since only the two smallest species survived, the effect on ciliate biomass was even larger (Fig. 2b). This negative effect on grazer biomass translated to the lower trophic level by leading to a less efficient resource use.
Local, regional, and beta diversity
Disturbance scale had a strong influence on beta diversity (Fig. 1d). Regional disturbances affected each local community to the same extent and thus resulted in high similarity of local communities. While we also expected high similarity of local communities in the undisturbed control since each local community was treated the same (i.e. not at all), similarity between undisturbed local communities decreased during the second half of the experiment. Similarly, Chase (2007) found drought disturbance to result in high similarity of pond communities, while species composition in undisturbed ponds was more variable. He suggested stochastic effects to be more important in undisturbed communities, leading to higher dissimilarity of communities, while disturbances provide a harsh environmental filter and increase the importance of niche-selective forces.
In contrast to regional disturbances, local disturbances led to an increase in beta diversity by creating a mosaic of differently disturbed patches. With strong local disturbance, this increase was only temporal and beta diversity quickly decreased as soon as the disturbance-intolerant species had gone extinct. Weak local disturbances were applied only every other week and resulted in pronounced fluctuations of beta diversity, with dissimilarity depending on the time since last disturbance. The amplitude of the fluctuations decreased with increasing time of the experiment, when slow colonizers had either died out or built up populations large enough to rapidly colonize disturbed patches. Since regional diversity depends on both local and beta diversity, the high dissimilarity of local communities with local disturbance let us expect an increase of regional relative to local diversity. However, there were only marginal differences between local and regional diversity: with strong local disturbance, the initial decline in species richness was faster for local than for regional richness. By comparing absolute species’ abundances of the four local communities, Bray–Curtis dissimilarity probably detected fine-scale differences that were not appearent when comparing local and regional richness or diversity.
Other studies comparing the effects of local disturbances on local, regional, and beta diversity also found a negative effect of disturbance on local diversity (Warren 1996; Östman et al. 2006; Cadotte 2007). However, results for dissimilarity and regional diversity are inconsistent. Similar to our experiment, Warren (1996) found a negative effect of disturbance on regional diversity despite a positive effect on beta diversity, at least under low dispersal rates. The reason was that disturbance reduced local richness to a stronger extent than regional richness. Therefore, strong disturbances resulted in a comparatively large difference between local and regional diversity, and thus high beta diversity. In contrast, an increase of both beta diversity and regional diversity with at least intermediate disturbance has also been found (Östman et al. 2006; Cadotte 2007).
Disturbance–diversity relationship
We found a negative relationship between disturbance intensity and diversity, irrespective of the spatial scale of disturbance and of whether local or regional diversity was regarded. One basic requirement for a unimodal relationship is the presence of a strong competitive hierarchy. When comparing communities with strong versus weak competitive hierarchy, Svensson et al. (2009) found a unimodal disturbance–diversity relationship in the former, but negative or nonsignificant patterns in the latter communities. In our model community, the competitive hierarchy was rather flat, with large variability of competitive ranks (Table 2). No competitive exclusions occurred in the undisturbed control, yet, without competitive displacement at undisturbed conditions, an increase in species richness with intermediate disturbances is not possible.
Apart from a strong competitive hierarchy, the presence of a trade-off between colonization and competitive ability is a prerequisite for the IDH to be operating (Haddad et al. 2008). Even the presence of such a trade-off does not guarantee a unimodal disturbance–diversity relationship when the number of good colonizers and good competitors is not balanced (Cadotte 2007). In our community, however, the species that were best at colonizing new habitats due to their high growth rates were also the species that finally dominated in the undisturbed control. Thus, succession was rather circular instead of serial, with the same species dominating at the beginning and at the end of succession.
