Abstract
Many concepts and theories in ecology are highly debated, because it is often difficult to design decisive tests with sufficient replicates. Examples include biodiversity theories, succession concepts, invasion theories, coexistence theories, and concepts of life history strategies. Microbiological tests of ecological concepts are rapidly accumulating, but have yet to tap into their full potential to complement traditional macroecological theories. Taking the example of microbial communities on leaf surfaces (i.e. the phyllosphere), we show that most explorations of ecological concepts in this field of microbiology focus on autecology and population ecology, while community ecology remains understudied. Notable exceptions are first tests of the island biogeography theory and of biodiversity theories. Here, the phyllosphere provides the unique opportunity to set up replicated experiments, potentially moving fields such as biogeography, macroecology, and landscape ecology beyond theoretical and observational evidence. Future approaches should take advantage of the great range of spatial scales offered by the leaf surface by iteratively linking laboratory experiments with spatial simulation models.
Electronic supplementary material
The online version of this article (doi:10.1007/s00442-011-2138-2) contains supplementary material, which is available to authorized users.
Keywords: Ecological theories, Diversity, Biogeography, Niche, Leaf surface
Introduction
Ecological concepts, theories, and models have traditionally been investigated from a perspective centered on macroorganisms. However, many tests of ecological theories using macroorganisms have not been decisive because it is difficult to find sufficient true replicates and to implement the necessary experiments. Recently, more and more studies have appeared in the literature that use microorganisms to test ecological concepts (Konopka 2006). They cover a wide range of microbiomes, among them the plant leaf surface, also referred to as the phyllosphere (Ruinen 1961). The phyllosphere supports numerous microorganisms, including bacteria, fungi, yeast, and protozoa (reviewed in Andrews and Harris 2000; Beattie and Lindow 1995; Hirano and Upper 2000; Kinkel 1997; Leveau 2006; Lindow and Leveau 2002; Lindow and Brandl 2003; Whipps et al. 2008). Bacteria are the most abundant members of the phyllosphere community, and have been shown to colonize leaves at densities of up to 108 cells cm−2 (Leveau 2006). The description of microbial community structure and the quantification of microbial diversity associated with leaf surfaces has been much improved by the application of culture-independent methods. These have provided many new insights into the microbial species that are common and unique to all plant leaf surfaces, the specific adaptations that bacteria and fungi possess and express to meet the chemical and physical challenges of the phyllosphere environment, and the factors that determine community composition, such as the plant species and weather conditions.
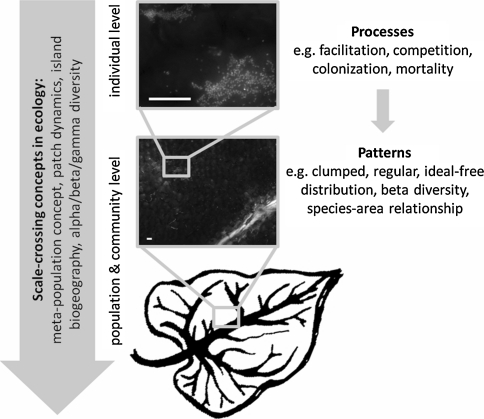
There are several reasons for considering the phyllosphere as a model system for testing ecological concepts and theories. One is its great environmental heterogeneity. For example, the availability of nutrients to microorganisms on leaves is highly variable in space and time (Leveau and Lindow 2001; Monier and Lindow 2003), thus providing ideal grounds for investigating environmental variation as a major property of ecological interactions. This spatiotemporal variability covers a great range of scales (Fig. 1), from micrometer leaf sections to individual leaves on a plant, and from leaves on different plant individuals or species up to whole plant communities. This notion aligns well with the growing awareness of the importance of scales for ecological patterns and processes (Levin 1992; Meyer et al. 2010). Testing ecological concepts in the phyllosphere can thus make a major contribution to landscape ecology (Andrews and Harris 2000). Phyllosphere microbiology also has the potential to test highly controversial ecological theories such as theories of biodiversity (e.g. Hubbell 2003; Ricklefs 2003). Moreover, the phyllosphere is highly accessible to experimental manipulation, facilitating experiments with sufficient replication and therefore statistical tests with considerable power. Visualization techniques such as confocal and epifluorescence microscopy and tools based on fluorescent protein markers improve the experimental options even further. The use of green fluorescent protein (GFP) has truly revolutionized our ability to monitor the whereabouts, behaviours and interactions of individual bacterial cells colonizing the leaf surface (Leveau and Lindow 2001; Remus-Emsermann and Leveau 2010). These and other molecular tools make microbal communities of the phyllosphere more tractable for experimental tests of concepts ranging from landscape ecology and macroecology to biogeography. Contrary to traditional macroecological approaches, the manipulation of island size or shape is relatively easy in the phyllosphere. Finally, the considerable commercial interest in the prevention of foliar diseases in agriculturally important crops and the growing concern over unwanted human pathogens on leafy greens adds extra weight to the need for ecological studies in the phyllosphere (Whipps et al. 2008).
Fig. 1.
