Abstract
We have quantified vegetable growers’ exposure to fungal bioaerosol components including (1→3)-β-d-glucan (β-glucan), total fungal spores, and culturable fungal units. Furthermore, we have evaluated factors that might affect vegetable growers’ exposure to fungal bioaerosols and airborne dust. Investigated environments included greenhouses producing cucumbers and tomatoes, open fields producing cabbage, broccoli, and celery, and packing facilities. Measurements were performed at different times during the growth season and during execution of different work tasks. Bioaerosols were collected with personal and stationary filter samplers. Selected fungal species (Beauveria spp., Trichoderma spp., Penicillium olsonii, and Penicillium brevicompactum) were identified using different polymerase chain reaction-based methods and sequencing. We found that the factors (i) work task, (ii) crop, including growth stage of handled plant material, and (iii) open field versus greenhouse significantly affected the workers’ exposure to bioaerosols. Packing of vegetables and working in open fields caused significantly lower exposure to bioaerosols, e.g. mesophilic fungi and dust, than harvesting in greenhouses and clearing of senescent greenhouse plants. Also removing strings in cucumber greenhouses caused a lower exposure to bioaerosols than harvest of cucumbers while removal of old plants caused the highest exposure. In general, the exposure was higher in greenhouses than in open fields. The exposures to β-glucan during harvest and clearing of senescent greenhouse plants were very high (median values ranging between 50 and 1500 ng m−3) compared to exposures reported from other occupational environments. In conclusion, vegetable growers’ exposure to bioaerosols was related to the environment, in which they worked, the investigated work tasks, and the vegetable crop.
Keywords: agriculture, air sampling, beta-glucan, bioaerosols, exposure assessment, fungi, glasshouse, glucan, occupational environment
INTRODUCTION
Workers in agriculture are exposed to airborne dust and fungal spores due to the handling of organic materials (Swan and Crook, 1998; Lee et al., 2006). However, concerning vegetable production in open fields or greenhouses, only few studies have focused on growers’ exposure to bioaerosols. In outdoor environments, weather conditions affect the prevalence of atmospheric bioaerosols (Jones and Harrison, 2004), whereas outdoor weather conditions have a negligible impact on greenhouse environments (Illing, 1997). Instead, the confined environment of a greenhouse is considered to strongly influence growers’ exposure to bioaerosols (fungal spores, bacteria, pollen, etc.) (Illing, 1997). An additional source of exposure to microorganisms might be microbial pest control agents (MPCAs), which are beneficial microorganisms applied in crop production (Jensen et al., 2002; Hansen et al., 2010a,b; Li and LaMondia, 2010).
As plants grow, the density of the biomass inside the greenhouse increases, and this biomass increase is expected to have a significant effect on the greenhouse environment. Greenhouse crops vary in size from small ornamental plants and low vegetable-producing plants like squash (Cucurbita pepo L.) to tall plants such as cucumber (Cucumis sativus L.) and tomato plants (Solanum lycopersicum L.), which reach above head height when mature. In Danish greenhouses, tomato plants are grown for ∼10 months while cucumber plants grow and bear fruit for ∼5 months. Leaves on tomato plants are removed continuously after ∼2 months of leaf growth, whereas cucumber leaves are left on the plants for the duration of the plants’ life span. Crops like cabbage (Brassica oleracea L. var. capitata), broccoli (B. oleracea L. var. italica), and celery (Apium graveolens L.) in open fields are ∼50 cm in height and can be considered low vegetation.
Inhalation of bioaerosols such as dust and fungal spores may cause respiratory symptoms and lung function impairment (Lacey and Dutkiewicz, 1994; Eduard et al., 2001; Atkinson et al., 2006; Bush et al., 2006). Occupational asthma and rhinitis have been reported for greenhouse workers sensitized to workplace flowers or moulds (Monsó et al., 2002; Monsó, 2004; Riu et al., 2008). The structural cell wall component (1→3)-β-D-glucan (β-glucan) of fungi may be associated with respiratory symptoms as well (Douwes, 2005). Some fungal species, which may be present in working environments, e.g. Aspergillus fumigatus Fresenius (Ascomycota: Eurotiales), can infect immunosuppressed individuals (Latgé, 1999). To evaluate whether occupational exposure to a bioaerosol component should be considered an occupational hazard, the exposure level of that particular component should be compared to accepted or suggested occupational exposure limits (OELs) (Eduard, 2009; Madsen et al., 2009).
The aim of this study was to quantify vegetable growers’ exposure to naturally occurring fungal bioaerosol components in greenhouses and open fields and to evaluate factors that might affect the level of exposure to these components as well as to airborne dust. We focused on work tasks and the crops produced, including growth stage of the plants. Furthermore, we aimed to compare open fields with greenhouse environments. The investigations were performed during regular work days at commercial, vegetable-producing greenhouses. In order to properly describe the effects of work activities in different settings and stages of crop production, we have drawn mainly upon unpublished data but also included data calculated from raw data previously used in Hansen et al. (2010b) and in Madsen et al. (2009). The genera Trichoderma and Beauveria (Ascomycota: Hypocreales) were included since species of these genera are used in Denmark as MPCAs. The Penicillium species (Ascomycota: Eurotiales) Penicillium olsonii Bainier & Sartory and Penicillium brevicompactum Dierckx were also quantified because they were found to be highly prevalent in the investigated environments.
MATERIALS AND METHODS
Working environments
Investigations of Danish vegetable growers’ working environment were performed in 2007–2008. Five greenhouses were investigated, of which three produced tomatoes and two produced cucumbers. One cucumber producer also produced poinsettia (Euphorbia pulcherrima Willd. ex Klotzsch). The remaining production environments included four open fields growing broccoli, cabbage, and celery and two packing facilities (Table 1). In tomato greenhouse T-3, the MPCA Supresivit® based on Trichoderma harzianum Rifai had been applied earlier in the growth season (Hansen et al., 2010b). Application of fungal MPCAs was not reported for any other investigated environments. In Table 1, the numbers of stationary samples and personal samples collected are shown.
Table 1.
