Abstract
Much of the developing world, particularly sub-Saharan Africa, has high levels of morbidity and mortality associated with infectious diseases. The greatest risk of invasive disease is in the young, the malnourished and HIV-infected individuals. In many regions in Africa these vulnerable groups and the wider general population are under constant immune pressure from a range of environmental factors, under-nutrition and multiple concurrent infections from birth through to adulthood. Intermittent microbial exposure during childhood is required for the generation of naturally acquired immunity capable of protection against a range of infectious diseases in adult life. However, in the context of a resource-poor setting, the heavy burden of malarial, diarrhoeal and respiratory infections in childhood may subvert or suppress immune responses rather than protect, resulting in sub-optimal immunity. This review will explore how poor maternal health, HIV exposure, socio-economic and seasonal factors conspire to weaken childhood immune defences to disease and discuss the hypothesis that recurrent infections may drive immune dysregulation, leading to relative immune senescence and premature immunological aging.
Keywords: acquired immunity, human, infectious disease, malnutrition, maternal health
Introduction
The burden of infectious diseases is greatest in countries with scant resources where poor access to clean water, good sanitation, hygiene and basic health care are still widespread. Worldwide, around 10 million children die each year, many of these deaths are in the poorest countries and most are avoidable. Apart from deaths caused by HIV and tuberculosis (TB), infectious disease-related mortality is greatest in the under-5s,1 most commonly as the result of malaria, diarrhoeal diseases and lower respiratory tract infections (Fig. 1). Fetal and infant immunity develops from the point of conception and is greatly influenced by the maternal uterine environment and passive immunity, respectively.2–4 This review will focus on how poor maternal health and socio-economic circumstances in African settings leave children immunologically disadvantaged and susceptible to a cycle of concurrent infections. We will evaluate the evidence that supports the hypothesis that long-term immune dysregulation is caused by the heavy burden of childhood infection.
Figure 1.
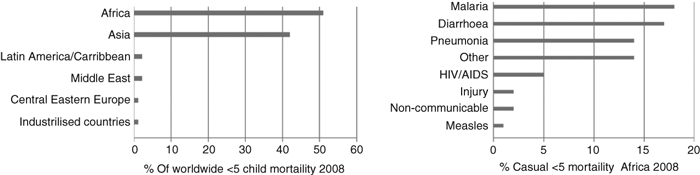
Regional distribution of mortality in the under-5s and causes of mortality in the under-5s in Africa, 2008 (adapted from ref. 1; neonatal mortality excluded).
High burden of infectious disease in children < 5 years of age
In sub-Saharan Africa, Plasmodium falciparum, Streptococcus pneumoniae and non-typhoidal Salmonella (NTS) are among the most frequent causes of life-threatening infectious disease in children < 5 years of age. Within the region, uncomplicated P. falciparum malaria infections can reach 260/1000 children < 5 years/year,5 bacteraemia caused by S. pneumoniae and NTS can reach 213/100 000 and 175/100 000 children < 2 years/year, respectively.6–8 The high prevalence of symptomatic malaria in childhood is in part the result of the frequency of exposure.9,10 Similarly, the high incidence of invasive pneumococcal disease is exacerbated by increased nasopharyngeal colonization by S. pneumoniae, which reaches up to 50% in the first month of life and remains high compared to industrialized countries.8,11 In this setting, the intensity of antigen exposure coupled with external immune pressures may drive microbial competition and adaptation, indeed a novel variant of NTS has emerged as a common cause of invasive disease in African populations.12 From the host perspective, poor maternal health can directly influence the programming of fetal and infant immunity leaving children vulnerable to highly adapted pathogens. To help to explain the high burden of childhood infections in developing countries, even in HIV-uninfected children who readily adhere to national childhood vaccine regimens, it is important to consider the intimate relationship between maternal health and the development of childhood immunity.
Impact of maternal health on childhood immunity
Good maternal health during pregnancy is critical for fetal growth and development. Babies born to women with HIV, placental malaria, malnutrition or micronutrient deficiencies are predisposed to low birthweight (LBW) (weighing < 2500 g) with an altered immune phenotype (Table 1).13–15 Maternal health is also essential for newborn immunity, which is largely acquired passively from the maternal placenta and breast milk. Poor maternal health and infection expose the fetus to transplacental transfer of soluble antigens and inflammatory cytokines, reduces fetal growth factors,16 and compromises passive immunity17,18 leaving infants immunologically vulnerable. These processes are all amplified by exposure to HIV infection, placental malaria and maternal malnutrition and will be discussed below.
Table 1.