Scales in the phyllosphere. Patterns such as clumped spatial distributions of bacterial populations or communities arise from ecological processes that occur at the individual level. Scale-explicit ecological concepts such as patch dynamics or species-area relationships can link the different levels across scales, but have not been explored fully for the phyllosphere yet. The two photographs show individual bacteria of two different strains (green and red) that have been inoculated on bean leaves, with the leaf topography visible in the background and a leaf vein in light grey in the lower right corner of the lower photograph. White bars correspond to 20 μm. Photographs by Daniel Esser (color figure online)
The aims of this review are to (1) compile and evaluate the conclusions that have been drawn in studies addressing ecological concepts in the phyllosphere to date, (2) identify gaps in these studies that would benefit from increased research efforts, and (3) pinpoint essential characteristics of future approaches to testing ecological concepts in the phyllosphere for the benefit of micro- and macroecology.
Ecological concepts addressed in the phyllosphere
Thus far, the focus of phyllosphere microbiologists has been predominantly on concepts from autecology and population ecology, such as fitness, habitat, niche, population dynamics, and competition, as listed in detail in Electronic supplementary material (ESM) 1 and 2. For instance, bacterial profiles of nutrient source utilization in vitro have been used to estimate niche overlap and to quantify the degree of ecological similarity or niche differentiation of bacterial species (Wilson and Lindow 1994). Among the autecological concepts, the application of life-history strategies to phyllosphere organisms is noteworthy. Originally designed for the classification of plants, concepts such as Grime’s (2001) C-S-R triangle theory on the trade-offs between competitiveness (C), stress tolerance (S), and a combination of high reproduction and low longevity (R, for ruderality) were applied to phyllosphere fungi (Nix-Stohr et al. 2008), for which generally an S strategy is assumed, which involves maximizing stress tolerance. Contrary to this expectation, however, these fungi were found to maximize the occupation and exploration of resources, thus implying R and C strategies instead (Nix-Stohr et al. 2008).
In phyllosphere population ecology, population dynamics and competition are the predominating concepts (ESM 2). Studies on population dynamics are plentiful, but very few identify spatiotemporal patterns or go beyond reporting static population densities. In one of the few studies that do (Ellis et al. 1999), populations of fluorescent pseudomonads were sampled from sugar beet leaves to reveal a dynamic, nonrandom and continuous turnover of ribotypes within that population. Such cyclic population dynamics and their underlying mechanisms are a highly debated topic in ecology (Turchin and Hanski 2001), which future phyllosphere studies may help to elucidate.
A recurring pattern in phyllosphere population dynamics that might benefit from further investigation is the great temporal variability in population sizes (Dreux et al. 2007; Nix et al. 2008). Only recently have techniques been developed that allow the quantification of the fate and reproductive success of individual bacteria on leaf surfaces. For example, Remus-Emsermann and Leveau (2010) showed that individual immigrants to leaf surfaces contribute unequally to population sizes.
Competition has not only been inferred from nutrient overlap indices (Wilson and Lindow 1994), but also from pre-emptive exclusion of competitors by primary colonizers of the leaf surface (Lindow and Leveau 2002; Lindow and Brandl 2003), which can be an important mechanism underlying the success of biocontrol against plant pathogens (Mohamed and Caunter 1995). This is particularly true when the pathogen is an r-strategist with a fast reproduction and low competitiveness (Marois and Coleman 1995), or when the germination of spores of pathogenic fungi depends on nutrients (Elad 1996). Due to the interest in biological control applications, the two population ecology concepts of parasitism and commensalism have traditionally have had a strong position in phyllosphere microbiology. The disease cycle of a pathogen such as P. syringae pv syringae features both a commensal stage (where it colonizes the leaf surface) and a parasitic stage (where it internalizes in the leaf apoplast and causes disease symptoms). Strong competition in the first stage may shorten or preclude the parasitic phase of such a pathogen (Fernando et al. 1996).
In microbial phyllosphere populations, trophic interactions are rare, but interactions between insect herbivores and microbial colonizers of plant leaves have been explored. This leads us into the realms of community ecology, i.e. the ecology of groups of different taxa. Leaf herbivory by insect larvae was shown to stimulate the size and diversity of microbial communities (Muller et al. 2003). Considering interactions between macroorganisms and microorganisms in ecological studies will enhance the explanatory power of future ecological studies. Relatively underexplored types of trophic interactions are represented by bacteriophage infection of epiphytic bacteria (Iriarte et al. 2007) and protozoan grazing (Bamforth 1980).
Most concepts from community ecology have received little attention in phyllosphere microbiology, but there are exceptions (ESM 3). Probably the best-known spatial community concept is MacArthur and Wilson’s (1967) island biogeography theory, where the processes of extinction, immigration and emigration lead to an equilibrium number of species in an island community depending on the size of the island and the distance to the mainland. The island biogeography theory is still strongly debated, particularly since it forms part of Hubbell’s (2001) controversial neutral theory of biodiversity. In their seminal test of island biogeography predictions, Kinkel et al. (1987) found evidence for species turnover and equilibrium in the phyllosphere, but not for a species–area relationship.