Given the flat competitive hierarchy, lack of a trade-off between competition and colonization ability and absence of a serial succession, the negative disturbance–diversity relationship is unsurprising. A model community with more species might have been a more robust test of the IDH, but this was not the main aim of our study. Rather than describing the relationship between disturbance intensity and diversity, we wanted to test whether the pattern of this relationship differed between local and regional disturbances. Our results suggest that local disturbances provide a harsher environmental filter that fewer species are able to pass than regional disturbances.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank M. Gasperl, C. Lorenz and A. Pitt for help with sampling and laboratory work. M. Prast built the sampler for removal of tiles and W. Foissner helped with species identification. We thank two anonymous reviewers for valuable comments on this manuscript. Funding was provided by the Austrian Science Fund, FWF, grant P19117-B17 to S. Wickham and a Marie-Andessner scholarship of the University of Salzburg to R. Limberger.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Bonis A, Lepart J, Grillas P. Seed bank dynamics and coexistence of annual macrophytes in a temporary and variable habitat. Oikos. 1995;74:81–92. doi: 10.2307/3545677. [DOI] [Google Scholar]
- Cáceres CE. Temporal variation, dormancy, and coexistence: a field test of the storage effect. Proc Nat Acad Sci USA. 1997;94:9171–9175. doi: 10.1073/pnas.94.17.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadotte MW. Competition–colonization trade-offs and disturbance effects at multiple scales. Ecology. 2007;88:823–829. doi: 10.1890/06-1117. [DOI] [PubMed] [Google Scholar]
- Cates RG, Orians GH. Successional status and the palatability of plants to generalized herbivores. Ecology. 1975;56:410–418. doi: 10.2307/1934971. [DOI] [Google Scholar]
- Chase JM. Drought mediates the importance of stochastic community assembly. Proc Nat Acad Sci USA. 2007;104:17430–17434. doi: 10.1073/pnas.0704350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Glenn SM. Intermediate disturbance and its relationship to within- and between-patch dynamics. N Z J Ecol. 1997;21:103–110. [Google Scholar]
- Connell JH. Diversity in tropical rain forests and coral reefs. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- Denslow JS. Tropical rainforest gaps and tree species diversity. Annu Rev Ecol Syst. 1987;18:431–451. doi: 10.1146/annurev.es.18.110187.002243. [DOI] [Google Scholar]
- Fussmann GF, Loreau M, Abrams PA. Eco-evolutionary dynamics of communities and ecosystems. Funct Ecol. 2007;21:465–477. doi: 10.1111/j.1365-2435.2007.01275.x. [DOI] [Google Scholar]
- Guo Q. Effects of bannertail kangaroo rat mounds on small-scale plant community structure. Oecologia. 1996;106:247–256. doi: 10.1007/BF00328605. [DOI] [PubMed] [Google Scholar]
- Haddad NM, Holyoak M, Mata TM, Davies KF, Melbourne BA, Preston K. Species’ traits predict the effects of disturbance and productivity on diversity. Ecol Lett. 2008;11:348–356. doi: 10.1111/j.1461-0248.2007.01149.x. [DOI] [PubMed] [Google Scholar]
- Hairston NGJ, Ellner SP, Geber MA, Yoshida T, Fox JA. Rapid evolution and the convergence of ecological and evolutionary time. Ecol Lett. 2005;8:1114–1127. doi: 10.1111/j.1461-0248.2005.00812.x. [DOI] [Google Scholar]
- Jiang L, Patel SN. Community assembly in the presence of disturbance: a microcosm experiment. Ecology. 2008;89:1931–1940. doi: 10.1890/07-1263.1. [DOI] [PubMed] [Google Scholar]
- Keeley SC, Keeley JE, Hutchinson SM, Johnson AW. Postfire succession of the herbaceous flora in southern California chaparral. Ecology. 1981;62:1608–1621. doi: 10.2307/1941516. [DOI] [Google Scholar]
- Limberger R, Wickham SA (2011) Competition–colonization trade-offs in a ciliate model community. Oecologia. doi:10.1007/s00442-011-2013-1 (in press) [DOI] [PMC free article] [PubMed]
- Mackey RL, Currie DJ. The diversity–disturbance relationship: is it generally strong and peaked? Ecology. 2001;82:3479–3492. [Google Scholar]