Effects of poor maternal health on childhood immunity
| Maternal complication | Impact on mother | Outcome for child |
|---|---|---|
| HIV infection | In utero inflammation Weight loss Poor T-cell help for B-cell antibody production | Skewed/activated T cells Low birthweight Thymic atrophy Poor placental immunity |
| Placental Malaria | Placental inflammation Transplacental passage of soluble Plasmodium falciparum | Low birthweight Thymic atrophy, Deceased antibody transfer Th2 skew, with decreased interferon-γ and increased interleukin-10 CD4+ CD25+ Foxp3+ T reg |
| Maternal malnutrition | Weight loss Gestational zinc deficiency | Reduced breast milk Low birthweight Thymic atrophy Reduced transfer of maternal IgM, IgG |
Th2, T helper type 2; Treg, regulatory T.
Infant exposure to HIV infection
Seroprevalence for HIV in antenatal clinics can reach 30% in HIV-endemic countries in Africa,19 making HIV infection a considerable burden to child health. Although the rapidly expanding availability of anti-retroviral therapy to pregnant HIV-infected women in several countries20 has dramatically reduced perinatal transmission of HIV from mother to baby, there is an increasing group of HIV-negative infants being born to HIV-positive mothers. These ‘sero-reverters’ are becoming recognized as a susceptible group with LBW,13 reduced placental antibody titres21 and increased susceptibility to infections.22 Regardless of the causal mechanism (such as poor maternal health, HIV infection or anti-retroviral therapy), there is increasing evidence to suggest that sero-reverted children are immunologically different. Clerici et al.14 found increased production of thymopoietic interleukin-7 (IL-7) and altered T-cell populations, with increased numbers of activated CD8 CD38 T cells, normal CD4 T-cell numbers but a skewed memory : naive CD4 T-cell ratio present at birth. This phenotype persisted in older HIV-exposed uninfected children. An increase in activated T cells may generate compensatory regulation or premature immunological senescence, which has direct implications for the generation of vaccine-induced and naturally acquired immunity. Vaccine-induced immunity in these individuals has shown contrasting results, while Malawian children had impaired CD4 T-cell responses to bacillus Calmette–Guérin (BCG) vaccine,23 a South African study found comparable antigen-specific IgG titres to all routine childhood vaccines in HIV-exposed and unexposed children.21 Impaired BCG responses were associated with elevated effector and senescent CD57+, programme death-1+ T cells which may undermine the longevity of BCG vaccination, whereas subtle T-cell phenotypic defects may not dramatically alter IgG antibody production to a short-term conjugate vaccine at 9 months. The degree or longevity of immune defects in sero-reverted children remains undefined. Whether the immune dysfunction described is caused by in utero HIV exposure,24in utero exposure to a maternal cytokine storm driven by HIV,25,26 or more generalized poor maternal health remains to be determined.
Placental malaria
During pregnancy, P. falciparum-infected erythrocytes sequester in the placenta where they induce inflammation causing placental insufficiency, decreased maternal insulin-like growth factor-1 levels (critical for fetal growth),16 which increases the risk of LBW infants, future infant malaria infections27 and sepsis.28 Studies investigating the effect of placental malaria on maternal–fetal antibody transfer have consistently shown a decrease in antibody titres to tetanus,17 measles and S. pneumoniae.18 One study categorized placental malaria into active-acute, active-chronic and past infections and found the biggest impact on placental antibody transfer was in those women who had chronic infection because they were more likely to have placental damage and high lymphocyte infiltration.17 Uterine inflammation and transplacental passage of soluble P. falciparum antigens during placental malaria alter neonatal adaptive and innate immunity in a number of ways.29–31 Placental malaria decreases cord blood mononuclear cell tumour necrosis factor-α (TNF-α) production in response to lipopolysaccharide–Toll-like receptor-4 (LPS-TLR-4) ligation.29 Placental infection also skews neonatal adaptive immunity by biasing T helper type 2 (Th2) cytokines, reducing interferon-γ (IFN-γ) and increasing IL-10 in response to P. falciparum, which appears to be under the control of CD4+ CD25+ Foxp3+ regulatory T cells.30 This skew in neonatal P. falciparum-specific Th1/Th2 cytokines and involvement of regulatory T cells31 may partly explain why infants born to women with placental malaria are predisposed to P. falciparum infection in their first year of life.27 In Malawi, the intervention of impregnated bed nets by pregnant women was shown to reduce the incidence of placental malaria from 25·2% to 6·8% and LBW infants from 14·1% to 8·9% during 1997–1998 and 2005–2006, respectively.32 Importantly, the decrease in placental malaria was more dramatic than the drop in LBW infants, highlighting the role of other confounders such as maternal nutrition and HIV status.
