Abstract
Global climate change is expected to affect terrestrial ecosystems in a variety of ways. Some of the more well-studied effects include the biogeochemical feedbacks to the climate system that can either increase or decrease the atmospheric load of greenhouse gases such as carbon dioxide and nitrous oxide. Less well-studied are the effects of climate change on the linkages between soil and plant processes. Here, we report the effects of soil warming on these linkages observed in a large field manipulation of a deciduous forest in southern New England, USA, where soil was continuously warmed 5°C above ambient for 7 years. Over this period, we have observed significant changes to the nitrogen cycle that have the potential to affect tree species composition in the long term. Since the start of the experiment, we have documented a 45% average annual increase in net nitrogen mineralization and a three-fold increase in nitrification such that in years 5 through 7, 25% of the nitrogen mineralized is then nitrified. The warming-induced increase of available nitrogen resulted in increases in the foliar nitrogen content and the relative growth rate of trees in the warmed area. Acer rubrum (red maple) trees have responded the most after 7 years of warming, with the greatest increases in both foliar nitrogen content and relative growth rates. Our study suggests that considering species-specific responses to increases in nitrogen availability and changes in nitrogen form is important in predicting future forest composition and feedbacks to the climate system.
Electronic supplementary material
The online version of this article (doi:10.1007/s00442-011-2133-7) contains supplementary material, which is available to authorized users.
Keywords: Soil warming, Net nitrogen mineralization, Nitrification, Species-specific nutrient acquisition strategies
Introduction
Climate change is predicted to play a significant role in altering the function and structure of forest ecosystems (Melillo et al. 1990). Many studies have addressed how changes in major processes such as photosynthesis and plant and microbial respiration are projected to affect ecosystem carbon (C) balance (e.g., Melillo et al. 1993, 2002, 2011; Ryan 1991; Ainsworth and Long 2005), water balance (Zavaleta et al. 2003), and cycling rates of key nutrients including nitrogen (N) and phosphorus (P) (Van Cleve et al. 1990; Peterjohn et al. 1994; Rustad et al. 2001; Melillo et al. 2002). A growing number of studies have examined how these functional changes within ecosystems can feedback to the climate system (Peterjohn et al. 1994; Shaver et al. 2000; Field et al. 2007; Melillo et al. 2011).
Direct and indirect feedbacks to the climate system as a result of warming are predicted to occur, with the direction (positive or negative) and magnitude being dependent on specific ecosystem conditions such as nutrient availability, moisture regime and species composition (Shaver et al. 2000; Field et al. 2007). In addition, ecosystem responses can vary over time, as organisms acclimate to changes in the environment or as community shifts in composition. For example, increased microbial activity, a characteristic response to warming, can further drive climate change by accelerating the decay of soil organic matter and thereby releasing carbon dioxide (CO2), a greenhouse gas, to the atmosphere (Peterjohn et al. 1994; Rustad and Fernandez 1998; Shaver et al. 2000; Luo et al. 2001; Rustad et al. 2001; Melillo et al. 2002, 2011; Eliasson et al. 2005). This response to warming is reported to decrease over time as labile carbon is depleted or as the microbial communities adapt to warmer conditions (Melillo et al. 2002, 2011; Bradford et al. 2008). However, over the long term, it is unclear if warming will cause additional changes in ecosystems, further altering the magnitude or direction of the microbial community’s response to climate change. Varying and interacting responses of ecosystem processes to warming make it difficult to predict the overall scale and direction of long-term feedbacks to the climate system.
A number of climate change experiments in forests (e.g., Peterjohn et al. 1994; Rustad et al. 2001) have also shown that soil warming increases net N mineralization, the transformation of organically bound N to ammonium (NH4 +) and nitrification, the transformation of NH4 + to nitrate (NO3 −). With increases in nitrification, ecosystems could experience gaseous and solution losses of N, potentially affecting water quality and creating a positive feedback to the climate system (Aber et al. 1989).
In an ecosystem where plant growth is limited by N availability, an increase in N has the potential to enhance photosynthetic rates and carbon storage in trees (Melillo et al. 2002, 2011). This can happen through increases N deposition in precipitation (Melillo and Gosz 1983; Thomas et al. 2009). Increased N availability to plants can also occur in response to soil warming (Melillo et al. 1995, 2002, 2011) as N is moved from the soil where the C:N mass ratio is often less than 30:1, to the plants where the C:N mass ratio in woody tissue is 200–300:1 (Melillo et al. 2002, 2011). Warming responses that cause enhanced carbon storage in woody tissue produce negative feedbacks to the climate system, which consequently slow the rate of atmospheric CO2 accumulations (Melillo et al. 2011).
Changes in N cycling in response to warming are also likely to have long-term consequences for plant community structure. Tree species vary in their abilities to acquire and retain N based on characteristics such as root morphology and physiology (Marschner and Dell 1994), fungal associations (Lambers et al. 2008), preferred form of N uptake (NO3 vs. NH4 vs. amino acids; Schimel and Bennett 2004; Finzi and Berthrong 2005) and N resorption efficiency (Killingbeck 1996; Kobe et al. 2005). As human-induced environmental changes continue to affect forest ecosystems, species-specific strategies and responses to changes in the N cycle may become increasingly important in determining plant–soil interactions, forest species composition and the associated long-term feedbacks to the climate system.
Models that project the redistribution of tree species in response to climate change have often used a “climate envelope” approach, with species tracking shifts in key climate parameters including temperature and moisture over space and time (e.g., VEMAP Members 1995; Iverson and Prasad 1998; Iverson et al. 2008; Mohan et al. 2009). Climate-driven changes in the nitrogen cycle have not routinely been considered in vegetation redistribution models. A recent review by Ostle et al. (2009) argues that this may be a serious omission and urges that this potential shortcoming be addressed in new modeling efforts.