Description of the investigated environments.
| Producer, crop | Production environment | Number of stationary samplers | Number of personal samplers | Work tasks | Plant stage | Date for air sampling |
| T-1, tomato | Greenhouse | 3 | 6 | Harvesting, nurturing | Mature | 22 May 2007 |
| T-1, tomato | Greenhouse | 3 | 5 | Clearinga | Senescent | 12 November 2007 |
| T-2, tomato | Greenhouse | 3 | 9 | Harvesting, nurturing | Mature | 19 June 2007 |
| T-2, tomato | Greenhouse | 3 | 7 | Clearinga | Senescent | 14 November 2007 |
| T-3, tomato | Greenhouse | 3 | 8 | Nurturing | Premature | 20 and 26 February 2008 |
| T-3, tomato | Greenhouse | 3 | 8 | Harvesting, nurturing | Mature | 20 May 2008 |
| C-1, cucumber | Greenhouse | 3 | 10 | Harvest, nurturing | Mature | 13 June 2007 |
| C-1, cucumber | Greenhouse | 3 | 5 | Harvesting | Senescent | 09 October 2007 |
| C-1, cucumber | Greenhouse | 2 | 8 | Clearinga | Senescent | 11 October 2007 |
| C-2, cucumber | Greenhouse | 3 | 7 | Harvesting, nurturing | Mature | 26 June 2007 |
| C-2, poinsettia | Packing facility | 1 | 6 | Pottingb | Young | 08 August 2007 |
| F-1, broccoli, cabbage | Open field | 2 | 5 | Harvesting | Mature | 22 August 2007 |
| F-1, cabbage | Open field | 2 | 3 | Harvesting | Mature | 27 October 2008 |
| F-2, cabbage | Open field | 2 | 4 | Harvesting | Mature | 23 August 2007 |
| F-3, broccoli | Packing facility | 1 | 3 | Wrapping | Mature | 14 August 2007 |
| F-3, celery | Open field | 1 | 3 | Harvesting | Mature | 12 September 2007 |
| F-3, broccoli | Open field | 2 | 5 | Harvesting | Mature | 12 September 2007 |
Removal of senescent plants.
Potting of young poinsettia in a cucumber packing facility using a potting machine.
Working activities
Growers’ work tasks depended on the growth stage of the crop plants. In the text below, the labels for work tasks as used in Tables 2 and 3 and are presented in brackets. Harvesting (harvesting) was the main task included in this study, but some growers in greenhouses were also occupied with nurturing of plants (nurturing). After the growing season, greenhouse plants were removed (clearing). The following procedure for clearing of plants was observed in cucumber greenhouses: the stems of senescent cucumber plants were cut with a scythe. Then they were released from their support strings with a knife and placed on wagons to be transported outside (removing plants). In the cleared greenhouse, old support strings were cut away (cutting strings). In tomato greenhouses, the long plant stems and support strings were cut down (cutting plants) and then bundled together in the aisles between plant rows (turning plants). Thereafter, a grower used a netting machine to net the plant stems together, before the bundle of stems was coiled onto a large spool on a tractor and transported outside (working machines). All windows and doorways were open in the greenhouses during clearing of senescent plants. Additional measurements were performed during potting of poinsettia in the sliding doorway of a cucumber packing facility (potting) and during wrapping of broccoli in plastic foil in a packing facility (wrapping). In open fields, the only investigated work task was harvest of the crop (harvesting).
Table 2.
Characterization of investigated environments. Concentrations and exposures are presented as median value, (range) and (multiplicative standard deviation).
| Investigated environments |
Stationary samplers, × 104 cfu m−3 |
Personal samplers, × 104 cfu m−3 |
||||||||||
| Env. # | Crop, facility, n | Crop stage (main task) | Temperature °C | RH % | n | Mesophilic fungi | n | Mesophilic fungi | Penicillium olsonii/Penicillium brevicompactum | Aspergillus fumigatus | Beauveria spp. | Trichoderma spp. |
| 1 | Poinsettia, packing facility, 1 | Young (potting) | ND | ND | 1 | 3.5b,c | 6 | 5.7c (1.2–28) (3.28) | ND | BD (BD–0.008) | BD (BD–0.01) | BD |
| 2 | Tomato, greenhouse, 1 | Premature (nurturing) | 25 (16–38) | 61 (32–84) | 6 | 0.34c (0.19–4.3)(4.23) | 14 | 0.61‡d (0.19–3.5) (2.48) | 0.17 (BD–2.5) (14.3) | BD (BD–0.008) | BD | BD‡ |
| 3 | Tomato, greenhouse, 3 | Mature (harvesting) | 28 (21–36) | 51 (22–69) | 8 | 1.7b,c (0.20–4.3) (2.83) | 23 | 32b,c (2.0–440) (3.96) | 1.6 (BD–58) (19.2) | BD (BD–0.009) | BD | BD |
| 4 | Tomato, greenhouse, 2 | Senescent (clearing) | 8 (6–9) | 50 (48–62) | 6 | 22a (5.0–120) (4.08) | 14 | 320a (8.6–450) (3.41) | ND | BD | BD | BD |
| 5 | Cucumber, greenhouse, 2 | Mature (harvesting) | 28 (22–38) | 48 (28–75) | 5 | 1.7b (0.47–4.2) (1.98) | 17 | 72b (BD–480) (18.60) | BD (BD–13) (45.4) | BD (BD–0.038) | BD (BD–0.008) | BD |
| 6 | Cucumber, greenhouse, 1 | Senescent (harvesting) | 21 (19–23) | 87 (85–88) | 3 | 0.96b,c (0.66–4.2) (2.65) | 4 | 52a,b (16–130) (1.20) | 1.9 (0.7–3.1) (1.98) | BD | BD | BD (BD–7.7) |
| 7 | Cucumber, greenhouse, 1 | Senescent (clearing) | 16 (13–18) | 70 (66–77) | 2 | 41a (35–48) (1.26) | 4 | 1000a (250–3800) (2.75) | ND | BD (BD–0.025) | BD (BD–0.004) | BD |
| 8 | Cucumber, greenhouse, 1 | No plants (removing strings) | ND | ND | 0 | ND | 4 | 53b,c (16–130) (3.98) | ND | BD | BD | BD |
| 9 | Cabbageθ, open field, 5 | Mature (harvesting) | 17 (5–21) | 80 (51–99) | 7 | 0.10d (BD–0.18) (4.72) | 22 | 0.97d (0.11–14) (3.46) | 0.035 (0.019–0.049) (1.61) | BD (BD–0.027) | BD | BD (BD–1.7) |
| 10 | Broccoli, packing facility, 1 | Harvested vegetables (wrapping) | 18 (17–23) | 60 (45–64) | 1 | 2.2a,b,c | 3 | 1.4c,d (0.49–1.5) (1.88) | 0.01 (BD–0.026) (2.55) | 0.01 (BD–0.013) | BD | 0.28 (0.13–0.43) (1.91) |
BD, below detection level (stationary samplers: dust, 0.031 mg m−3; mesophilic fungi, 88 cfu m−3. Personal samplers: culturable microorganisms, 80 cfu m−3, however, 24 cfu m−3 for Trichoderma spp.). ND, not determined. Data within columns which are not significantly different from each other (α = 0.05) are marked with the same letters in superscript.