Maternal malnutrition
Outside times of famine in sub-Saharan Africa, malnutrition or under-nutrition in adults is commonly linked to HIV infection33 and is exacerbated by seasonal fluctuations in the availability of food. There is increasing evidence that maternal under-nutrition not only affects birthweight but also causes persistent immune defects in the newborn. A Gambian study (where HIV seroprevalence is low) found that adults (< 25 years age) born during the months of July–December when rainfall is high and food availability is low (hungry season) were 10 times more likely to die from an infection-related illness than those born throughout the rest of the year. Although maternal malaria and diarrhoea infections peak in the hungry season and could contribute to the long-term effects on health, premature adult infection-related mortality remained high outside the August to November peak in placental malaria. The predominance of deaths from infectious diseases therefore suggests a permanent immunological defect caused by the reduced availability of food during critical periods of early development.34
A study comparing breast milk from Zairian malnourished women with healthy controls found comparable secretory IgA (sIgA) antibody titres against rotavirus, respiratory syncytial virus, Escherichia coli, S. pneumoniae and Haemophilus influenzae but an approximately 30% decrease in the amount of breast milk.35 Hence, the quantity of antibodies and innate factors delivered to the newborn in maternal milk was reduced. When lactoferrin, lysozyme and sIgA levels were serially evaluated in breast milk from mothers with healthy babies and mothers with septicaemic babies, the breast milk from mothers with healthy babies had rapidly declining sIgA titres that were replaced by lactoferrin and lysozyme (blocks bacterial binding and lyses bacterial cell walls). In contrast, milk samples from women whose babies developed septicaemia had sustained levels of sIgA and low levels of lactoferrin and lysozyme,36 implicating lactoferrin and lysozyme rather than sIgA in the control of infection in the newborn.
Micronutrient deficiencies, including vitamin A and zinc, are widespread in resource-poor settings. Although vitamin A deficiency is known to cause immunological defects,37 a review of vitamin A supplementation trials in pregnant women showed no beneficial effects on prenatal and postnatal infant mortality, stillbirth or LBW babies.38 In contrast, although zinc supplementation given to all pregnant women irrespective of deficiency had no impact on the reduction of LBW babies, it did reduce the risk of infant diarrhoea, dysentery and impetigo in LBW infants,39 implying an effect on maternal passive immunity. Indeed, zinc-deficient mothers are known to produce low levels of natural immunoglobulins with a persistent defect in IgM, associated with transiently low levels of IgA and IgG2 in the neonate.40 Maternal zinc deficiency not only affects the mother but is linked with LBW babies15 and infant thymic atrophy.41 In a study conducted in Bangladesh, antenatal zinc supplementation improved newborn BCG vaccine responses in LBW babies but surprisingly not responses to H. influenzae type-b vaccine.42 Although Akman et al.43 found no link between gestational zinc deficiency and LBW babies they reported reduced neonatal zinc levels, which is critical to macrophage oxidative burst,44 dendritic cell function45 and thymic cell function in the newborn.46 The discrepancies between zinc-related maternal and child immune defects and the variable benefits of zinc supplementation highlight the complexities of micronutrient deficiencies and the effects of supplementation.
Immunological disruption caused by concurrent infections
Poor maternal health and consequential defects in fetal and newborn immunity are likely to predispose infants to multiple recurrent or concurrent infections. Although exposure to antigens is required for the generation of naturally acquired immunity and long-lasting immune protection, excessive recurrent or concurrent infections during periods of immune development may independently alter normal development, increasing the individual's susceptibility to further infections. This will be particularly important in sub-Saharan Africa where infection is endemic. For example, effective clearance of concurrent bacterial and helminth infections may be hampered by a predominant Th1 response inhibiting Th2 immunity and vice versa or because of bystander immune regulation. Furthermore, the inflammatory environment and tissue damage caused by one pathogen may enhance microbial attachment and dissemination of another.47,48 However, the impact of recurrent/concurrent infection as a potential cause of immune dysfunction and long-term morbidity/mortality has not been fully investigated.
Studies in Malawi,49 The Gambia50 and Mozambique5 all describe a link between underlying P. falciparum infection and lethal NTS septicaemia in children. Although NTS infections are usually limited to the intestinal tract, the increase in bacterial dissemination in those co-infected with malaria suggests a defect in the gut mucosal barrier as well as a splenic defect.51,52 In a mouse model of S. typhimurium-malaria, co-infection resulted in attenuated neutrophil influx and pro-inflammatory cytokine responses to NTS within the intestinal tissue.51 Using the same murine model of co-infection, Roux et al.52 have shown that macrophages were unresponsive to NTS because of the overwhelming uptake of damaged red blood cells as a consequence of malaria-induced haemolytic anaemia. In addition, antigen-presenting cells infected with Salmonella have limited ability to present antigen and induce subsequent T-cell responses.53 Similarly, in vitro malaria-infected erythrocytes inhibit the maturation of dendritic cells and reduce their capacity to stimulate T cells.54 In general, but not exclusively, co-infections result in a poorer prognosis. A study in Ghanaian children co-infected with rotavirus and enteropathogenic E. coli were shown to suffer from more severe diarrhoea and dehydration, with diarrhoea often lasting longer than in children infected with rotavirus alone.55 Together these data highlight the commonality of co-infections and demonstrates the added immune pressure that is common in sub-Saharan Africa.