Here, we report the results of a large soil warming study at the Harvard Forest in central Massachusetts, USA, designed to explore the complex links among climate change, ecosystem biogeochemistry and plant responses. In a mixed deciduous stand, we have increased soil temperature 5°C using buried resistance cables (Melillo et al. 2002, 2011). For 7 years, we measured biogeochemical and plant responses in 900-m2 heated and control (unwarmed) areas to examine how a temperate forest ecosystem is affected by warming-induced changes in the N cycle. Our measurements have included in situ net N mineralization and nitrification, soil–water N concentrations, nitrous oxide (N2O) emissions from the soil surface to the atmosphere, N concentration in green leaves and leaf litter, nitrate reductase activity in leaves of dominant species, and growth responses of the canopy trees. By examining how warming influences various fluxes and pools of N within the ecosystem, we document some short- and longer-term effects on ecosystem structure and function.
Materials and methods
Study site
The research site was an even-aged, mixed deciduous forest in central Massachusetts, USA (42°28′N, 72°10′W). Historical records, stonewalls and soil-horizon characteristics indicate that the area was used for either pastureland or low-intensity agriculture prior to 1908. White pines dominated the site by the early 1900s, but were destroyed in the 1938 hurricane. Blow downs were salvaged and the area was left to regrow naturally to its current state: a mid-aged stand of mixed hardwoods dominated by red oaks (Quercus rubra). Soils are mainly Canton series (coarse-loamy over sandy or sandy-skeletal, mixed, semi-active, mesic Typic Dystrudepts) with a surface pH of 5.2, and subsurface pH of 5.5. The average bulk density is 0.37 g cm−3 in the organic layer and 0.78 g cm−3 in the mineral layer. The climate is cool, temperate and humid. The mean weekly air temperature varies from a high of about 20°C in July to a low of about −6°C in January. Precipitation is distributed evenly throughout the year and averages about 108 cm annually.
During the summer and fall of 2001, about 5 km of heating cable were installed by hand to minimize disturbance in a 30 × 30 m area (Melillo et al. 2011). Cables were buried at 10 cm depth, spaced 20 cm apart. An adjacent 900-m2 area was delineated to serve as the control area, with a 5-m buffer inbetween the areas. Results from our previous nearby soil-warming experiment using 5-m2 plots confirmed that the soil disturbance associated with the installation of heating cables had no effect on soil temperatures and only minor and variable impacts on soil moisture and growth of small-statured woody vegetation (Melillo et al. 2002). Pre-treatment biogeochemical data were collected during the 2002 growing season and heating commenced in May 2003.
When supplied with 240 VAC, the heating cables have a power output of 13.6 W m−1 and produce a power density of about 77 W m−2. Within the heated area, there were 160 resistance cables, each of which is approximately 30 m long. A typical resistance per cable was 96 Ω. The heated area was monitored with 80 thermistors installed at 5 cm depth and 6 moisture probes at 10 cm depth, while the control area had 12 thermistors and 6 moisture probes at analogous depths. Each minute, heating cables were turned on or off automatically to maintain a 5°C temperature differential between heated and control areas.
Due to the scale and cost of the experiment, plot-level replication was not possible. Pre-treatment data were collected during the 2002 growing season to compare the baseline biogeochemistry for the two large areas, control and heated. Our pre-treatment measurements indicated that the control and heated areas were structurally and functionally similar prior to initiating treatment in the heated area (Melillo et al. 2011). In 2002, the tree biomass in the two areas was similar (202 Mt ha−1 in the control area and 192 Mt ha−1 in the heated area). Woody increment in the pre-treatment year was 1.73 Mt C ha−1 in the control area and 1.69 Mt C ha−1 in the heated area. N mineralization in the two areas was also similar during the pre-treatment year as determined by in situ incubations (method described below).
Net N mineralization and nitrification
Using the in situ buried bag incubation method (Eno 1960; Melillo 1977), we measured the rates of net N mineralization and nitrification in plots in the heated and control areas. We divided each area into 36 plots, each 5 × 5 m, and randomly chose 10 plots per treatment for the N mineralization and nitrification study. For each sampling, we took two 10 cm-deep soil cores from each plot in each treatment. One core, designated as the “initial” sample, was split into organic and mineral horizons and each horizon was placed into a gas-permeable polyethylene bag and transported to the laboratory for analysis. The second core, designated as the “final” sample, was placed intact inside another gas-permeable polyethylene bag and positioned back in the ground. The final bags were incubated for 5 weeks April–November and for 5 months over the winter and then harvested and transported to the laboratory for analysis.
In the laboratory, soils were separated into organic and mineral horizons and sieved through a 5.6-mm screen to remove rocks and roots. A subsample of the soil was weighed and dried at 105°C for 24 h for soil moisture analyses. Approximately 10 g of soil were placed in 100 mL of 2 M KCl, extracted for 36 h and filtered. The extracts were analyzed for nitrite/nitrate (NO2 − + NO3 −–N) and ammonium (NH4 +–N) using a Lachat QuikChem FIA + 8000 Series Flow Injection Analyzer with Omnion 3.0 software. Using these techniques, the detection limit for NO2 − + NO3 −–N was 0.02 mg L−1 and the detection limit for NH4 +–N was 0.005 mg L−1.
For each sample, extractable NO2 − + NO3 −–N and NH4 +–N were scaled to kg N ha−1 using sample mass, moisture content and bulk density. Net mineralization rates were calculated as final minus initial extractable NO2 − + NO3 −–N + NH4 +–N divided by incubation length. Net nitrification rates were calculated as final minus initial extractable NO2 − + NO3 −–N divided by incubation length.