Cabbage, broccoli, or celery.
Results calculated from raw data also used in Hansen et al. (2010b).
Table 3.
Personal exposure during different work tasks. Exposures are presented as median value, (range) and (multiplicative standard deviation).
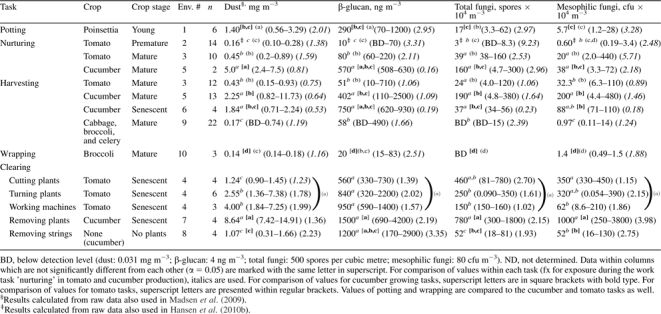 |
Air sampling
For personal samples, Gesamtstaubprobenahme (GSP) samplers (Conical Inhalable Sampler, BGI, Waltham, MA, USA) were attached to the growers’ clothing as close as possible to the breathing zone. Airflow was adjusted to 3.5 l min−1 and checked every second hour. Twice a day, samplers were turned off during scheduled breaks.
Stationary air samplers, Millipore 25 mm closed-face cassettes (Millipore, Bedford, MA, USA), were positioned between plant rows 1.5 m above ground. Airflow was adjusted to 1.9 l min−1 corresponding to an inlet velocity of 1.25 m s−1, and the airflow was checked every second hour. Outdoor reference samples were also collected by stationary air samplers, which were placed 50–100 m upwind from the greenhouse or field. Samplers ran throughout the entire working day. During sampling, the growers carried out their regular work tasks. Each grower and each stationary sampling station carried two of the described samplers, one with a polycarbonate filter (pore size 1 μm, Osmonics Inc., Minnetonka, MN, USA) for culturable counts, total counts, and quantification of β-glucan and one with a Teflon filter (pore size 1 μm; Millipore) for gravimetric analysis.
Gravimetric and β-glucan analysis
The mass of dust was quantified by gravimetric analysis of Teflon filters as previously described by Madsen et al. (2009). Extracts from polycarbonate filters were analysed in duplicate for β-glucan using the kinetic, chromatic Fungitic G Test (Seikaga Co., Tokyo, Japan). The triple helix structure of the β-glucan was made water soluble by adding 0.3 M NaOH (Fogelmark et al., 2001) and incubating for 10 min. The detection level was 4 ng ml−1.
Quantification of selected culturable microorganisms
The day after each sampling, collected materials were extracted by placing filters in 10 ml (personal samples) or in 6 ml (stationary samples) sterile solution (0.05% Tween 80, 0.85% NaCl) followed by orbital shaking for 15 min (500 rpm) at room temperature. Ten-fold dilution series were prepared and 100 μl aliquots were plated on agar plates. Unless otherwise stated, agar plates were incubated for 7 days under conditions indicated below.
Quantifications of mesophilic fungi and A. fumigatus were obtained from DG-18 plates (DG-18 agar, Oxoid, Basingstoke, UK) with chloramphenicol (100 mg l−1, Oxoid, Hampshire, UK) at 25°C and 45°C, respectively. Colony-forming units (cfu) of Trichoderma spp. were quantified on T. harzianum selective medium (THSM) after incubation at 22°C for 5–10 days as described by Williams et al. (2003). This medium contains a range of antibiotics with both antibacterial and antifungal activities, increasing the specificity toward Trichoderma spp. Colonies with varying morphology grew on the THSM plates. Hence, polymerase chain reaction (PCR) amplification of DNA was used for verification of the identification based on morphological features and for exclusion of colonies from the MPCA product Supresivit® as described in Hansen et al. (2010b).
Beauveria spp. were quantified on a selective agar medium as described by Meyling and Eilenberg (2006). Colonies morphologically resembling Beauveria spp. were cultured in liquid medium, lyophilized, and DNA extracted as described by Meyling et al. (2009). PCR amplifications spanning the internal transcribed spacers 1 and 2 (ITS1–5.8S–ITS2 region) of the ribosomal RNA gene cluster using primers ITS5 and ITS4 (White et al., 1990) were performed in reaction volumes of 25 μl containing 1× Dynazyme buffer (Finnzymes, Espoo, Finland), 200 μM dNTP, 0.5 μM of each primer, 2U Dynazyme II polymerase, and 10 ng genomic DNA. Cycling conditions consisted of 3 min initial denaturation at 94°C, 35 cycles of 1 min at 94°C, 1 min at 55°C, and 1.5 min at 72°C followed by final extension for 7 min at 72°C. PCR products were purified using the GFX PCR DNA and Gel band purification kit (GE Healthcare, Chalfont St Giles, UK) according to the manufacturer’s instructions. Sequencing was performed by MWG (Ebersberg, Germany). Sequence alignments were generated using ClustalW and Sequence Alignment Editor in BioEdit ver. 7.0.9.0 (Hall, 1999) and compared to reference sequences published by Rehner and Buckley (2005) and retrieved from GenBank [accession numbers (isolate number) AY532017 (ARSEF 32), AY532046 (ARSEF 753), AY532051 (ARSEF 812), and AY532053 (ARSEF 816)]. Beauveria spp. were characterized by molecular methods to distinguish detected colonies from Beauveria strains, which might have been applied to the environments as an MPCA.