Persistent microbial exposure can result in the regulation of the immune response as the host attempts to limit detrimental effects of inflammation. Conversely, bacteria such as Neisseria meningitidis56 and Helicobacter pylori57 regulate adaptive responses for their own persistence. In the context of recurrent infectious inflammation and high levels of commensalism in a resource-poor setting, regulation is a strong contender to hamper immunity to other pathogens or reduce vaccine efficacy. Although children suffer the greatest mortality from P. falciparum malaria, most experience only mild disease. Frequent episodes of uncomplicated parasitaemia in children are thought to generate regulatory T cells that suppress anti-parasitic responses resulting in uncontrolled parasitic load and susceptibility to complicated malaria.58,59 Similarly, the induction of regulatory T cells to one infection may impact on others in a bystander fashion. Lung IL-10 production during influenza virus infection has been shown to predispose mice to secondary infection following pneumococcal or meningococcal challenge by impairing phagocytic bacterial clearance.60,61 Interestingly, IL-10 appears late in the immune response to influenza and is sustained after the virus is cleared. Bacterial, viral and parasitic organisms such as mycobacteria, measles virus and helminths62 can skew or suppress immunity to new pathogenic stimuli via high levels of suppressive cytokines (transforming growth factor-β and IL-10) or Th2/IL-10 subverted responses.
A variety of viral infections63 also have the potential to induce localized inflammation and tissue damage to the lung and gut epithelium in resource-poor settings. Both processes favour microbial pathogenesis, exposing surface molecules and cell receptors to which bacteria readily adhere. Bacteria attach more effectively because of increased expression of epithelial adhesion molecules,47,64,65 exposure of epithelial basement membrane and reduced mucociliary velocity.66 Pneumococci express PspA which binds to the polymeric immunoglobulin receptor (pIgR) on respiratory epithelial cells.48 Expression of pIgR is greatly increased by IFN-γ67 and coincidently influenza-induced IFN-γ correlates with reduced pneumococcal clearance68 probably because of enhanced bacterial attachment. Although the incidence of respiratory viral co-infections are not widely reported in sub-Saharan Africa, a recent study in South African children ≤5 years has revealed the prevalence of respiratory infections in HIV-infected and uninfected children.69 Whether certain viral infections predispose individuals to invasive pneumococcal disease in areas of high pneumococcal colonization remains to be determined. Cytomegalovirus (CMV) is another common viral infection with seroprevalence that reaches up to 100% in Africa with increased rates of CMV-associated pneumonia in HIV-positive children.70 In the elderly, CMV infections are associated with advanced aging of the immune system,71 and recently it was shown that adults thymectomized in early childhood had a similar phenotype to an aging population with decreased naive T cells, increased markers of inflammation and highly differentiated CD57+ cells.72 Features of premature immunological aging were even more pronounced in those individuals who were CMV seropositive, suggesting a synergistic effect of lack of thymic activity and immune pressure exerted by CMV on promoting premature exhaustion of the T-cell repertoire.
Although intermittent microbial exposure is required for the generation of naturally acquired immunity, the antigen load must be controlled. We propose that multiple antigenic challenges in the short-term generate immune memory which is potentially undermined by immune regulation and premature immune exhaustion and relative ‘immunological aging’ caused by excessive immunological activation. Hence, intense antigen exposure in immunologically disadvantaged children in a resource-poor setting may lead to repeated episodes of malarial, gastrointestinal and respiratory infections.
Environmental impact on childhood susceptibility to infections
In addition to poor maternal health and multiple co-infections in childhood, common socio-economic, seasonal and environmental factors together with childhood malnutrition amplify pathogen exposure with the potential to further undermine immune function, in sub-Saharan Africa.
Socio-economic and seasonal factors
Socio-economic and seasonal factors converge to increase the risk of infection. Socio-economic status determines the size of home; smaller dwellings become crowded during the cold winter season, which increases pathogen exposure and transmission. Likewise, better quality and stability of pit latrines cause less water contamination in the rainy season and subsistence farming and food yield is influenced by seasonality. Studies from West Africa have shown high faecal carriage of Salmonella among rural populations during the rainy season (8–16%) and high rates of faecal excretion in the malnourished (29%).73 With the coincidental peak in malarial infections in the rainy season, the likelihood of lethal NTS septicaemia is increased. In relation to access to clean water, enteric infections are especially common during the rainy season; these alter gut integrity, which leads to impaired absorption of nutrients; coupled with the irregular food yield a vicious cycle of infection–malnutrition–infection is a frequent outcome. Seasonality influences disease rates in part because of optimal conditions for pathogenic survival but also because immunity is weakened by malnutrition and co-infections.