Soil solution chemistry
In the fall of 2001, a total of 14 porous cup tension lysimeters were installed at a 50 cm depth (7 control, 7 heated). Lysimeter collections began in June 2002, approximately 8 months after installation, in order to minimize the effects of soil disturbance caused by lysimeter installation. Soil solution samples were collected once a month by draining any existing water from lysimeters using a syringe, and then applying a tension of 0.05 MPa with a hand pump. Approximately 24 h later, samples were collected, filtered, placed into scintillation vials and frozen for later analysis of NO2 − + NO3 −–N and NH4 +–N.
Filtered samples were analyzed for NO2 − + NO3 −–N and NH4 +–N using a Lachat QuikChem FIA + 8000 Series Flow Injection Analyzer with Omnion 3.0 software. Using these techniques, the detection limit for NO2 − + NO3 −–N was 0.02 mg L−1 and the detection limit for NH4 +–N was 0.005 mg L−1.
N2O soil efflux
Nitrous oxide was measured monthly April through November 2002–2007 using head-space air extracted with syringes from closed chambers of permanently installed gas collars and analyzed using electron capture gas chromatography similar to the procedure used by Bowden et al. (1990). On each sampling date, fluxes were measured in the afternoon and then analyzed using a Shimadzu model GC8A gas chromatograph (GC) equipped with a 3-m × 3.2-mm-diameter stainless steel column filled with Poropak Q (80/100 mesh) and a 63Ni detector. Carrier gas was N2 at 20 mL min−1. Column and detector temperatures were 65 and 310°C, respectively. Standards and samples were transferred into the GC using a 14-port valve (Valco Instruments Incorporated) equipped with a 1-mL sample loop. A 20-mm × 4-mm-ID Silica gel (0.5 g, 80/100 mesh) water trap attached to the inlet of the injection valve was used to reduce water vapor carried into the GC (Bowden et al. 1990). Certified N2O standards of 310 and 999 ppbv (Scott Specialty Gases, Plumsteadville, PA, USA) were used for calibration. Bowden et al. (1990) showed that standard curves are linear over this range and we found little variation throughout the day.
Foliar N
Green foliage was collected once per year from 2003 to 2009 during July in the heated and control areas. Sun leaves from red oak (Quercus rubra), red maple (Acer rubrum), white ash (Fraxinus Americana), sugar maple (Acer saccharum), hemlock (Tsuga canadensis) and birch (Betula lenta) were shot down from the canopy and dried at 60°C for 24 h and ground using a Wiley Mill. A subsample of the leaf litter collected each fall was ground using a Wiley Mill in preparation for analysis. Both green and brown leaf samples were dried again at 60°C prior to analysis and analyzed for %N using Perkin-Elmer CHN analyzer. Average percent resorption was calculated as:
 |
Nitrate reductase enzyme activity
We sampled nitrate reductase activity (NRA) during an expected peak in seasonal NO3 − availability. Our methods were derived primarily from Downs et al. (1993). Green leaf samples were collected from red oak, red maple and white ash canopy trees July 6, 2009 within 2 h of solar noon on a sunny day. The samples were washed and cut into 10- to 15-mm2 sections, excluding midribs. A buffered solution (pH 7.0) of 40 μM KNO3 with 1.5% propanol solution was added to the leaf tissues, and a 100-kPa vacuum was applied to each sample for 3 min and released. The tissues were incubated in the dark for 2 h at 30°C. A 1.0-mL aliquot of each sample was then diluted 1:2 with deionized water. After adding 0.5 mL each of 1% sulfanilamide in 1:5 HCl and 0.1% n-napthylethylene diamine dihydrochloride, the samples were colorimetrically analyzed for nitrite using a Shimadzu UV 1201 spectrophotometer at 535 nm. Percent moisture was calculated gravimetrically using a subsample of each leaf. To normalize for changes in canopy mass with warming, NRA was multiplied by leaf mass for each species.
Growth responses of trees by species
Allometric equations were applied to monthly measurements of dendrometer bands on all trees >5 cm DBH in order to calculate changes in above- and belowground woody biomass and vegetation carbon storage over time (Jenkins et al. 2003). Relative growth rates were calculated as:
 |
where t equals time, Biomass t is the woody biomass at time t and Biomass t+1 is the biomass at time t + 1.
Litterfall was collected throughout the fall in permanent litter baskets (0.32 m2 in size from 2003 to 2009 and 0.21 m2 in size from 2006 to 2010). Litter was sorted by species, dried at 60°C for 24 h, and weighed. Annual litterfall by species was used as a proxy for canopy mass in subsequent analyses.
Statistical analyses
Annual net N mineralization rates were compared between the heated and control areas using Friedman’s test, a non-parametric repeated measures analysis in SAS (v.9.1.3). Similarly, relative growth rates were compared between heated and control areas using a Friedman’s test. In the cases where our samples were not collected from the same individual through time, including NRA, foliar %N, N resorption and litterfall, we compared data in the heated versus control areas using non-parametric (Wilcoxon) rank-sum tests. Results are reported on a “warming year” basis beginning in May, to coincide with heating cable activation (e.g., May 2002–April 2003 is the “pre-treatment” year, May 2003–April 2004 is “year 1,” and so on).