Biodiversity has been a hot topic in community ecology for a long time and studies addressing diversity in microbial ecosystems abound. Trees in tropical regions are hot spots of fungal species diversity, with common fungal species associated with large host ranges and rare species associated with small host ranges (Arnold and Lutzoni 2007). Most of these studies remain descriptive by focussing on species richness in different habitats. One exception is the finding that plants with different genetic backgrounds can drive bacterial diversity on their leaf surface (Balint-Kurti et al. 2010). Another exception is the derivation of log-normal species–abundance curves for a pseudomonad community (Ellis et al. 1999), which is not in agreement with the zero-sum multinomial curve predicted by Hubbell’s (2001) neutral theory of biodiversity. Similarly, a recent study on the diversity of fungal phyllosphere communities shows log-normal rank-abundance curves for the subset of more common species and log-series distributions of the abundances of the subset of the rarer species (Unterseher et al. 2011). A growing number of studies report on the use of next-generation sequencing to reveal the bacterial and fungal diversity on plant leaf surfaces in a culture-independent manner (e.g., Redford et al. 2010; Jumpponen and Jones 2010).
Succession and invasion are two spatiotemporal concepts from community ecology that are ideally suited for testing in the phyllosphere. Succession has been addressed in the phyllosphere (reviewed e.g. in Redford and Fierer 2009), but mostly in a purely descriptive way and often reporting mere seasonal variation rather than true successional shifts in community composition. In contrast, invasions have been approached with more attention to the underlying mechanisms. For instance, antagonists can prevent the invasion of leaf pathogens if they are allowed to pre-emptively colonize the leaf (Giddens et al. 2003). With a future emphasis on the mechanisms of ecological succession and invasion, fundamental and applied ecology are likely to benefit. Biocontrol managers may be able to directly apply results from invasions research to the colonization of biocontrol agents (Andrews 1990).
Points of departure for future investigations of ecological concepts in the phyllosphere
Major gaps in testing autecological concepts with organisms of the phyllosphere are resource uptake concepts such as optimal foraging theory and ideal-free distribution and the trait-based concepts of phenotypic plasticity and trade-offs (Table 1). Both optimal foraging and ideal free distribution are spatial theories and are therefore particularly suitable for tests in the phyllosphere.
Table 1.
Ecological concepts underaddressed in the phyllosphere
| Concept | Relevance | Issues |
|---|---|---|
| Autecology | ||
| Niche | Core concept for predicting outcomes of interactions such as competition or facilitation | The term “niche” has repeatedly been used to refer only to a spatial location, while the classic niche definition applies to a combination of resources and environmental conditions that allow survival and reproduction (including space, but not exclusively) |
| Resource uptake | ||
| Optimal foraging theory | The patchily distributed resources in the phyllosphere are ideal to test whether micro- and macroorganisms have an equally optimal energy intake over time… | |
| Ideal-free distribution | …and build up population sizes proportional to the amount of resources in a patch | |
| Phenotypic plasticity | Contributes to the flexibility of the reaction to environmental changes such as climate change | |
| Trade-offs | Limit the flexibility of evolutionary responses to abiotic and biotic changes | |
| Population ecology | ||
| Allee effect | Mechanism causing overproportional reduction in survival and reproduction at small population densities | |
| Allelopathy | Mechanism similar to antibiosis that could enhance pre-emptive competition | The focus of the only available study (Vokou 2007) was on allelochemistry rather than the ecological mechanism |
| Competition | ||
| Density dependence | Regulation of intraspecific population dynamics with consequences for coexistence | Density-dependent processes such as mortality have been identified, but forms of density dependence (e.g. over- or undercompensating for density increase) have not yet been explored |
| Scramble/contest | Mechanisms of sharing resources among all competitors (typical for r-strategists) versus monopolizing resources by the best competitor (K-strategists, territoriality), particularly relevant under varying spatiotemporal conditions | |
| Apparent competition | Improving the ecological realism by studying more complex interactions such as competition mediated by an enemy attacking both competitors | |
| Competitive release | Scope for microecological corroboration of this strongly debated concept of niche expansion after release from competition | |
| Resource depletion zone | Exploring the zone of resource reduction by the consumer may provide spatial explanations for competitive outcomes | |
| Cycles | Widespread population dynamics in ecology affecting the type and degree of interactions, e.g. predator–prey cycles | Only the potential for population cycles has been highlighted based on seasonality of environmental conditions across temporal scales (Hirano and Upper 2000); a demonstration is lacking |
| Metapopulation | Spatiotemporal mechanism for the prevention of extinction based on a set of patchy subpopulations that are linked by dispersal | There have been no explicit tests yet, but some implicit treatments (Ives et al. 2004; Woody et al. 2007). A test requires patchy subpopulations that go extinct asynchronously due to demographic stochasticity, but which are linked by dispersal ensuring metapopulation persistence |
| Community ecology | ||
| Community assembly rules | Mechanisms underlying species presence and absence in space and time | Community assembly rules have been mentioned (Nix-Stohr et al. 2008), but a more explicit identification of community assembly rules is needed |
| Diversity theories | Explain diversity patterns and provide underlying mechanisms [e.g., neutral theory of biodiversity (Hubbell 2001), intermediate disturbance hypothesis (Connell 1978)] | |
| Ecosystem engineers and keystone species | Provide a focus for biological control research and for understanding an ecosystem | |
| Food web, food chain, multitrophic interactions, connectance | Enhancing the ecological realism of research and the likelihood of discovering mechanisms by including more complex biotic interactions | |