Penicillium olsonii and P. brevicompactum (P. olsonii/P. brevicompactum) were quantified on nutrient agar (Oxoid) with cycloheximide (50.0 mg l−1, Applichem, Darmstadt, Germany) incubated at 25°C. The species identification of eight isolates was verified by Accugenix Inc. (Newark, DE, USA) by sequencing of the internal transcribed spacer region of the ribosomal operon.
The detection limit for culturable microorganisms was 80 cfu m−3 for personal samplers (6 h of sampling) and 88 cfu m−3 for stationary samplers (6 h of sampling).
Total counts of fungal spores
Total counts of fungal spores (total fungi) were performed after staining filters in 20 ppm acridine orange (DIFCO, Sparks, MD, USA) in acetate buffer for 30 s with subsequent filtration through a polycarbonate filter (0.4 μm; Nuclepore, Cambridge, MA, USA). The number of fungal spores was counted using an epifluorescence microscope at ×1250 magnification (Orthoplan, Leitz, Wetzlar, Germany). Particles exhibiting the morphology of fungal spores with a diameter of 1.8–10 μm were defined as such. Only samples from personal samplers were investigated by microscopy. The detection limit for total fungi was 500 spores per cubic metre (6 h of sampling).
Temperature and relative humidity
In the investigated environments, temperature and relative humidity were measured with data loggers [Tinytag TGP-1500, Plus Data Logger, Gemini Data Loggers (UK) Ltd, UK].
Data analysis
Similar working environments were grouped together, depending on facility and crop, making 10 categories of environments (Table 2). Each environment was evaluated for ambient temperature, relative humidity, and amounts of mesophilic fungi. To evaluate growers’ exposure to bioaerosols and dust, data collected for specific work tasks within each environment were processed together (Table 3). Measurements from personal and stationary samplers are termed ‘exposures’ and ‘concentrations’, respectively, in the following. Both were calculated as time-weighted averages. Data are presented with median values, ranges, and multiplicative standard deviations (s*) as described by Limpert et al. (2001). If 50% or more of measurements were below the detection limit, s* was not calculated. Exposure and concentration data for dust and fungal bioaerosol components were logarithmically transformed for statistical analysis to approximate normality. Variance analysis was used to compare the quantified concentrations and exposures to bioaerosols by using Proc GLM analysis in SAS where α = 0.05 (SAS software, version 8e, SAS Institute, Cary, NC, USA).
RESULTS
Bioaerosols in the investigated environments
Characteristics of the 10 environments are presented in Table 2. Measurements of airborne mesophilic fungi showed that the median values of airborne fungi measured by personal samplers were 15–52 times higher than the concentrations measured by stationary samplers in greenhouses with mature and senescent plants. The difference was less pronounced in a greenhouse with premature plants and in open fields, respectively, 2 and 10 times.
Stationary samplers.
Comparing the investigated environments (Env.), the highest concentrations of mesophilic fungi were measured in environments where senescent greenhouse plants were being removed (Env. 4 and Env. 7; Table 2). In tomato greenhouses, the concentrations of mesophilic fungi did not differ significantly between greenhouse environments with premature plants (Env. 2) and greenhouse environments with mature plants (Env. 3) (P = 0.09). In cucumber greenhouses, there was no significant difference between the concentrations of mesophilic fungi whether in environments where crops were harvested from mature (Env. 5) or senescent plants, respectively (Env. 6) (P = 0.65). Concentrations of mesophilic fungi did not differ significantly whether in environments with mature cucumbers or mature tomatoes (Env. 5 and Env. 3, respectively) (P = 0.32). No difference between concentrations of mesophilic fungi was observed during clearing whether in environments with senescent tomato (Env. 4) or senescent cucumber plants (Env. 7) (P = 0.56). Reference measurements outside greenhouses showed the concentration of mesophilic fungi to be 3.4 × 103 cfu m−3 (median) with a range between 3.8 × 102 and 8.9 × 103 cfu m−3 during summer months (May–August) and 3.9 × 102 cfu m−3 (median) with a range between 4.4 × 101 and 3.1 × 103 cfu m−3 during winter months (October–February).
Personal samplers.
Among the mesophilic fungi, Penicillium was the most common genus, followed by Cladosporium (Ascomycota: Capnodiales) (data not shown). Spores of P. olsonii/P. brevicompactum were not quantified for all environments but were detected in each of the investigated environments (Table 2). They constituted from <0.061‰ to 28% of the mesophilic fungi in the investigated environments. The fungus A. fumigatus was detected in the personal samples from 7 of the 10 environments, but only in low concentrations, ranging up to 380 cfu m−3 (Table 2). Beauveria spp. exposure was only found in samples from growers working in environments with cucumber production, including during potting of poinsettia in a cucumber packing facility, and only in low concentrations (reaching 100 cfu m−3) (Table 2). DNA sequencing of selected Beauveria isolates (n = 11) from two greenhouses of this study revealed that the Beauveria strains isolated belonged to two major clades, Clade A (n = 8) and Clade C (n = 3), as defined by Rehner and Buckley (2005). The three isolates of Clade C had similar ITS sequences and were found in both greenhouses while three different ITS sequences were found for Clade A isolates with each greenhouse harbouring two of these. Exposure to Trichoderma spp. could be observed for growers working in a greenhouse with senescent cucumber plants, a field with cabbage, and in a packing hall where broccoli were being wrapped in plastic foil (Table 2). By PCR analysis, it was found that the Trichoderma spp. isolates reported in this study did not originate from applied MPCAs.
Exposure related to work tasks
Personal exposures of tomato growers and cucumber growers to bioaerosols during different work tasks at different crop stages were compared (Table 3). For tomato growers in greenhouses with mature plants (Env. 3), there was no difference in exposure to bioaerosol components (dust, β-glucans, total, or mesophilic fungi) whether harvesting crop or nurturing plants (P > 0.32). However, growers nurturing premature tomato plants (Env. 2) were exposed to lower amounts of bioaerosol components than growers nurturing or harvesting from the mature plants (Env. 3) (P < 0.05). In tomato production, exposures to the studied bioaerosol components were highest during clearing disregarding specific tasks (P < 0.05). During clearing of cucumber plants, the exposures to most bioaerosol components were higher than exposures during all other work tasks earlier in the year (P < 0.05). Exceptions were exposure to dust and β-glucan while nurturing mature plants (P > 0.1) and exposure to β-glucan during harvest from senescent plants.