Results
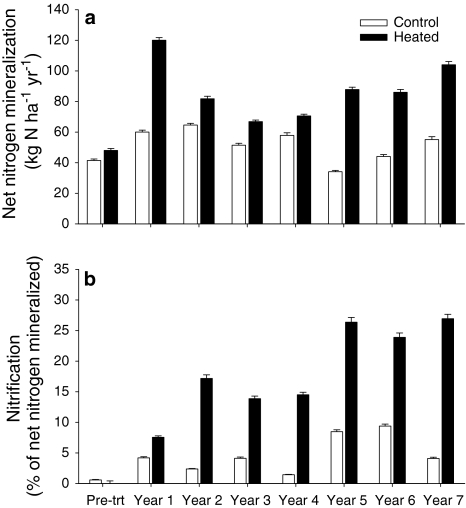
Based on in situ soil incubations, we found that warming significantly increased annual rates of net N mineralization (P < 0.01) (Fig. 1a). The average increase of net N mineralization in the heated area relative to the control area was about 45% over 7 years of warming; a mean annual increase in the net N mineralization of 27.4 kg N ha−1 in the organic and mineral soil (0–10 cm) horizons. We observed a progressive increase in NO3 − production over the 7-year study period (Fig. 1b). By the 5th year of warming, the production of NO3 − had increased by a factor of 3 in the heated area, with 25% of the NH4 + produced during the mineralization process transformed to NO3 −.
Fig. 1.
a Net N mineralization in the control (open bars) and heated (filled bars) areas. Bars represent mean net N mineralization rates of subplots (n = 10) in kg N ha−1year−1 + 1SE. b Net nitrification as a percent of total net N mineralized. Bars represent mean net N mineralization rates in percent of subplots (n = 10) +1SE
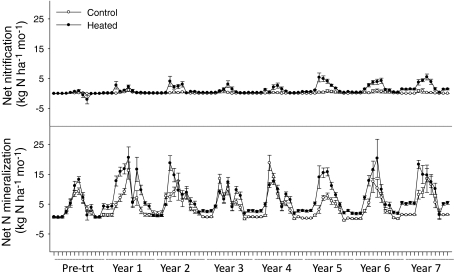
Net N mineralization and nitrification varied both annually and seasonally in the heated and control areas. We defined spring as April–May, summer as June–August, autumn as September–October and winter as November–March. Peaks in net N mineralization occurred in the summer months, averaging 45 and 37% of the annual totals in the control and heated areas, respectively (Fig. 2). In the spring, net mineralization averaged 21% of the annual total in the control area and 23% in the heated area. In the fall, mineralization averaged 30% in the control area and 31% in the heated area. Over the winter, mineralization averaged 4% in the control area and 9% in the heated area. These seasonal trends were also observed for net nitrification, with peaks occurring in the summer months, averaging 55 and 49% of the annual totals in the control and heated areas, respectively (Fig. 2). In the spring, net nitrification averaged 25% of the annual total in the control area and 32% in the heated area. In the fall, nitrification averaged 18% in the control area and 11% in the heated area. In the winter, nitrification averaged 3% in the control area and 8% in the heated area. While the overall trends were the same for both areas, the proportion of N mineralized in the summer decreased in response to warming, while the proportion of N mineralized in the winter increased with warming. The proportion of net nitrification occurring in the spring and winter increased in response to warming and decreased in the fall and summer.
Fig. 2.
a Monthly net nitrification in the control (white circles) and heated (filled circles) areas. Points represent mean net nitrification rates of subplots (n = 10) in kg N ha−1year−1 + 1SE. b Monthly net mineralization in the control (white circles) and heated (filled circles) areas. Points represent mean net N mineralization rates of subplots (n = 10) in kg N ha−1year−1 + 1SE
Over 7 years of warming, we saw no significant solution or gaseous losses of N. While we saw occasional spikes in NH4 + and NO3 − solution losses in the heated and control areas, there was no significant effect of warming on solution losses through time. Gaseous N2O fluxes from both heated and control areas were near detection limits, and no discernable pattern emerged over years of warming (Online Resource 1).
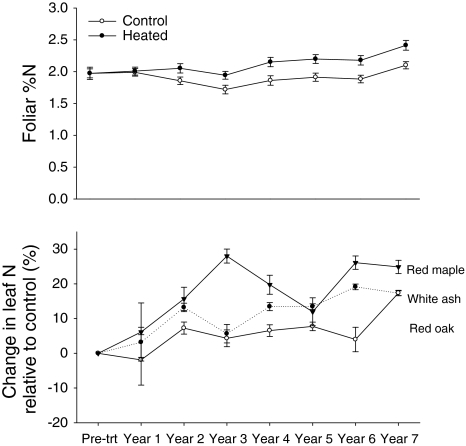
Foliar percent N has been significantly higher in the heated area since the 2nd year of warming (P < 0.05 for each year) (Fig. 3a). While the dominant species (oak, red maple and white ash) all showed higher percent N with warming in most years, red maple had the largest and most consistent response to warming. When foliar N was expressed as a percent increase over the controls, red maple shows a 25% increase in leaf N by year 6 (Fig. 3b).