| Island biogeography | Explains diversity dynamics on islands such as leaves or habitable patches within leaves | To our knowledge, only one explicit test exists to date (Kinkel et al. 1987), the conclusions of which are mixed. To establish generality, more tests are needed |
| Patch dynamics | Scale-explicit spatiotemporal coexistence mechanism, probably enhancing biodiversity | |
| Specialization and omnivory | Determines the connectedness of communities that can influence community resilience and resistance | |
| Species (or rank) abundance curves | Patterns for the validation of diversity theories such as Hubbell’s (2001) neutral theory | Ellis et al. (1999) report lognormal species abundance curves. To establish the generality of a diversity theory, more patterns have to be evaluated |
| Species–area relationships | Basis for island biogeography theory; related to community assembly and diversity theories | Kinkel et al. (1987) did not find a relationship between number of species and area, but the tested fungal species were probably not limited by area. Hence, testing other species would be valuable to establish generality |
| Stability | Measure of the resilience or resistance of a community to perturbations such as invasions by exotic species | |
| Top-down and bottom-up regulation | Knowledge of the regulation of an organism via antagonists or via resources is a key to understanding and manipulating community organization | |
Issues are only mentioned if concepts have been addressed, but insufficiently for our purpose of testing or applying the respective concept in the phyllosphere. “Concept” is used here in the wider sense, including theories. For a definition of the ecological concepts, see the glossary of an ecological textbook, e.g. http://www.blackwellpublishing.com/begon/
In population ecology, the most frequently addressed concepts of population dynamics and competition also bear the most apparent gaps in phyllosphere microbiology (Table 1). Concerning population dynamics, the two interesting phenomena of compensatory density dependence and the Allee effect remain largely untreated in the phyllosphere. Since compensation is rarely perfect, the degree of over- or undercompensation is an important property of the population dynamics of a species, allowing forecasts of population dynamics under changing environmental conditions. In the same way, the Allee effect may offer an explanation for unexpected patterns of population dynamics in experiments with small population sizes. Competitive release and niche expansion of the released competitor is a strongly debated concept in ecology that is highly suitable for microbial corroboration in the phyllosphere. Similarly, the concepts of scramble and contest competition are rarely questioned in ecology, despite the lack of conclusive evidence for the hypothesis that r-strategists exemplify scramble competition and K-strategists show contest competition. The outcome of a competitive interaction can depend on the impact of other biotic interactions, as in the case of apparent competition. Exploring apparent competition in the phyllosphere, e.g. by exposing two competing bacterial strains to a third one that is antagonistic to both of them, would add ecological realism to the existing setups.
In community ecology, we identify the greatest opportunities for phyllosphere microbiology to test ecological concepts. The description of communities is quite advanced with respect to diversity, but community stability, assembly rules, and top-down versus bottom-up regulation have not yet been explored (Table 1). The relationship between community stability and ecosystem functioning is a matter of lively discussions in ecology (Bezemer and Van der Putten 2007). Stability can be measured as resilience or resistance of a community to perturbations such as the introduction of an exotic species (Grimm and Wissel 1997). In the phyllosphere, such an exotic species could simply be represented by a bacterial cell or fungal spore that does not originate from the same plant or even environment. Community assembly rules aim to predict spatial species distributions. They often strongly rely on biotic interactions (Diamond 1975), but their generality has been debated (Gotelli and McCabe 2002). Their strong spatial reference makes them highly amenable to phyllosphere studies. Despite the good coverage of diversity descriptions in phyllosphere community studies, explicit tests of diversity theories are lacking (Table 1). One way to test diversity theories such as Hubbell’s (2001) neutral theory or Tilman’s (2004) stochastic niche theory is to assemble species abundance curves (see one of the few phyllosphere examples thus far: Ellis et al. 1999) and compare them with the predictions of the theories. Moreover, the exploration of coexistence mechanisms such as scale-explicit patch dynamics (Table 1) may aid our understanding of the processes underlying community diversity.
Investigations of trophic interactions in phyllosphere communities have only just begun. An explicit consideration of food chains, even food webs and their associated properties such as connectance, may be beneficial and more realistic in scenarios where trophic interactions occur (Table 1). Finally, since bacteria can manipulate their environment (Brown et al. 2009), the topic of ecosystem engineering is a very promising avenue to explore. If keystone species can be identified, research efforts aimed at gaining a mechanistic understanding of community structure and dynamics can be concentrated on these species.
Assessments of microbial biodiversity in the phyllosphere can use a descriptive or a mechanistic approach. If pure descriptions of diversity are needed, diversity indices that include the dominance structure of the community should be used instead of simple species richness measures. For instance, the range of ecosystem services that a phyllosphere community can provide depends not only on the number of functional groups present, but also on the relative abundances of the species that perform those functions. This can be investigated with diversity indices but not with species richness measures. To take advantage of the great range of scales offered by the phyllosphere (Fig. 1), future studies could also explore alpha, beta, and gamma diversity, referring to the diversity within a location (e.g. a leaf), between two locations (e.g. two leaves), and within a larger-scale region (e.g. pooled leaves from different parts of an agricultural field or forest), respectively.