In the text below, work task exposures are presented in relation to the different environments as presented in Table 3.
Nurturing.
While nurturing plants in Env. 2, 3, and 5, the highest median exposure to dust and β-glucan was detected in cucumber greenhouses with mature plants. The lowest exposure to all bioaerosol components was observed in tomato greenhouses with premature plants, which was significantly lower than exposures in tomato greenhouses with mature plants (P < 0.05).
Harvesting.
During harvest in Env. 3, 5, 6, and 9, the highest exposure to all bioaerosol components was observed in cucumber greenhouses (P < 0.05), except for total fungi, for which no significant difference was measured between cucumber harvest and tomato harvest (P > 0.11). No statistically significant difference could be observed between exposures of growers in greenhouses with mature or senescent cucumber plants (P > 0.2). The lowest exposure to bioaerosols during harvest activities was observed in open fields (P < 0.05), with one exception: no significant difference was measured for β-glucan exposure during harvest in open field and harvest in tomato greenhouses (P = 0.45).
Clearing of senescent plants.
In Env. 4, 7, and 8, growers removed senescent plants. During removal of cucumber plants, exposure to dust and fungal spores was higher than during removal of old strings in a cucumber greenhouse. During removal of tomato plants (Env. 4), three work tasks were investigated. The exposure to dust during cutting of tomato plants was lower than for the other two tasks (turning plants and working machines) (P < 0.05). However, exposure to mesophilic fungi during cutting of support strings was higher than during working with machines (P < 0.05). When placing the tasks of removing tomato plants into one group, no significant difference in exposure to the investigated bioaerosol components could be observed between the exposure during removal of tomato plants and the exposure during removal of cucumber plants.
Since the work tasks of potting poinsettia and wrapping broccoli were only performed in one environment each, the exposures were compared to other work tasks within tomato and cucumber production (Table 3).
Potting.
Plants were only potted in Env. 1 (Table 3). Growers potting poinsettia were exposed to levels of dust and β-glucan, which were not significantly different from the exposure during harvest of cucumbers (P = 0.10 and P = 0.39, respectively). In contrast, the exposures during removal of cucumber plants were higher than during potting of poinsettia. For exposure to fungal spores during potting of poinsettia, the exposure was not significantly different from the exposure measured during nurturing of cucumber plants (total and mesophilic fungi) (P > 0.44) and harvesting from senescent plants (total fungi) (P = 0.8).
Wrapping broccoli.
Exposure during wrapping of broccoli was investigated in Env. 10 (Table 3). The growers wrapping broccoli were exposed to lower levels of bioaerosol components than growers producing cucumbers, clearing tomato plants (not shown), and potting poinsettia (P < 0.05). The exposure to the studied bioaerosol components was not significantly different from what was measured for growers nurturing premature tomato plants (P > 0.26) with exception of total fungi (Table 3).
DISCUSSION
We found that vegetable growers in several of the investigated working environments were exposed to levels of bioaerosols that exceed OELs suggested by researchers (Heida et al., 1995; Meyer et al., 2005; Eduard, 2009), and we identified tasks causing increased exposure. Generally, vegetable growers’ exposure to mesophilic fungi was found to be highest in environments where senescent tomato and cucumber plants were being removed, followed by environments in which tomatoes and cucumbers were harvested. Exposure to airborne mesophilic fungi in the investigated vegetable greenhouses was higher than what previously has been measured by personal samplers (Monsó et al., 2002; Radon et al., 2002) and stationary samplers (Li and LaMondia, 2010; Adhikari et al., 2011) in greenhouses with ornamental plants. Based on personal air samples, we found that growers potting poinsettia were exposed to a higher concentration of mesophilic fungi than growers working in open fields, even though they worked in a large, open doorway. Furthermore, we found that the exposure was similar to growers’ exposure in some of the greenhouse environments with high exposure levels. Aerosolized fungi from the peat and leaf surfaces may have contributed to the inhalable fungal bioaerosols.
For growers wrapping broccoli, exposures to mesophilic fungi were generally low compared to exposures in greenhouses with mature plants. In tomato and cucumber production, vegetables were packed in packing halls separate from the greenhouses. Since growers appear to be exposed to lower concentrations of some bioaerosol components in a packing hall, it might be possible to reduce the growers’ daily bioaerosol exposure through job rotation.
The lowest exposures to mesophilic fungi were observed in open fields during harvest; however, the exposure level did resemble the exposure in a greenhouse with premature tomato plants. In this study, as in many studies in agricultural environments, it has been a challenging task to increase the number of personal samples. Therefore, due to the small sample size, the possibility of statistical type II errors cannot be excluded. To evaluate the bioaerosol exposures measured in this study and to improve our understanding of vegetable growers’ exposure to bioaerosols, further studies are needed. To our knowledge, vegetable growers’ exposure to fungal bioaerosols has previously only been measured in a single tomato greenhouse (Hansen et al., 2010b).
It has been suggested that stationary samplers adequately can be used in the assessment of personal exposure to airborne fungi in confined agricultural environments (Adhikari et al., 2011). For stationary measurements performed in environments where personal sampling showed exposure to high levels of mesophilic fungi (e.g. clearing of senescent tomato plants), the concentration of mesophilic fungi measured by stationary samplers were higher than in environments where growers were exposed to lower levels of mesophilic fungi (e.g. harvesting of tomatoes) (Table 2). This observation indicates that there likely is a correlation between measurements performed by personal and stationary samples in vegetable production. However, in the greenhouses with relatively high personal exposures, air samples collected by stationary samplers did not sufficiently reflect the high personal exposure. In environments with low personal exposure (e.g. a greenhouse with premature plants), the measured exposure level was closer to the bioaerosol levels measured by stationary samplers. This observation indicates that personal samplers are an important tool for measuring growers’ exposure to bioaerosols in some plant-producing environments. The underestimation of exposure using stationary samplers in areas with relatively high personal exposures may also partly be caused by a lower degree of air mixing in greenhouses with tall plants. Particle size and wind velocity can affect the sedimentation speed and dispersal of particles in investigated environments. The sampler used in this study (GSP) has a high sampling efficiency for particles with aerodynamic diameters <50 μm (Kenny et al., 1997, 1999; Aizenberg et al., 2000). However, we have not further measured the sizes of particles within this inhalable fraction.