Fig. 3.
a Foliar %N in control (open circle) and heated (filled circle) leaves ±1SE. Percent N is not available for year 4. A pre-treatment correction factor was applied to the %N values. b Percent change in leaf N content in the heated area relative to control [(%Nheated − %Ncontrol) / %Ncontrol × 100] for white ash (filled circle) (n = 5 control, n = 4 heated), red maple (open circle) (n = 4 control, n = 5 heated), and oak (filled triangle) (n = 6 control, n = 8 heated) ±1SE. A pre-treatment correction factor was applied to the %N values
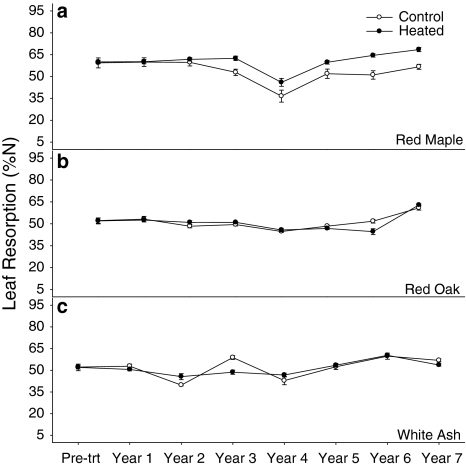
Overall, we saw no significant stand-level responses of leaf N resorption efficiency with warming. However, we did see significant species-level differences (Fig. 4a–c). Red maple had the highest leaf N resorption compared to red oak and white ash in the warmed area (P < 0.01 for each year), and its leaf N resorption efficiency increased relative to the control by year 3 (P < 0.05 for each year). By year 6, oaks had a significant decrease in N resorption in response to warming (P = 0.04). White ash had a variable response in leaf resorption, with a positive response to warming in year 2 and a negative response to warming in year 3 (P = 0.05 and 0.01, respectively).
Fig. 4.
Average percent N resorption for a red maple b red oak and c white ash in the control (open circles) and heated (filled circles) areas. Lines represent %N values retained in the trees during senescence by species ±1SE. A pre-treatment correction factor was applied to the resorption values
We sampled NRA during an expected peak in seasonal NO3 − availability in 2009. Overall, oaks had the highest NRA in the heated and control areas, followed by white ash and red maple (Table 1).
Table 1.
Nitrate reductase activity corrected for canopy mass in 2008 (μmoles NO2 − m−2 litter mass h−1) by species in the control and heated areas ±1SE; P = 0.77, 0.042, and 0.23 for red maple, white ash, and red oak, respectively
| Species | Heated | Control |
|---|---|---|
| Red maple | 10.3 ± 25.8 | 95.7 ± 107 |
| White ash | 35.0 ± 7.8 | 96.7 ± 22.7 |
| Red oak | 2,027.4 ± 271.4 | 2,524.1 ± 303.8 |
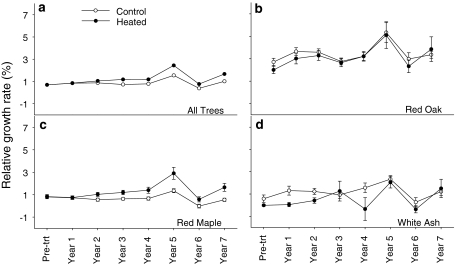
Warming had significant effects on plant C cycling. Overall, there was an increase in C storage with warming (Melillo et al. 2011). In addition, after correcting for pre-treatment differences, we saw a significant increase in the relative growth rate in trees on the heated area through time (P = 0.007) (Fig. 5a). We saw varied species-specific responses to warming. Red maple showed the largest and most consistent response to warming, doubling its relative growth rate in the warmed areas (P = 0.01) (Fig. 5b). While red oak had the greatest increase in biomass and trends toward increased relative growth rate in the heated area, the difference was not significant (P = 0.20) (Fig. 5c). White ash showed no consistent pattern of increased relative growth rates with warming (P = 0.47) (Fig. 5d).
Fig. 5.
a Average relative growth rate for heated (filled circle) (n = 74) and control (n = 83) (open circles) areas ±1SE. b Relative growth rate of oak species (red and black oaks) in control (open circles) (n = 10) and heated (filled circles) (n = 22) areas. c Relative growth rate of red maple in control (open circles) (n = 43) and heated (filled circles) (n = 33) areas. d Relative growth rate of white ash in control (open circles) (n = 7) and heated (filled circles) (n = 10) areas as a percent of initial growing season biomass ±1SE
In addition to increases in woody biomass, we saw overall increases in litterfall with warming (60 kg C ha−1). Litterfall was used here as a proxy for canopy mass. Oak litterfall showed the greatest increase in response to warming by year 5 (P = 0. 006), with an average annual increase in litterfall of 35% relative to the control over the past 7 years. The other dominant species showed more variation through time, with no clear pattern emerging.
Discussion
Over 7 years of treatment, we have seen significant warming-induced changes in the N cycle that include increases in net mineralization and nitrification and increases in N concentrations in leaves of the canopy trees. We saw no evidence of increases in gaseous or solution N losses from the heated area relative to the control; the system has maintained a closed N cycle in spite of warming. The increase in N availability in the warmed area has led to increases in leaf N and plant C storage relative to the control (Melillo et al. 2011) and to an increase in relative growth rates, especially for red maples. Relative to other tree species, red oak exhibited high rates of nitrate reductase activity, although the relatively high reductase activity was observed in trees growing in both the control and heated areas.
Soil nitrogen
The increase in N mineralization in response to warming that we documented in this study has also been observed in other studies; some in forests (Peterjohn et al. 1994; Hartley et al. 1999; Rustad et al. 2001; Melillo et al. 2002), some in grasslands (Shaw and Harte 2001) and some in tundra (Chapin et al. 1995). In our study, net N mineralized in the heated area remained elevated relative to the control for the full 7 years. This sustained increase in net N mineralization with warming has been accompanied by increases in net nitrification, which has also been reported by others (Hartley et al. 1999; Barnard et al. 2004; Emmett et al. 2004).
The increase in nitrification with soil warming is likely due to a combination of multiple interacting soil and plant responses, many of which we have not measured in this study. We have found that soil warming leads to decreases in labile soil organic matter and microbial biomass (Bradford et al. 2008; Frey et al. 2008). One possible implication of these changes in the labile C pool and microbial biomass is a decrease in microbial immobilization of N. This would cause an increase in NH4 + available to nitrifying bacteria and an increase in net nitrification.