Childhood malnutrition
Under-nutrition owing to inadequate breastfeeding, poor access to nutritious foods, recurrent enteric infections are potent causes of immunosuppression. In many countries in sub-Saharan Africa some degree of childhood under-nutrition is the norm ranging from those with micronutrient deficiencies to the severely malnourished (weight-for-age Z-score < − 3).74
Zinc deficiency contributes to pneumonia,7 diarrhoea,75 and possibly malaria.9 Supplementation is recommended by the World Health Orgnaization for the treatment of childhood diarrhoea in developing countries.75 The immunological basis for increased infections in zinc-deficient individuals is complex. Intracellular zinc comes in both bound (essential for < 300 enzymes) and free forms, and zinc supplementation may largely alter the availability of free zinc.76 Recently, dendritic cell activation was shown to decrease intracellular free zinc and, contrary to expectation, zinc supplementation hampered LPS-TLR-4-mediated expression of MHC class I, class II and CD86.45 In severely malnourished Zambian children dendritic cell number and function were evaluated on admission, during convalescence and at the 6-month follow-up.77 On admission, malnourished children had decreased numbers of dendritic cells and impaired capacity to produce IL-12 and up-regulate HLA-DR and CD86 in response to LPS; whether these defects were caused by altered zinc homeostasis was not addressed in this study. Maternal and infant zinc deficiency in human and animal models has been linked to infant thymic atrophy,41 impaired BCG responses78 and decreased mitogenic T-cell responses.79 In HIV-negative malnourished Rwandan children there was a correlation between zinc deficiency and low %CD4 T cells,80 perhaps caused by a combination of thymic atrophy and IL-7 unresponsiveness. Recently, Miz-1, a transcription regulator composed of 13 zinc finger domains was shown to control the downstream effects of thymopoietic IL-7; Miz-1-deficient mice have thymic atrophy and lack T-cell lineage precursors.81 A recent study in Tanzania found that 48·3% of well-nourished children were zinc deficient and that deficiency was associated with increased peripheral blood mononuclear cell TNF-α and IFN-γ responses in malaria-infected individuals.82 The increase in TNF-α may inadvertently cause greater harm by up-regulating the vascular endothelium promoting parasitic sequestration.83 Keeping parasitized erythrocytes in the circulation potentially increases transmission by maintaining an assessable pool of infected cells for the mosquito to ingest (Table 2).
Table 2.
Summary of environmental confounders on childhood immunity
| Confounder | Impact | Outcome for child |
|---|---|---|
| Socio-economic | Crowding | High antigen exposure and transmission |
| Seasonality | Instability of pit latrines Contaminated water Optimal pathogen survival | Recurrent enteric infections Malaria and non-typhoidal Salmonella co-infections |
| Childhood malnutrition | Zinc deficiency | Poor dendritic cell function Reduced T-cell numbers Thymic atrophy |
Evidence of long-term immunological defects and the ‘Barker Hypothesis’
In a series of studies, Barker introduced the hypothesis that prenatal environmental insults (such as maternal under-nutrition), manifested by low birthweight, programme the fetus for susceptibility to infectious diseases in infancy and development of chronic non-communicable diseases in adulthood.2 In parallel, evidence is accumulating to suggest that early events may also programme immune function. This is perhaps not surprising, because development of the immune system begins prenatally and it is likely that prenatal and early postnatal factors, such as nutritional resources and pathogen exposure, are important determinants of later immune function. Indeed, prenatal growth restriction has been associated with increased prevalence of atopic and autoimmune disease in adulthood.84 As previously discussed, a study in The Gambia showed that the season of birth was strongly linked to infectious disease mortality later in life.34 The implication is that prenatal under-nutrition (or possibly an infectious or toxic insult correlated with season of birth) caused permanent impairment of immune function. In an area of intense antigen exposure such as sub-Saharan Africa prenatal immune impairments will predispose infants to recurrent infections that prematurely age the immune system. A prospective study in Bangladesh showed that children who had been LBW infants had high T-cell turnover with shortened telomeres, increased concentrations of T-cell receptor rearrangement excision circles and a low proportion of CD3 cells, which are features of early immunological ageing.85 As chronic infections such as CMV and HIV frequently cause prolonged T-cell senescence,86 we propose that sub-optimal immunity and concurrent childhood infections may also drive irreversible premature ‘immunological aging’.