Differences in measurements between personal and stationary samplers might also be caused by differences in the contribution of bioaerosols to the bioaerosol load caused by working activities. Our data from stationary samplings indicate that the concentration of fungal bioaerosols in undisturbed air in greenhouses is independent of whether tomatoes or cucumbers are produced. However, results obtained from personal samplers indicate that factors in cucumber production increase the growers’ exposure to bioaerosol components, compared with that of their colleagues working with tomato production. Organic material accumulated on the leaves of cucumber plants may contribute to the higher exposure levels measured during working activities in the cucumber greenhouses due to release of dust from the leaves. It can be expected that growth of epiphytic fungi on plant surfaces affects growers’ exposure to fungal bioaerosols. Other factors that might affect vegetable growers’ exposure to bioaerosols could be (i) height of the plants, (ii) the plants’ leaf area index, (iii) leaf morphology, and (iv) particles settling on the leaves over time. However, future studies of these factors must be conducted to draw further conclusions on their importance for growers’ exposure to bioaerosols.
Penicillium and Cladosporium were the most frequently detected fungal genera in the present study, supporting findings from other greenhouse environments (Davies et al., 1988; Monsó et al., 2002; Radon et al., 2002; Okushima et al., 2004; Li and LaMondia, 2010; Magyar et al., 2011). Penicillium spp. culturable on nutrient agar plates with 50 μg ml−1 cycloheximide were identified as P. brevicompactum and P. olsonii and were observed in all investigated environments but appeared to be more prevalent in tomato greenhouses than in cucumber greenhouses and open fields. Previously, Penicillum spp. resistant to cycloheximide have been reported in samples from timber and air samples collected in Turin, Italy (Seifert and Giuseppin, 2000). Cycloheximide-resistant fungi might disrupt bacterial counts on agar plates that contain cycloheximide for suppression of fungal growth.
The fungus A. fumigatus was detected in 7 of the 10 investigated environments but only in concentrations <400 cfu m−3. As A. fumigatus is a thermotolerant fungus, it can be detected in environments where organic matter is decomposing, e.g. in biofuel plants and valerian root farms (Skorska et al., 2005; Madsen, 2006). The low concentrations of A. fumigatus observed in this study may indicate that particles from decomposing materials are not introduced into the air of vegetable productions in any large extent.
Fungal species from the genera Beauveria and Trichoderma, which both are used for biocontrol (Benítez et al., 2004; Zimmermann, 2007), were detected as naturally occurring in the investigated environments. However, Beauveria spp. were only detected in environments with cucumber production and only in concentrations <100 cfu m−3. Previously, Beauveria spp. have been isolated from the surface of cucumbers (5% of samples), but not from cucumber leaves or other materials from the investigated greenhouse (Ruiz et al., 1996). Other studies have found airborne Beauveria spp. in outdoor and indoor environments, where they only represented a minor fraction of the fungal microbiota (reviewed by Madsen et al., 2007). However, in horse stables, concentrations of airborne Beauveria spp. (250 cfu m−3) were higher (Nardoni et al., 2005) than in the cucumber production of this study and in other environments described in the literature. None of the investigated greenhouse environments were reported to have been treated with Beauveria spp.; thus, we expect the observed Beauveria strains to be indigenous to the environment, which also is indicated by the genetic diversity identified. Trichoderma spp. could be isolated from a broccoli packing hall, two open fields, and a cucumber greenhouse. Based on PCR analysis, it was concluded that the isolated Trichoderma spp. strains did not originate from the MPCA Supresivit®. The findings indicate that Trichoderma spp. mostly are not prevalent in vegetable productions, which is in accordance with a report of 42 Trichoderma spp. spores per cubic metre in a greenhouse with ornamental plants (Li and LaMondia, 2010) and an average concentration of 0.1 Trichoderma spp. spores per cubic metre in an orchid greenhouse (Magyar et al., 2011).
Although β-glucans have been shown to have inflammatory properties (Holck et al., 2007), no conclusions on associations between β-glucan exposure and health effects have been drawn at this point in regard to an appropriate OEL (Douwes, 2005). As a consequence, there is no OEL for interpreting β-glucan exposure data. We found that growers harvesting cucumbers and growers clearing either tomato or cucumber plants (Table 3) were exposed to higher levels of β-glucan (medians between 400 and 1500 ng m−3) than reported from most other working environments. In greenhouses with ornamental plants, concentrations of β-glucans have been reported to range between 5.0 and 55.8 ng m−3 near major work areas in summer time (Adhikari et al., 2011). In the paper industry, β-glucan exposure during different work processes (n = 20) varied between 4 and 366 ng m−3 (Rylander et al., 1999). In a horse stable, the concentration of β-glucan dropped from 362 to 85 ng m−3 when doors were opened (Elfman et al., 2009). For waste collectors, the exposure to β-glucan was similar to or less than what we observed for tomato growers in the present study (Thorn et al., 1998; Fogelmark et al., 2001; Heldal et al., 2003). In four offices, β-glucan concentrations ranged from 1.1 to 8.7 ng m−3 (Madsen et al., 2011). In the open fields investigated in the current study, we measured growers’ β-glucan exposure to range between <4 and 490 ng m−3 with a median value of 58 ng m−3. This level is comparable to the measurements in a paper factory and higher than what is found in working environments such as offices and schools (Rylander et al., 1998; Madsen et al., 2011).
CONCLUSIONS
In cucumber and tomato production, levels of fungal bioaerosols that exceed suggested OELs were evident during the labour-intensive harvest season and during clearing of senescent plants. We found vegetable growers’ exposures to bioaerosol components to be related to the environment in which they work, with the lowest exposures in open fields. In greenhouses, our measurements showed that cucumber growers in many working situations are exposed to higher levels of airborne fungi than tomato growers.