Tree responses to warming
Soil warming has led to changes in plant N cycling and relative growth rates. As soil N availability has increased with warming, the trees in the heated area have exhibited increased leaf N. By year 2, significant warming-induced changes in green leaf N content were apparent, especially for red maples. Leaf N is positively correlated to photosynthetic rate and carbon storage in many plants growing across the globe (Field and Mooney 1986; Reich et al. 1994, 1995, 1997; Ollinger et al. 2008). This relationship, observed in ecosystems around the world, suggests that the increases in leaf N we see with warming likely corresponds to increases in C assimilation in the heated area, which has implications for C cycling and storage.
Coupled with increases in leaf N, warming leads to general increases in the relative growth rate of trees, particularly for red maple. Recent studies have shown that red maple saplings increase in relative growth rate with increasing N availability (Finzi and Canham 2000; Zaccherio and Finzi 2007). Red maple’s strategy of taking up and storing more N, resulting in an increase in relative growth rate, suggests that it may have a competitive advantage in a warmer world.
Red oaks too may have a competitive advantage in a warmer world. This tree species had the greatest NRA, demonstrating its potential to take advantage of increases in NO3 − in a warmer world. However, the higher levels of NRA have not yet resulted in increased relative growth rate for oaks in the warmed areas.
Multiple factors
Interactions between increasing temperatures and other factors predicted to change in the future, such as CO2 concentrations, could reinforce the ecosystem responses to warming alone. Bazazz and Miao (1993) found that, when N was added to seedlings in growth chambers at Harvard Forest with elevated CO2 (700 μL/L CO2), biomass of red maple and red oak increased significantly relative to controls. Therefore, as CO2 concentrations increase and warming stimulates increases in N availability, it is possible that we may see further increases in growth rates of these species. Results from Duke University’s Free-Air Carbon Dioxide Enrichment experiment (FACE) in North Carolina show red maples also benefiting from CO2 enrichment (Mohan et al. 2007).
N cycling and forest community composition in a warmer world
Model-based predictions of future species composition in the northeastern USA in response to climate change have relied predominantly on variables such as temperature, precipitation, elevation, soil type and properties, and land use and fragmentation (Iverson and Prasad 2001, 2002; Hickler et al. 2004; Iverson et al. 2008). As suggested by Ostle et al. (2009), species’ responses to both form and abundance of N should be incorporated into species distribution models to better predict future forest composition. We have seen that the amount and form of N available may play a role in the growth rate and potential dominance of tree species in these temperate forests. We may be able to better predict the composition of our future forests by incorporating N availability and species-specific N form preference into species distribution models. By examining the N concentration in leaves, resorption of N during senescence, and the NRA in canopy leaves, in addition to the growth response of trees to warming, we can better predict how forested ecosystems will respond to climate change.
Conclusions
We have seen significant increases in net N mineralization and net nitrification with soil warming. The absence of solution and gaseous N losses suggest that the additional N mineralized as a result of warming is available for plant and microbial uptake. Warming significantly increases leaf N and growth of trees in the warmed area. The increases in available N have a significant effect on the relative growth rate of red maples, suggesting that this species will have a competitive advantage in taking up the additional N in a warmer world if the N economy is predominantly NH4 + based. Interestingly, the red maples do not take advantage of the growing NO3 − resource in the warmed area. Of the canopy dominants, oaks show the highest levels of NRA, suggesting that they are using much of the NO3 −. If the future N economy has a greater proportion of NO3 −, we would expect oaks to have a competitive advantage. By distinguishing species-specific nutrient acquisition strategies, we can begin to understand how canopy dominance and species composition may shift as the form and abundance of N changes in a warmer world.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the National Institute for Climate Change Research (DOE-DE-FCO2-06-ER64157), DOE BER (DE-SC0005421) and the Harvard Forest Long-Term Ecological Research program (NSF-DEB-0620443).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM. Nitrogen saturation in Northern forest ecosystems. Bioscience. 1989;39:378–386. doi: 10.2307/1311067. [DOI] [Google Scholar]
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? a meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Barnard R, Barthes L, Roux XL, Harmens H, Raschi A, Soussana J, Winkler B, Leadley PW. Atmospheric CO2 elevation has little effect on nitrifying and denitrifying enzyme activity in four European grasslands. Glob Change Biol. 2004;10:488–497. doi: 10.1111/j.1529-8817.2003.00746.x. [DOI] [Google Scholar]