These findings add to a growing body of evidence that events occurring in utero or early in postnatal life may permanently affect components of the immune system. Experimental data from several animal models support these observations. In the rat, maternal under-nutrition results in significant immune deficiencies in the offspring, and these impairments persist into adulthood, despite ad libitum feeding of the offspring.3,4 Interestingly, these deficits carry over into the next generation of offspring without further nutrient restriction, demonstrating that prenatal insults can have intergenerational effects, perhaps inherited epigenetically.87
Conclusion
In resource-poor settings, humans are exposed to a wealth of immunological pressures as they negotiate the transition from an in utero environment to one full of potential pathogens. We have described a range of factors specific to sub-Saharan Africa that may undermine the effectiveness of childhood immunity. Exposure to antigens is required for the generation of naturally acquired immunity and long-lasting immune protection. However, we propose that excessive exposure from concurrent childhood infections and high antigen burdens are detrimental and could cause immune dysregulation rather than protection. Maternal vaccination offers the potential to increase placental and breast-milk antibody titres, giving the infant greater protection against common pathogens. Huge progress has been made with the intervention of bednets32 and anti-retroviral therapy,20 dramatically reducing the incidence of placental malaria and mother-to-child transmission of HIV infection, respectively. However, better maternal and infant health is still undermined by gestational and childhood micronutrient deficiencies. A mechanistic understanding of how malnutrition effects maternal and child immunity is required to make advances in overcoming the long-term problem of poor nutrition within this setting. Furthermore, additional focus of how co-infections predispose children to recurrent disease may lead to further optimization of current and novel vaccine regimens or the generation of new therapeutic approaches.
Glossary
- BCG
bacille Calmette–Guérin
- CMV
cytomegalovirus
- IFN-γ
interferon-γ
- IL
interleukin
- LBW
low birthweight
- LPS
lipopolysaccharide
- NTS
non-typhoidal Salmonella
- pIgR
polymeric immunoglobulin receptor
- sIgA
secretory immunoglobulin A
- TB
tuberculosis
- Th2
T helper type 2
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
Disclosures
The authors have declared that no competing interests exist.
References
- 1.You D, Wardlaw T, Salama P, Jones G. Levels and trends in under-5 mortality, 1990–2008. Lancet. 2010;375:100–3. doi: 10.1016/S0140-6736(09)61601-9. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–28. [PubMed] [Google Scholar]
- 3.Beach RS, Gershwin ME, Hurley LS. Gestational zinc deprivation in mice: persistence of immunodeficiency for three generations. Science. 1982;218:469–71. doi: 10.1126/science.7123244. [DOI] [PubMed] [Google Scholar]
- 4.Chandra RK. Antibody formation in first and second generation offspring of nutritionally deprived rats. Science. 1975;190:289–90. doi: 10.1126/science.1179211. [DOI] [PubMed] [Google Scholar]
- 5.Bassat Q, Guinovart C, Sigauque B, et al. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Trop Med Int Health. 2009;14:1011–9. doi: 10.1111/j.1365-3156.2009.02326.x. [DOI] [PubMed] [Google Scholar]
- 6.Berkley JA, Lowe BS, Mwangi I, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 7.Greenwood B. The epidemiology of pneumococcal infection in children in the developing world. Philos Trans R Soc Lond B Biol Sci. 1999;354:777–85. doi: 10.1098/rstb.1999.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine OS, O'Brien KL, Knoll M, et al. Pneumococcal vaccination in developing countries. Lancet. 2006;367:1880–2. doi: 10.1016/S0140-6736(06)68703-5. [DOI] [PubMed] [Google Scholar]
- 9.O'Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, Snow RW, Newton CR, Marsh K. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–62. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceesay SJ, Casals-Pascual C, Erskine J, et al. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet. 2008;372:1545–54. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill PC, Cheung YB, Akisanya A, Sankareh K, Lahai G, Greenwood BM, Adegbola RA. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: a longitudinal study. Clin Infect Dis. 2008;46:807–14. doi: 10.1086/528688. [DOI] [PubMed] [Google Scholar]