In cucumber greenhouses, differences in exposure to airborne dust were detected between growers nurturing mature plants and growers harvesting cucumbers; thus, working activities in a greenhouse influence the exposure levels. In contrast, these differences between activities were not seen for fungal bioaerosols. Comparable findings were observed in tomato greenhouses, where growers cutting senescent plants were exposed to lower levels of dust than tomato growers performing other tasks during clearing of plants. In contrast, the former group was exposed to more mesophilic fungi than the growers working with machines during clearing of plants. However, for tomato growers, there was no difference in bioaerosol exposure whether growers nurtured mature plants or harvested tomatoes.
Vegetable growers were exposed to the naturally occurring counterparts of MPCAs in low concentrations. This shows that the fungi in question (Beauveria spp. and Trichoderma spp.) can be naturally present in the vegetable growing environments prior to use of the MPCAs.
FUNDING
Danish Environmental Protection Agency.
Acknowledgments
We want to thank Tina T. Olsen, Signe H. Nielsen, Anne Grethe Holm-Jensen, and Tina Thane for technical assistance and Kira Tendal for copyediting.
References
- Adhikari A, Gupta J, Wikins JR, III, et al. Airborne microorganisms, endotoxin, and (1→3)-beta-D-glucans exposure in greenhouses and assessment of respiratory symptoms among workers. Ann Occup Hyg. 2011;55:272–85. doi: 10.1093/annhyg/meq082. [DOI] [PubMed] [Google Scholar]
- Aizenberg V, Grinshpun SA, Willeke K, et al. Performance characteristics of the button personal inhalable aerosol sampler. Am Ind Hyg Assoc J. 2000;61:398–404. doi: 10.1080/15298660008984550. [DOI] [PubMed] [Google Scholar]
- Atkinson RW, Strachan DP, Anderson HR, et al. Temporal associations between daily counts of fungal spores and asthma exacerbations. Occup Environ Med. 2006;63:580–90. doi: 10.1136/oem.2005.024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez T, Rincón AM, Limón MC, et al. Biocontrol mechanisms of Trichoderma strains. Int Microbiol. 2004;7:249–60. [PubMed] [Google Scholar]
- Bush RK, Portnoy JM, Saxon A, et al. The medical effects of mold exposure. J Allergy Clin Immunol. 2006;117:326–33. doi: 10.1016/j.jaci.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Davies PDO, Jacobs R, Mullins J, et al. Occupational asthma in tomato growers following an outbreak of the fungus Verticillium albo-atrum in the crop. J Soc Occup Med. 1988;38:13–7. doi: 10.1093/occmed/38.1-2.13. [DOI] [PubMed] [Google Scholar]
- Douwes J. (1→3)-beta-D-glucans and respiratory health: a review of the scientific evidence. Indoor Air. 2005;15:160–9. doi: 10.1111/j.1600-0668.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- Eduard W. Fungal spores: A critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit Rev Toxicol. 2009;39:799–864. doi: 10.3109/10408440903307333. [DOI] [PubMed] [Google Scholar]
- Eduard W, Douwes J, Mehl R, et al. Short term exposure to airborne microbial agents during farm work: exposure-response relations with eye and respiratory symptoms. Occup Environ Med. 2001;58:113–8. doi: 10.1136/oem.58.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfman L, Riihimaki M, Pringle J, et al. Influence of horse stable environment on human airways. J Occup Med Toxicol. 2009;4:10. doi: 10.1186/1745-6673-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelmark B, Thorn J, Rylander R. Inhalation of (1→3)-beta-D-glucan causes airway eosinophilia. Mediators Inflamm. 2001;10:13–9. doi: 10.1080/09629350123707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–8. [Google Scholar]
- Hansen VM, Eilenberg J, Madsen AM. Occupational exposure to airborne Bacillus thuringiensis kurstaki HD1 and other bacteria in greenhouses and vegetable fields. Biocont Sci Technol. 2010a;20:605–19. [Google Scholar]
- Hansen VM, Winding A, Madsen AM. Exposure to bioaerosols during the growth season in an organic greenhouse tomato production using Supresivit (Trichoderma harzianum) and Mycostop (Streptomyces griseoviridis) Appl Environ Microbiol. 2010b;76:5874–81. doi: 10.1128/AEM.00446-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heida H, Bartman F, van der Zee SC. Occupational exposure and indoor air quality monitoring in a composting facility. Am Ind Hyg Assoc. 1995;56:39–43. doi: 10.1080/15428119591017295. [DOI] [PubMed] [Google Scholar]
- Heldal KK, Halstensen AS, Thorn J, et al. Upper airway inflammation in waste handlers exposed to bioaerosols. Occup Environ Med. 2003;60:444–50. doi: 10.1136/oem.60.6.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holck P, Sletmoen M, Stokke BT, et al. Potentiation of histamine release by microfungal (1→3)- and (1→6)-beta-D-glucans. Basic Clin Pharmacol Toxicol. 2007;101:455–8. doi: 10.1111/j.1742-7843.2007.00140.x. [DOI] [PubMed] [Google Scholar]
- Illing HP. Is working in greenhouses healthy? Evidence concerning the toxic risks that might affect greenhouse workers. Occup Med (Lond) 1997;47:281–93. doi: 10.1093/occmed/47.5.281. [DOI] [PubMed] [Google Scholar]
- Jensen GB, Larsen P, Jacobsen BL, et al. Bacillus thuringiensis in fecal samples from greenhouse workers after exposure to B. thuringiensis-based pesticides. Appl Environ Microbiol. 2002;68:4900–5. doi: 10.1128/AEM.68.10.4900-4905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Harrison RM. The effects of meteorological factors on atmospheric bioaerosol concentrations—a review. Sci Total Environ. 2004;326:151–80. doi: 10.1016/j.scitotenv.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Kenny LC, Aitken R, Chalmer C, et al. A collaborative European study of personal inhalable aerosol sampler performance. Ann Occup Hyg. 1997;41:135–53. doi: 10.1016/S0003-4878(96)00034-8. [DOI] [PubMed] [Google Scholar]