To improve our understanding of the mechanisms underlying community structure, it is also important to move beyond mere descriptions of diversity and relate diversity to mechanisms, i.e. time, space (species-area curves), or some measure of ecosystem function such as protection against invasion of a plant pathogen. This approach is going to be facilitated by recent developments of culture-independent methods suitable for the quantitative assessment of microbial diversity. Such methods include quantitative PCR to estimate the abundance of specific bacterial taxa on a plant (Zwielehner et al. 2008), rRNA gene amplicon pyrosequencing to assess fungal (Jumpponen and Jones 2009) and bacterial (Redford et al. 2010) diversity on tree foliage, and proteogenomics to uncover the most abundantly expressed genes in the phyllosphere environment (Delmotte et al. 2009). Diversity studies of this kind may also make a valuable contribution to the resolution of the longstanding diversity–ecosystem function debate in ecology (Cameron 2002). This is particularly important, because our knowledge of ecosystem functions and the relative importance of phyllosphere communities is limited.
Concluding remarks
An impressive amount of phyllosphere literature has already been devoted to the investigation of ecological concepts across all levels of organization. If gaps in these accounts are addressed in future studies, significant progress can be achieved not only in microbial ecology and applied phyllosphere research but also in the ecological study of macrobiomes. In the realm of autecology, a very promising line of future phyllosphere research is the study of trade-offs between phenotypic plasticity and bet-hedging in a rapidly changing environment like the leaf surface. In other words, does it make more sense for a bacterial population to respond to or to anticipate change? Based on Leibler and Kussell’s (2010) idea of responsive versus stochastic switching models, future experiments could expose bacteria with different genotypes to disturbances of differing frequency and intensity and monitor survival and reproductive success. In population ecology, a highly anticipated target for future research is interactions at the level of individuals, because the phyllosphere offers the necessary spatial variation at the scale of individuals and because the necessary tools for individual-based microbiology are now available. In this context, an interesting question to ask is: what does a concept like carrying capacity mean from the viewpoint of an individual bacterium—can carrying capacity be explained bottom-up, and what new insights would such an explanation allow us to gain in our attempts to mechanistically understand and predict the colonization of leaves or other environments? In community ecology, advances are most likely to come from variations on studies like that of Kinkel et al.’s (1987) island biogeography study, perhaps with a more inclusive focus on immigration and emigration processes, while taking full advantage of the latest methodologies such as pyrosequencing and proteogenomics. This emphasis on mechanisms rather than patterns should guide future phyllosphere research in general, and could, for instance, also be transferred to questions of community stability. Answers to these questions will ultimately be of practical value, e.g. a knowledge of the extent to which differently structured communities resist invasions by plant pathogens in a spatially and temporally variable environment such as the phyllosphere.
Future collaborations between microbiologists and ecologists will greatly benefit from a more unambiguous use of ecological terminology (see the column “Issues” in Table 1). For instance, assembly rules have been mentioned without a clear treatment of species distributions (e.g. Nix-Stohr et al. 2008), and the phenomenon of competitive exclusion has been termed niche exclusion (e.g. Giddens et al. 2003). The term “niche” is often used synonymously with habitat, to refer to a location in space only (e.g. Pereg et al. 1994), not including the range of all resources, environmental conditions and biotic interactions that limit the reproduction and survival of a species (Hutchinson 1957). Another definition problem is posed by the dissent over which species concept should be used when addressing species-specific concepts such as intra- and interspecific competition, or when measuring community patterns such as species diversity. Here, concepts grouping species according to function seem more appropriate (Konopka 2006). Moreover, there seems to be a need for independent replicate studies to establish the generality of some of the conclusions that have been reached, such as in the case of island biogeography theory and the species–area relationship. Explicit tests should be conducted in cases where conclusions on concepts such as metapopulations or rank abundance curves were only by-products of studies with a different focus. For example, for a full test of metapopulation dynamics, evidence is required for the spatial organisation of the population into discrete patches, the occasional extinction of subpopulations, and the coupling of the subpopulations in the patches via dispersal (Levins 1969).