- Bazazz FA, Miao SL. Successional status, seed size, and responses of tree seedlings to CO2, light, and nutrients. Ecology. 1993;74:104–112. doi: 10.2307/1939505. [DOI] [Google Scholar]
- Bowden RD, Steudler PA, Melillo JM, Aber JD. Annual nitrous oxide fluxes from temperate forest soils in the northeastern United States. J Geophys Res. 1990;95:13997–14005. doi: 10.1029/JD095iD09p13997. [DOI] [Google Scholar]
- Bradford MA, Davies CA, Frey SA, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Treseder KK, Wallenstein MD. Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett. 2008;11:1316–1327. doi: 10.1111/j.1461-0248.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- Chapin SF, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. Responses of arctic tundra to experimental and observed changes in climate. Ecology. 1995;76:694–711. doi: 10.2307/1939337. [DOI] [Google Scholar]
- Downs MR, Nadelhoffer KJ, Melillo JM, Aber JD. Foliar and fine root nitrate reductase activity in seedlings of four forest tree species in relation to nitrogen availability. Trees. 1993;7:233–236. doi: 10.1007/BF00202079. [DOI] [Google Scholar]
- Eliasson PE, McMurtrie RE, Pepper DA, Stromgre M, Sune L, Agren GI. The response of heterotrophic CO2 flux to soil warming. Glob Change Biol. 2005;11:167–181. doi: 10.1111/j.1365-2486.2004.00878.x. [DOI] [Google Scholar]
- Emmett BA, Beier C, Estiarte M, Tietema A, Kristensen HL, Williams D, Penuelas J, Schmidt I, Sowerby A. The response of soil processes to climate change: results from manipulation studies of shrublands across an environmental gradient. Ecosystems. 2004;7:625–637. doi: 10.1007/s10021-004-0220-x. [DOI] [Google Scholar]
- Eno CF. Nitrate production in the field by incubating the soil in polyethylene bags. Soil Sci Soc Am J. 1960;24:277–279. doi: 10.2136/sssaj1960.03615995002400040019x. [DOI] [Google Scholar]
- Field C, Mooney HA. The photosynthesis—nitrogen relationship in wild plants. In: Givnish TJ, editor. On the economy of plant form and function. Cambridge: Cambridge University Press; 1986. pp. 25–55. [Google Scholar]
- Field CB, Lobell DB, Peters HA, Chiariello NR. Feedbacks of terrestrial ecosystems to climate change. Annu Rev Environ Resour. 2007;32:1–29. doi: 10.1146/annurev.energy.32.053006.141119. [DOI] [Google Scholar]
- Finzi AC, Berthrong ST. Amino acid cycling in three cold-temperate forests on the northeastern USA. Soil Biol Biochem. 2005;38:861–869. [Google Scholar]
- Finzi AC, Canham CD. Sapling growth in response to light and nitrogen availability in a southern New England forest. For Ecol Manag. 2000;131:153–165. doi: 10.1016/S0378-1127(99)00206-6. [DOI] [Google Scholar]
- Frey SD, Drijber R, Smith H, Melillo J. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol Biochem. 2008;40:2904–2907. doi: 10.1016/j.soilbio.2008.07.020. [DOI] [Google Scholar]
- Hartley AE, Neill C, Melillo JM, Crabtree R, Bowles FP. Plant performance and soil nitrogen mineralization in response to simulated climate change in subarctic dwarf shrub heath. Oikos. 1999;86:331–343. doi: 10.2307/3546450. [DOI] [Google Scholar]
- Hickler T, Smith B, Sykes MT, Davis MB, Sugita S, Walker K. Using a generalized vegetation model to simulate vegetation dynamics in Northeastern USA. Ecology. 2004;85:519–530. doi: 10.1890/02-0344. [DOI] [Google Scholar]
- Iverson LR, Prasad AM. Predicting abundance of 80 tree species following climate change in the eastern United States. Ecol Monogr. 1998;68:465–485. doi: 10.1890/0012-9615(1998)068[0465:PAOTSF]2.0.CO;2. [DOI] [Google Scholar]
- Iverson LR, Prasad AM. Potential redistribution changes in tree species richness and forest community types following climate change. Ecosystems. 2001;4:186–199. doi: 10.1007/s10021-001-0003-6. [DOI] [Google Scholar]
- Iverson LR, Prasad AM. Potential redistribution of tree species habitat under five climate change scenarios in the eastern US. For Ecol Manag. 2002;155:205–222. doi: 10.1016/S0378-1127(01)00559-X. [DOI] [Google Scholar]
- Iverson L, Prasad A, Matthews S. Modeling potential climate change impacts on the trees in the northeastern United States. Mitig Adapt Strateg Glob Change. 2008;13:487–516. doi: 10.1007/s11027-007-9129-y. [DOI] [Google Scholar]
- Jenkins JC, Chojnacky DC, Heath LS, Birdsey RA. National-scale biomass estimators for United States tree species. For Sci. 2003;49:12–35. [Google Scholar]
- Killingbeck KT. Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology. 1996;77:1716–1727. doi: 10.2307/2265777. [DOI] [Google Scholar]
- Kobe RK, Lepczyk CA, Iyer M. Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology. 2005;86:2780–2792. doi: 10.1890/04-1830. [DOI] [Google Scholar]
- Lambers H, Raven JA, Shaver GR, Smith SE. Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol. 2008;23:95–103. doi: 10.1016/j.tree.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Luo Y, Wan S, Hui D, Wallace LL. Acclimatization of soil respiration to warming in a tall grass prairie. Nature. 2001;413:622–625. doi: 10.1038/35098065. [DOI] [PubMed] [Google Scholar]
- Marschner H, Dell B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil. 1994;159:89–102. [Google Scholar]
- Melillo JM (1977) Mineralization of nitrogen in northern forest ecosystems. PhD dissertation, Yale University, New Haven, CT
- Melillo JM, Gosz JR. Interactions of biogeochemical cycles in forest ecosystems. In: Bolin B, Cook RB, editors. The major biogeochemical cycles and their interactions. New York: Wiley; 1983. pp. 177–222. [Google Scholar]