- Kenny LC, Aitken RJ, Baldwin PEJ, et al. The sampling efficiency of personal inhalable aerosol samplers in low air movement environments. J Aerosol Sci. 1999;30:627–38. [Google Scholar]
- Lacey J, Dutkiewicz J. Bioaerosols and occupational lung disease. J Aerosol Sci. 1994;25:1371–404. [Google Scholar]
- Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–50. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Adhikari A, Grinshpun SA, et al. Personal exposure to airborne dust and microorganisms in agricultural environments. J Occup Environ Hyg. 2006;3:118–30. doi: 10.1080/15459620500524607. [DOI] [PubMed] [Google Scholar]
- Li D-W, LaMondia J. Airborne fungi associated with ornamental plant propagation in greenhouses. Aerobiologia. 2010;26:2615–28. [Google Scholar]
- Limpert EW, Stahel A, Abbt M. Log-normal distributions across the sciences: keys and clues. Bioscience. 2001;51:341–52. [Google Scholar]
- Madsen AM. Exposure to airborne microbial components in autumn and spring during work at Danish biofuel plants. Ann Occup Hyg. 2006;50:821–31. doi: 10.1093/annhyg/mel052. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Frederiksen MW, Allerman L, et al. (1→3)-beta-D-glucan in different background environments and seasons. Aerobiologia. 2011;27:173–79. [Google Scholar]
- Madsen AM, Hansen VM, Meyling NV, et al. Human exposure to airborne fungi from genera used as biocontrol agents in plant production. Ann Agric Environ Med. 2007;14:5–24. [PubMed] [Google Scholar]
- Madsen AM, Hansen VM, Nielsen SH, et al. Exposure to dust and endotoxin of employees in cucumber and tomato nurseries. Ann Occup Hyg. 2009;53:129–38. doi: 10.1093/annhyg/men073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar D, Eszéki ER, Oros G, et al. The air spora of an orchid greenhouse. Aerobiologia. 2011;27:121–34. [Google Scholar]
- Meyer HW, Jensen KA, Nielsen KF, et al. Double blind placebo controlled exposure to molds: exposure system and clinical results. Indoor Air. 2005;15:73–80. doi: 10.1111/j.1600-0668.2005.00351.x. [DOI] [PubMed] [Google Scholar]
- Meyling NV, Eilenberg J. Isolation and characterisation of Beauveria bassiana isolates from phylloplanes of hedgerow vegetation. Mycol Res. 2006;110:188–95. doi: 10.1016/j.mycres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Meyling NV, Lübeck M, Buckley EP, et al. Community composition, host-range and genetic structure of the fungal entomopathogen Beauveria in adjoining agricultural and semi-natural habitats. Mol Ecol. 2009;18:1282–93. doi: 10.1111/j.1365-294X.2009.04095.x. [DOI] [PubMed] [Google Scholar]
- Monsó E. Occupational asthma in greenhouse workers. Curr Opin Pulm Med. 2004;10:147–50. doi: 10.1097/00063198-200403000-00010. [DOI] [PubMed] [Google Scholar]
- Monsó E, Magarolas R, Badorrey I, et al. Occupational asthma in greenhouse flower and ornamental plant growers. Am J Respir Crit Care Med. 2002;165:954–60. doi: 10.1164/ajrccm.165.7.2106152. [DOI] [PubMed] [Google Scholar]
- Nardoni S, Mancianti F, Sgorbini M, et al. Identification and seasonal distribution of airborne fungi in three horse stables in Italy. Mycopathologia. 2005;160:29–34. doi: 10.1007/s11046-005-2669-3. [DOI] [PubMed] [Google Scholar]
- Okushima L, Ishii M, Sase S, et al. An evaluation of floating dust particles and molds in commercial greenhouses. 2004. Conference Proceeding. In Relf D, editor. Proceedings of the XXVI IHC - Horticulture, Human Well-being and Life Quality. Acta Hort 639 ISHS 2004. [Google Scholar]
- Radon K, Danuser B, Iversen M, et al. Air contaminants in different European farming environments. Ann Agric Environ Med. 2002;9:41–8. [PubMed] [Google Scholar]
- Rehner SA, Buckley EP. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Riu E, Monso E, Marin A, et al. Occupational risk factors for rhinitis in greenhouse flower and ornamental plant growers. Am J Rhinol. 2008;22:361–4. doi: 10.2500/ajr.2008.22.3186. [DOI] [PubMed] [Google Scholar]
- Ruiz JA, Bentabol A, Gallego C, et al. Aflatoxin-producing strains of Aspergillus flavus in the mould flora of the different greenhouse substrates for the cultivation of cucumber (Cucumis sativus, L) Int J Food Microbiol. 1996;29:193–9. [Google Scholar]
- Rylander R, Norrhall M, Engdahl U, et al. Airways inflammation, atopy, and (1→3)-beta-D-glucan exposures in two schools. Am J Respir Crit Care Med. 1998;158:1685–7. doi: 10.1164/ajrccm.158.5.9712139. [DOI] [PubMed] [Google Scholar]
- Rylander R, Thorn J, Attefors R. Airways inflammation among workers in a paper industry. Eur Respir J. 1999;13:1151–7. doi: 10.1034/j.1399-3003.1999.13e35.x. [DOI] [PubMed] [Google Scholar]
- Seifert KA, Giuseppin S. Cycloheximide tolerance as a taxonomic character in Penicillium. In: Samson RA, Pitt JI, editors. Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Amsterdam, the Netherlands: Hardwood Academic Publishers; 2000. pp. 259–63. [Google Scholar]
- Skorska C, Sitkowska J, Krysinska-Traczyk E, et al. Exposure to airborne microorganisms, dust and endotoxin during processing of valerian roots on farms. Ann Agric Environ Med. 2005;12:119–26. [PubMed] [Google Scholar]
- Swan JM, Crook B. Airborne microorganisms associated with grain handling. Ann Agric Environ Med. 1998;5:7–15. [PubMed] [Google Scholar]
- Thorn J, Beijer L, Rylander R. Airways inflammation and glucan exposure among household waste collectors. Am J Ind Med. 1998;33:463–70. doi: 10.1002/(sici)1097-0274(199805)33:5<463::aid-ajim5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols. San Diego, CA: Academic Press; 1990. pp. 315–22. [Google Scholar]
- Williams J, Clarkson JM, Mills PR, et al. A selective medium for quantitative reisolation of Trichoderma harzianum from Agaricus bisporus compost. Appl Environ Microbiol. 2003;69:4190–1. doi: 10.1128/AEM.69.7.4190-4191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocont Sci Technol. 2007;17:553–96. [Google Scholar]