In our attempt to advance microecological theories and concepts in the phyllosphere, it will be important to complement the empirical approaches listed above with theoretical approaches and models. Since one unique feature of the phyllosphere is the great range of spatial scales covered, the behaviour of ecological processes along scale transitions will be a key point to start from. Adopting an individual-based, bottom-up approach would then reflect the awareness that larger-scale community patterns emerge from individual-level interactions (Fig. 1). Kreft et al. (1998, 2001) have simulated microbial interactions and population dynamics in biofilms, and these models can easily be adapted to represent phyllosphere conditions. The majority of these models implement an individual-based approach (Grimm et al. 2005). Addressing individual variation in phyllosphere models is justified because individual properties such as spatial position (Kinkel et al. 2002; Monier and Lindow 2003) and fitness (Grimm and Railsback 2005) play an important role in microbial interactions and in the majority of ecological concepts and theories (Durrett and Levin 1994). A phyllosphere simulation model should above all be data driven, which will require strong feedbacks between modellers and empiricists (for a good example, see Ives et al. 2004). There are two conceptual colonization models that have been developed for the phyllosphere (Beattie and Lindow 1999; Nix et al. 2008). These offer good starting points, as do earlier models specifically formulated to address microbial survival, plasmid transfer, and dispersal in the phyllosphere (Knudsen et al. 1988; Knudsen 1989).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Martin Schädler, Mitja Remus-Emsermann, Hans van Veen, Tanja Scheublin, and an anonymous reviewer for helpful input or exchanges of ideas during the preparation of the manuscript. We acknowledge funding from NWO VIDI-grant 864.06.002 to JHJL. KMM was partly funded by the State of Lower Saxony (Ministry of Science and Culture; Cluster of Excellence “Functional Biodiversity Research”). This is NIOO publication number 5105.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Andrews JH (1990) Biological control in the phyllosphere—realistic goal or false hope? Can J Plant Pathol Rev Can Phytopathol 12:300–307
- Andrews JH, Harris RF. The ecology and biogeography of microorganisms of plant surfaces. Annu Rev Phytopathol. 2000;38:145–180. doi: 10.1146/annurev.phyto.38.1.145. [DOI] [PubMed] [Google Scholar]
- Arnold AE, Lutzoni F. Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology. 2007;88:541–549. doi: 10.1890/05-1459. [DOI] [PubMed] [Google Scholar]
- Balint-Kurti P, Simmons SJ, Blum JE, Ballare CL, Stapleton AE. Maize leaf epiphytic bacteria diversity patterns are genetically correlated with resistance to fungal pathogen infection. Mol Plant-Microbe Interact. 2010;23:473–484. doi: 10.1094/MPMI-23-4-0473. [DOI] [PubMed] [Google Scholar]
- Bamforth SS. Terrestrial Protozoa. J Protozool. 1980;27:33–36. [Google Scholar]
- Beattie GA, Lindow SE. The secret life of foliar bacterial pathogens on leaves. Annu Rev Phytopathol. 1995;33:145–172. doi: 10.1146/annurev.py.33.090195.001045. [DOI] [PubMed] [Google Scholar]
- Beattie GA, Lindow SE. Bacterial colonization of leaves: a spectrum of strategies. Phytopathology. 1999;89:353–359. doi: 10.1094/PHYTO.1999.89.5.353. [DOI] [PubMed] [Google Scholar]
- Bezemer TM, Van der Putten WH. Diversity and stability in plant communities. Nature. 2007;446:E6–E8. doi: 10.1038/nature05749. [DOI] [PubMed] [Google Scholar]
- Brown SP, Inglis RF, Taddei F. Evolutionary ecology of microbial wars: within-host competition and (incidental) virulence. Evolut Appl. 2009;2:32–39. doi: 10.1111/j.1752-4571.2008.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron T. 2002: the year of the ‘diversity-ecosystem function’ debate. TREE. 2002;17:495–496. [Google Scholar]
- Connell JH. Diversity in tropical rain forests and coral reefs—high diversity of trees and corals is maintained only in a non-equilibrium state. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- Delmotte N, Knief C, Chaffron S, Innerebner G, Roschitzki B, Schlapbach R, von Mering C, Vorholt JA (2009) Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. PNAS 106:16428–16433. doi:10.1073/pnas.0905240106 [DOI] [PMC free article] [PubMed]
- Diamond JM. Assembly of species communities. In: Cody ML, Diamond JM, editors. Ecology and evolution of communities. Cambridge: Harvard University Press; 1975. pp. 342–444. [Google Scholar]
- Dreux N, Albagnac C, Carlin F, Morris CE, Nguyen-The C. Fate of Listeria spp. on parsley leaves grown in laboratory and field cultures. J Appl Microbiol. 2007;103:1821–1827. doi: 10.1111/j.1365-2672.2007.03419.x. [DOI] [PubMed] [Google Scholar]
- Durrett R, Levin SA. The importance of being discrete (and spatial) Theor Popul Biol. 1994;46:363–394. doi: 10.1006/tpbi.1994.1032. [DOI] [Google Scholar]
- Elad Y. Mechanisms involved in the biological control of Botrytis cinerea incited diseases. Eur J Plant Pathol. 1996;102:719–732. doi: 10.1007/BF01877146. [DOI] [Google Scholar]