- Matthaei CD, Peacock KA, Townsend CR. Patchy surface stone movement during disturbance in a New Zealand stream and its potential significance for the fauna. Limnol Oceanogr. 1999;44:1091–1102. doi: 10.4319/lo.1999.44.4.1091. [DOI] [Google Scholar]
- Matthaei CD, Arbuckle CJ, Townsend CR. Stable surface stones as refugia for invertebrates during disturbance in a New Zealand stream. J North Am Benthol Soc. 2000;19:82–93. doi: 10.2307/1468283. [DOI] [Google Scholar]
- Östman Ö, Kneitel JM, Chase JM. Disturbance alters habitat isolation’s effect on biodiversity in aquatic microcosms. Oikos. 2006;114:360–366. doi: 10.1111/j.2006.0030-1299.14521.x. [DOI] [Google Scholar]
- Pacala SW, Rees M. Models suggesting field experiments to test two hypotheses explaining successional diversity. Am Nat. 1998;152:729–737. doi: 10.1086/286203. [DOI] [PubMed] [Google Scholar]
- Paine RT, Levin SA. Intertidal landscapes: disturbance and the dynamics of pattern. Ecol Monogr. 1981;51:145–178. doi: 10.2307/2937261. [DOI] [Google Scholar]
- Pake CE, Venable DL. Seed banks in desert annuals: implications for persistence and coexistence in variable environments. Ecology. 1996;77:1427–1435. doi: 10.2307/2265540. [DOI] [Google Scholar]
- Platt WJ. The colonization and formation of equilibrium plant species associations on badger disturbances in a tall-grass prairie. Ecol Monogr. 1975;45:285–305. doi: 10.2307/1942425. [DOI] [Google Scholar]
- Questad EJ, Foster BL. Vole disturbances and plant diversity in a grassland metacommunity. Oecologia. 2007;153:341–351. doi: 10.1007/s00442-007-0734-y. [DOI] [PubMed] [Google Scholar]
- Rees M, Condit R, Crawley MJ, Pacala SW, Tilman D. Long-term studies of vegetation dynamics. Science. 2001;293:650–655. doi: 10.1126/science.1062586. [DOI] [PubMed] [Google Scholar]
- Roxburgh SH, Shea K, Wilson JB. The intermediate disturbance hypothesis: patch dynamics and mechanisms of species coexistence. Ecology. 2004;85:359–371. doi: 10.1890/03-0266. [DOI] [Google Scholar]
- Scholes L, Warren PH, Beckerman AP. The combined effects of energy and disturbance on species richness in protist microcosms. Ecol Lett. 2005;8:730–738. doi: 10.1111/j.1461-0248.2005.00777.x. [DOI] [Google Scholar]
- Sousa WP. Disturbance in marine intertidal boulder fields: the nonequilibrium maintenance of species diversity. Ecology. 1979;60:1225–1239. doi: 10.2307/1936969. [DOI] [Google Scholar]
- Sousa WP. Intertidal mosaics: patch size, propagule availability, and spatially variable patterns of succession. Ecology. 1984;65:1918–1935. doi: 10.2307/1937789. [DOI] [Google Scholar]
- Sousa WP. The role of disturbance in natural communities. Annu Rev Ecol Syst. 1984;15:353–391. doi: 10.1146/annurev.es.15.110184.002033. [DOI] [Google Scholar]
- Svensson JR, Lindegarth M, Pavia H. Equal rates of disturbance cause different patterns of diversity. Ecology. 2009;90:496–505. doi: 10.1890/07-1628.1. [DOI] [PubMed] [Google Scholar]
- Tilman D. Competition and biodiversity in spatially structured habitats. Ecology. 1994;75:2–16. doi: 10.2307/1939377. [DOI] [Google Scholar]
- Turner MG. Disturbance and landscape dynamics in a changing world. Ecology. 2010;91:2833–2849. doi: 10.1890/10-0097.1. [DOI] [PubMed] [Google Scholar]
- Turner MG, Baker WL, Peterson CG, Peet RK. Factors influencing succession: lessons from large, infrequent natural disturbances. Ecosystems. 1998;1:511–523. doi: 10.1007/s100219900047. [DOI] [Google Scholar]
- Van den Brink FWB, De Leeuw JPHM, Van der Velde G, Verheggen GM. Impact of hydrology on the chemistry and phytoplankton development in floodplain lakes along the Lower Rhine and Meuse. Biogeochemistry. 1993;19:103–128. doi: 10.1007/BF00000798. [DOI] [Google Scholar]
- Warren PH. Dispersal and destruction in a multiple habitat system: an experimental approach using protist communities. Oikos. 1996;77:317–325. doi: 10.2307/3546071. [DOI] [Google Scholar]
- Wellborn GA, Skelly DK, Werner EE. Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst. 1996;27:337–363. doi: 10.1146/annurev.ecolsys.27.1.337. [DOI] [Google Scholar]
- Wilson JB. The ‘intermediate disturbance hypothesis’ of species coexistence is based on patch dynamics. N Z J Ecol. 1994;18:176–181. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.