- 12.Kingsley RA, Msefula CL, Thomson NR, et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 2009;19:2279–87. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bailey RC, Kamenga MC, Nsuami MJ, Nieburg P, St Louis ME. Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. Int J Epidemiol. 1999;28:532–40. doi: 10.1093/ije/28.3.532. [DOI] [PubMed] [Google Scholar]
- 14.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–71. [PubMed] [Google Scholar]
- 15.Meadows NJ, Ruse W, Smith MF, Day J, Keeling PW, Scopes JW, Thompson RP, Bloxam DL. Zinc and small babies. Lancet. 1981;2:1135–7. doi: 10.1016/s0140-6736(81)90587-0. [DOI] [PubMed] [Google Scholar]
- 16.Umbers AJ, Boeuf P, Clapham C, et al. Placental malaria-associated inflammation disturbs the insulin-like growth factor axis of fetal growth regulation. J Infect Dis. 2011;203:561–9. doi: 10.1093/infdis/jiq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumberland P, Shulman CE, Maple PA, Bulmer JN, Dorman EK, Kawuondo K, Marsh K, Cutts FT. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis. 2007;196:550–7. doi: 10.1086/519845. [DOI] [PubMed] [Google Scholar]
- 18.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79:F202–5. doi: 10.1136/fn.79.3.f202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montana LS, Mishra V, Hong R. Comparison of HIV prevalence estimates from antenatal care surveillance and population-based surveys in sub-Saharan Africa. Sex Transm Infect. 2008;84(Suppl 1):i78–84. doi: 10.1136/sti.2008.030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21(Suppl 4):S65–71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 21.Cartwright K, Jones DM. Investigation of meningococcal disease. J Clin Pathol. 1989;42:634–9. doi: 10.1136/jcp.42.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epalza C, Goetghebuer T, Hainaut M, Prayez F, Barlow P, Dediste A, Marchant A, Levy J. High incidence of invasive group B streptococcal infections in HIV-exposed uninfected infants. Pediatrics. 2010;126:e631–8. doi: 10.1542/peds.2010-0183. [DOI] [PubMed] [Google Scholar]
- 23.Miles DJ, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guerin (BCG) vaccine in HIV-uninfected infants. Immunology. 2010;129:446–54. doi: 10.1111/j.1365-2567.2009.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn L, Meddows-Taylor S, Gray G, Tiemessen C. Human immunodeficiency virus (HIV)-specific cellular immune responses in newborns exposed to HIV in utero. Clin Infect Dis. 2002;34:267–76. doi: 10.1086/338153. [DOI] [PubMed] [Google Scholar]
- 25.Chougnet C, Kovacs A, Baker R, et al. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J Infect Dis. 2000;181:1590–7. doi: 10.1086/315458. [DOI] [PubMed] [Google Scholar]
- 26.Borges-Almeida E, Milanez HM, Vilela MM, Cunha FG, Abramczuk BM, Reis-Alves SC, Metze K, Lorand-Metze I. The impact of maternal HIV infection on cord blood lymphocyte subsets and cytokine profile in exposed non-infected newborns. BMC Infect Dis. 2011;11:38. doi: 10.1186/1471-2334-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz NG, Adegnika AA, Breitling LP, et al. Placental malaria increases malaria risk in the first 30 months of life. Clin Infect Dis. 2008;47:1017–25. doi: 10.1086/591968. [DOI] [PubMed] [Google Scholar]
- 28.Dzwonek AB, Neth OW, Thiebaut R, et al. The role of mannose-binding lectin in susceptibility to infection in preterm neonates. Pediatr Res. 2008;63:680–5. doi: 10.1203/PDR.0b013e31816fdbff. [DOI] [PubMed] [Google Scholar]
- 29.Adegnika AA, Kohler C, Agnandji ST, et al. Pregnancy-associated malaria affects toll-like receptor ligand-induced cytokine responses in cord blood. J Infect Dis. 2008;198:928–36. doi: 10.1086/591057. [DOI] [PubMed] [Google Scholar]
- 30.Bisseye C, van der Sande M, Morgan WD, Holder AA, Pinder M, Ismaili J. Plasmodium falciparum infection of the placenta impacts on the T helper type 1 (Th1)/Th2 balance of neonatal T cells through CD4(+)CD25(+) forkhead box P3(+) regulatory T cells and interleukin-10. Clin Exp Immunol. 2009;158:287–93. doi: 10.1111/j.1365-2249.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, King CL. Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol. 2011;186:2780–91. doi: 10.4049/jimmunol.1001188. [DOI] [PubMed] [Google Scholar]
- 32.Feng G, Simpson JA, Chaluluka E, Molyneux ME, Rogerson SJ. Decreasing burden of malaria in pregnancy in Malawian women and its relationship to use of intermittent preventive therapy or bed nets. PLoS ONE. 2010;5:e12012. doi: 10.1371/journal.pone.0012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semba RD, Miotti PG, Chiphangwi JD, Liomba G, Yang LP, Saah AJ, Dallabetta GA, Hoover DR. Infant mortality and maternal vitamin A deficiency during human immunodeficiency virus infection. Clin Infect Dis. 1995;21:966–72. doi: 10.1093/clinids/21.4.966. [DOI] [PubMed] [Google Scholar]