- Ellis RJ, Thompson IP, Bailey MJ. Temporal fluctuations in the pseudomonad population associated with sugar beet leaves. Fems Microbiol Ecol. 1999;28:345–356. doi: 10.1111/j.1574-6941.1999.tb00589.x. [DOI] [Google Scholar]
- Fernando WGD, Watson AK, Paulitz TC. The role of Pseudomonas spp, and competition for carbon, nitrogen and iron in the enhancement of appressorium formation by Colletotrichum coccodes on velvetleaf. Eur J Plant Pathol. 1996;102:1–7. doi: 10.1007/BF01877110. [DOI] [Google Scholar]
- Giddens SR, Houliston GJ, Mahanty HK. The influence of antibiotic production and pre-emptive colonization on the population dynamics of Pantoea agglomerans (Erwinia herbicola) Eh1087 and Erwinia amylovora in planta. Environ Microbiol. 2003;5:1016–1021. doi: 10.1046/j.1462-2920.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- Gotelli NJ, McCabe DJ. Species co-occurrence: a meta-analysis of J. M. Diamond’s assembly rules model. Ecology. 2002;83:2091–2096. doi: 10.1890/0012-9658(2002)083[2091:SCOAMA]2.0.CO;2. [DOI] [Google Scholar]
- Grime JP. Plant strategies, vegetation processes, and ecosystem properties. New York: Wiley; 2001. [Google Scholar]
- Grimm V, Railsback SF. Individual-based modeling and ecology. Princeton: Princeton University Press; 2005. [Google Scholar]
- Grimm V, Revilla E, Berger U, Jeltsch F, Mooij WM, Railsback SF, Thulke HH, Weiner J, Wiegand T, DeAngelis DL. Pattern-oriented modeling of agent-based complex systems: lessons from ecology. Science. 2005;310:987–991. doi: 10.1126/science.1116681. [DOI] [PubMed] [Google Scholar]
- Grimm V, Wissel C. Babel, or the ecological stability discussions: an inventory and analysis of terminology and a guide for avoiding confusion. Oecologia. 1997;109:323–334. doi: 10.1007/s004420050090. [DOI] [PubMed] [Google Scholar]
- Hirano SS, Upper CD (2000) Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev 64:624–653 [DOI] [PMC free article] [PubMed]
- Hubbell SP. The unified neutral theory of biodiversity and biogeography. Princeton: Princeton University Press; 2001. [DOI] [PubMed] [Google Scholar]
- Hubbell SP (2003) Modes of speciation and the lifespans of species under neutrality: a response to the comment of Robert E. Ricklefs. Oikos 100:193–199. doi:10.1034/j.1600-0706.2003.12450.x
- Hutchinson GE (1957) Concluding remarks. Cold Spring Harbor Symp Quant Biol 22:415–427
- Iriarte FB, Balogh B, Momol MT, Smith LM, Wilson M, Jones JB. Factors affecting survival of bacteriophage on tomato leaf surfaces. Appl Environ Micorbiol. 2007;73:1704–1711. doi: 10.1128/AEM.02118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives AR, Woody ST, Nordheim EV, Andrews JH. The synergistic effects of stochasticity and dispersal on population densities. Am Nat. 2004;163:375–387. doi: 10.1086/381942. [DOI] [PubMed] [Google Scholar]
- Jumpponen A, Jones KL. Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol. 2009;184:438–448. doi: 10.1111/j.1469-8137.2009.02990.x. [DOI] [PubMed] [Google Scholar]
- Jumpponen A, Jones KL. Seasonally dynamic fungal communities in the Quercus macrocarpa phyllosphere differ between urban and nonurban environments. New Phytol. 2010;186:496–513. doi: 10.1111/j.1469-8137.2010.03197.x. [DOI] [PubMed] [Google Scholar]
- Kinkel LL. Microbial population dynamics on leaves. Annu Rev Phytopathol. 1997;35:327–347. doi: 10.1146/annurev.phyto.35.1.327. [DOI] [PubMed] [Google Scholar]
- Kinkel LL, Andrews JH, Berbee FM, Nordheim EV. Leaves as islands for microbes. Oecologia. 1987;71:405–408. doi: 10.1007/BF00378714. [DOI] [PubMed] [Google Scholar]
- Kinkel LL, Newton MR, Leonard KJ. Resource aggregation in the phyllosphere: implications for microbial dynamics across spatial scales. In: Lindow SE, Hecht-Poinar EI, Elliott VJ, editors. Phyllosphere microbiology. St. Paul: The American Phytopathological Society; 2002. pp. 317–340. [Google Scholar]
- Knudsen GR. Model to predict aerial dispersal of bacteria during environmental release. Appl Environ Microbiol. 1989;55:2641–2647. doi: 10.1128/aem.55.10.2641-2647.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen GR, Walter MV, Porteous LA, Prince VJ, Armstrong JL, Seidler RJ. Predictive model of conjugative plasmid transfer in the rhizosphere and phyllosphere. Appl Environ Microbiol. 1988;54:343–347. doi: 10.1128/aem.54.2.343-347.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka A. Microbial ecology: searching for principles. Microbe. 2006;1:175–179. [Google Scholar]
- Kreft JU, Booth G, Wimpenny JWT. BacSim, a simulator for individual-based modelling of bacterial colony growth. Microbiology. 1998;144:3275–3287. doi: 10.1099/00221287-144-12-3275. [DOI] [PubMed] [Google Scholar]
- Kreft JU, Picioreanu C, Wimpenny JWT, van Loosdrecht MCM (2001) Individual-based modelling of biofilms. Microbiology 147:2897–2912 [DOI] [PubMed]
- Leibler S, Kussell E (2010) Individual histories and selection in heterogeneous populations. Proc Natl Acad Sci USA 107:13183–13188. doi:10.1073/pnas.0912538107 [DOI] [PMC free article] [PubMed]
- Leveau JHJ. Microbial communities in the phyllosphere. In: Riederer M, Müller C, editors. Biology of the plant cuticle. Oxford: Blackwell; 2006. pp. 334–367. [Google Scholar]