- Melillo JM, Callaghan TV, Woodward FI, Salati E, Sinha ESK. The IPCC Scientific Assessment. In: Houghton JT, Jenkins GJ, Ephraums JJ, editors. Climate change. New York: Cambridge University Press; 1990. pp. 282–310. [Google Scholar]
- Melillo JM, McGuire AD, Kicklighter DW, Moore B, III, Vorosmarty CJ, Schloss AL. Global climate change and terrestrial net primary production. Nature. 1993;363:234–240. doi: 10.1038/363234a0. [DOI] [Google Scholar]
- Melillo JM, Kicklighter DW, McGuire AD, Peterjohn WT, Newkirk K. Global change and its effects on soil organic carbon stocks. In: Zepp R, Sonntag C, editors. Report of the Dahlem workshop on the role of nonliving organic matter in the earth’s carbon cycle. Chichester: Wiley; 1995. pp. 175–189. [Google Scholar]
- Melillo JM, Steudler PA, Aber JD, Newkirk K, Lux H, Bowles FP, Catricala C, Ahrens T, Morrisseau S. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298:2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- Melillo JM, Butler SM, Johnson JE, Mohan JE, Lux H, Burrows E, Bowles FP, Smith RM, Vario CL, Hill T, Burton AJ, Zhou Y, Tang J. Soil warming, carbon-nitrogen interactions and forest carbon budgets. Proc Natl Acad Sci USA. 2011;108:9508–9512. doi: 10.1073/pnas.1018189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan JE, Clark JS, Schlesinger WH. Long-term CO2 enrichment of a forest ecosystem: implications for forest regeneration and succession. Ecol Appl. 2007;17:1198–1212. doi: 10.1890/05-1690. [DOI] [PubMed] [Google Scholar]
- Mohan JE, Cox RM, Iverson LR. Composition and carbon dynamics of forests in northeastern North America in a future, warmer world. Can J For Res. 2009;39:213–230. doi: 10.1139/X08-185. [DOI] [Google Scholar]
- Ollinger SV, Richardson AD, Martin ME, Hollinger DY, Frolking SE, Reich PB, Plourde LC, Katul GG, Munger JW, Oren R, Smith M-L, Paw UKT, Bolstad PV, Cook BD, Day MC, Martin TA, Monson RK, Schid HP. Canopy nitrogen, carbon assimilation, and albedo in temperate and boreal forests: functional relations and potential climate feedbacks. Proc Natl Acad Sci USA. 2008;105:19336–19341. doi: 10.1073/pnas.0810021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostle NJ, Smith P, Fisher R, Woodward FI, Fisher JB, Smith JU, Galbraith D, Levy P, Meir P, McNamara NP, Bardgett RD. Integrating plant-soil interactions into global carbon models. J Ecol. 2009;97:851–863. doi: 10.1111/j.1365-2745.2009.01547.x. [DOI] [Google Scholar]
- Peterjohn WT, Melillo JM, Steudler PA, Newkirk KM, Bowles FP, Aber JD. The response of trace gas fluxes and N availability to elevated soil temperatures. Ecol Appl. 1994;4:617–625. doi: 10.2307/1941962. [DOI] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS, Uhl C. Photosynthesis-nitrogen relations in Amazonian tree species. Oecologia. 1994;97:62–72. doi: 10.1007/BF00317909. [DOI] [PubMed] [Google Scholar]
- Reich PB, Kloeppel BD, Ellsworth DS, Walters MB. Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia. 1995;104:24–30. doi: 10.1007/BF00365558. [DOI] [PubMed] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustad LE, Fernandez IJ. Soil warming: consequences for foliar litter decay in a spruce-fir forest in Maine, USA. Soil Sci Soc Am J. 1998;62:1072–1081. doi: 10.2136/sssaj1998.03615995006200040031x. [DOI] [Google Scholar]
- Rustad LE, Campbell JL, Marion GM, et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth response to experimental ecosystem warming. Oecologia. 2001;126:543–562. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- Ryan M. Effects of climate change on plant respiration. Ecol Appl. 1991;1:157–167. doi: 10.2307/1941808. [DOI] [PubMed] [Google Scholar]
- Schimel JP, Bennett J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 2004;85:591–602. doi: 10.1890/03-8002. [DOI] [Google Scholar]
- Shaver GR, Canadell J, Chapin FS, Gurevitich J, Harte J, Henry G, Ineson P, Jonasson S, Melillo J, Pitelka L, Rustad L. Global warming and terrestrial ecosystems: a conceptual framework for analysis. Bioscience. 2000;50:871–882. doi: 10.1641/0006-3568(2000)050[0871:GWATEA]2.0.CO;2. [DOI] [Google Scholar]
- Shaw RM, Harte J. Response of nitrogen cycling to simulated climate change: differential responses along a subalpine ecotone. Glob Change Biol. 2001;7:193–210. doi: 10.1046/j.1365-2486.2001.00390.x. [DOI] [Google Scholar]
- Thomas RQ, Canham CD, Weathers KC, Goodale CL. Increased tree carbon storage in response to nitrogen deposition in the US. Nat Geosci. 2009;3:13–17. doi: 10.1038/ngeo721. [DOI] [Google Scholar]
- Van Cleve KV, Oechel WC, Hom JL. Response of black spruce (Piceamariana) ecosystems to soil temperature modification in interior Alaska. Can J For Res. 1990;20:1530–1535. doi: 10.1139/x90-203. [DOI] [Google Scholar]
- VEMAP Members Vegetation/ecosystem modeling and analysis project: comparing biogeography and biogeochemistry models in a continental-scale study of terrestrial ecosystem responses to climate change and CO2 doubling. Global Biogeochem Cycles. 1995;9:407–437. doi: 10.1029/95GB02746. [DOI] [Google Scholar]
- Zaccherio MT, Finzi AC. Atmospheric deposition may affect northern hardwood forest composition by altering soil nutrient supply. Ecol Appl. 2007;17:1929–1941. doi: 10.1890/06-2067.1. [DOI] [PubMed] [Google Scholar]
- Zavaleta ES, Thomas BD, Chiariello NR, Asner GP, Shaw MR, Field CB. Plants reverse warming effect on ecosystem water balance. Proc Natl Acad Sci USA. 2003;100:9892–9893. doi: 10.1073/pnas.1732012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.