- Leveau JHJ, Lindow SE. Predictive and interpretive simulation of green fluorescent protein expression in reporter bacteria. J Bacteriol. 2001;183:6752–6762. doi: 10.1128/JB.183.23.6752-6762.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SA. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. doi: 10.2307/1941447. [DOI] [Google Scholar]
- Levins R. Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Assoc Am. 1969;15:237–240. [Google Scholar]
- Lindow SE, Brandl MT. Microbiology of the phyllosphere. Appl Environ Microbiol. 2003;69:1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow SE, Leveau JH. Phyllosphere microbiology. Curr Opin Biotechnol. 2002;13:238–243. doi: 10.1016/S0958-1669(02)00313-0. [DOI] [PubMed] [Google Scholar]
- MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
- Marois JJ, Coleman PM (1995) Ecological succession and biological control in the phyllosphere. Can J Bot Rev Can Bot 73:S76–S82
- Meyer KM, Jopp F, Münkemüller T, Reuter H, Schiffers K (2010) Crossing scales in ecology. Basic Appl Ecol 11:561–562. doi:10.1016/j.baae.2010.08.003
- Mohamed S, Caunter IG (1995) Isolation and characterization of a Pseudomonas fluorescens strain suppressive to Bipolaris maydis. J Phytopathol (Phytopathol Z) 143:111–114. doi:10.1111/j.1439-0434.1995.tb00241.x
- Monier JM, Lindow SE (2003) Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc Natl Acad Sci USA 100:15977–15982. doi:10.1073/pnas.2436560100 [DOI] [PMC free article] [PubMed]
- Muller T, Muller M, Behrendt U, Stadler B. Diversity of culturable phyllosphere bacteria on beech and oak: the effects of lepidopterous larvae. Microbiol Res. 2003;158:291–297. doi: 10.1078/0944-5013-00207. [DOI] [PubMed] [Google Scholar]
- Nix-Stohr S, Moshe R, Dighton J. Effects of propagule density and survival strategies on establishment and growth: further investigations in the phylloplane fungal model system. Microb Ecol. 2008;55:38–44. doi: 10.1007/s00248-007-9248-8. [DOI] [PubMed] [Google Scholar]
- Nix SS, Burpee LL, Jackson KL, Buck JW. Short-term temporal dynamics of yeast abundance on the tall fescue phylloplane. Can J Microbiol. 2008;54:299–304. doi: 10.1139/W08-012. [DOI] [PubMed] [Google Scholar]
- Pereg LL, Lipkin Y, Sar N. Different niches of the Halophila stipulacea seagrass bed harbor distinct populations of nitrogen-fixing bacteria. Marine Biol. 1994;119:327–333. doi: 10.1007/BF00347529. [DOI] [Google Scholar]
- Redford AJ, Bowers RM, Knight R, Linhart Y, Fierer N. The ecology of the phyllosphere: geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Envrion Microbiol. 2010;12:2885–2893. doi: 10.1111/j.1462-2920.2010.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redford AJ, Fierer N. Bacterial succession on the leaf surface: a novel system for studying successional dynamics. Microb Ecol. 2009;58:189–198. doi: 10.1007/s00248-009-9495-y. [DOI] [PubMed] [Google Scholar]
- Remus-Emsermann MNP, Leveau JHJ. Linking environmental heterogeneity and reproductive success at single-cell resolution. ISME J. 2010;4:215–222. doi: 10.1038/ismej.2009.110. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. A comment on Hubbell’s zero-sum ecological drift model. Oikos. 2003;100:185–192. doi: 10.1034/j.1600-0706.2003.12018.x. [DOI] [Google Scholar]
- Ruinen J. The phyllosphere I. An ecologically neglected milieu. Plant Soil. 1961;15:81–109. doi: 10.1007/BF01347221. [DOI] [Google Scholar]
- Tilman D (2004) Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. Proc Natl Acad Sci USA 101:10854–10861. doi:10.1073/pnas.0403458101 [DOI] [PMC free article] [PubMed]
- Turchin P, Hanski I. Contrasting alternative hypotheses about rodent cycles by translating them into parameterized models. Ecol Lett. 2001;4:267–276. doi: 10.1046/j.1461-0248.2001.00204.x. [DOI] [Google Scholar]
- Unterseher M, Jumpponen A, Öpik M, Tedersoo L, Moora M, Dormann CF, Schnittler M. Species abundance distributions and richness estimations in fungal metagenomics—lessons learned from community ecology. Mol Ecol. 2011;20:275–285. doi: 10.1111/j.1365-294X.2010.04948.x. [DOI] [PubMed] [Google Scholar]
- Vokou D. Allelochemicals, allelopathy and essential oils: a field in search of definitions and structure. Allelopath J. 2007;19:119–133. [Google Scholar]
- Whipps JM, Hand P, Pink D, Bending GD. Phyllosphere microbiology with special reference to diversity and plant genotype. J Appl Microbiol. 2008;105:1744–1755. doi: 10.1111/j.1365-2672.2008.03906.x. [DOI] [PubMed] [Google Scholar]
- Wilson M, Lindow SE. Coexistence among epiphytic bacterial populations mediated through nutritional resource partitioning. Appl Environ Microbiol. 1994;60:4468–4477. doi: 10.1128/aem.60.12.4468-4477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ST, Ives AR, Nordheim EV, Andrews JH. Dispersal, density dependence, and population dynamics of a fungal microbe on leaf surfaces. Ecology. 2007;88:1513–1524. doi: 10.1890/05-2026. [DOI] [PubMed] [Google Scholar]
- Zwielehner J, Handschur M, Michaelsen A, Irez S, Demel M, Denner EBM, Hasiberger AG. DGGE and real-time PCR analysis of lactic acid bacteria in bacterial communities of the phyllosphere of lettuce. Mol Nutr Food Res. 2008;52:614–623. doi: 10.1002/mnfr.200700158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.