- 34.Moore SE, Cole TJ, Collinson AC, Poskitt EM, McGregor IA, Prentice AM. Prenatal or early postnatal events predict infectious deaths in young adulthood in rural Africa. Int J Epidemiol. 1999;28:1088–95. doi: 10.1093/ije/28.6.1088. [DOI] [PubMed] [Google Scholar]
- 35.Brussow H, Barclay D, Sidoti J, Rey S, Blondel A, Dirren H, Verwilghen AM, Van Geert C. Effect of malnutrition on serum and milk antibodies in Zairian women. Clin Diagn Lab Immunol. 1996;3:37–41. doi: 10.1128/cdli.3.1.37-41.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ella EE, Ahmad AA, Umoh VJ, Ogala WN, Balogun TB, Musa A. Studies on the interaction between IgA, lactoferrin and lysozyme in the breastmilk of lactating women with sick and healthy babies. Journal of Infectious Diseases and Immunity. 2011;3:24–9. [Google Scholar]
- 37.Glennie SJ, Williams NA, Heyderman RS. Mucosal immunity in resource-limited setting: is the battle ground different? Trends Microbiol. 2011;18:487–93. doi: 10.1016/j.tim.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 38.van den Broek N, Dou L, Othman M, Neilson JP, Gates S, Gulmezoglu AM. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2010;11:CD008666. doi: 10.1002/14651858.CD008666.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Osendarp SJ, van Raaij JM, Darmstadt GL, Baqui AH, Hautvast JG, Fuchs GJ. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: a randomised placebo controlled trial. Lancet. 2001;357:1080–5. doi: 10.1016/s0140-6736(00)04260-4. [DOI] [PubMed] [Google Scholar]
- 40.Favier AE. The role of zinc in reproduction. Hormonal mechanisms. Biol Trace Elem Res. 1992;32:363–82. doi: 10.1007/BF02784623. [DOI] [PubMed] [Google Scholar]
- 41.Golden MH, Jackson AA, Golden BE. Effect of zinc on thymus of recently malnourished children. Lancet. 1977;2:1057–9. doi: 10.1016/s0140-6736(77)91888-8. [DOI] [PubMed] [Google Scholar]
- 42.Osendarp SJ, Fuchs GJ, van Raaij JM, Mahmud H, Tofail F, Black RE, Prabhakar H, Santosham M. The effect of zinc supplementation during pregnancy on immune response to Hib and BCG vaccines in Bangladesh. J Trop Pediatr. 2006;52:316–23. doi: 10.1093/tropej/fml012. [DOI] [PubMed] [Google Scholar]
- 43.Akman I, Arioglu P, Koroglu OA, Sakalli M, Ozek E, Topuzoglu A, Eren S, Bereket A. Maternal zinc and cord blood zinc, insulin-like growth factor-1, and insulin-like growth factor binding protein-3 levels in small-for-gestational-age newborns. Clin Exp Obstet Gynecol. 2006;33:238–40. [PubMed] [Google Scholar]
- 44.Wirth JJ, Fraker PJ, Kierszenbaum F. Zinc requirement for macrophage function: effect of zinc deficiency on uptake and killing of a protozoan parasite. Immunology. 1989;68:114–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Kitamura H, Morikawa H, Kamon H, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–7. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 46.Wellinghausen N. Immunobiology of gestational zinc deficiency. Br J Nutr. 2001;85(Suppl 2):S81–6. [PubMed] [Google Scholar]
- 47.McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187:1000–9. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 48.Luo R, Mann B, Lewis WS, et al. Solution structure of choline binding protein A, the major adhesin of Streptococcus pneumoniae. EMBO J. 2005;24:34–43. doi: 10.1038/sj.emboj.7600490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bronzan RN, Taylor TE, Mwenechanya J, et al. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 50.Mackenzie G, Ceesay SJ, Hill PC, et al. A decline in the incidence of invasive non-typhoidal Salmonella infection in The Gambia temporally associated with a decline in malaria infection. PLoS ONE. 2010;5:e10568. doi: 10.1371/journal.pone.0010568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butler B. Davis: University of California; 2010. Effects of malaria on mucosal barrier function in the gut and the host response to non-typhoidal Salmonella. Ph.D Thesis. [Google Scholar]
- 52.Roux CM, Butler BP, Chau JY, Paixao TA, Cheung KW, Santos RL, Luckhart S, Tsolis RM. Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to Nontyphoidal Salmonella enterica serovar typhimurium infection in mice. Infect Immun. 2010;78:1520–7. doi: 10.1128/IAI.00887-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albaghdadi H, Robinson N, Finlay B, Krishnan L, Sad S. Selectively reduced intracellular proliferation of Salmonella enterica serovar typhimurium within APCs limits antigen presentation and development of a rapid CD8 T cell response. J Immunol. 2009;183:3778–87. doi: 10.4049/jimmunol.0900843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urban BC, Ferguson DJ, Pain A, Willcox N, Plebanski M, Austyn JM, Roberts DJ. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–7. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 55.Hori H, Akpedonu P, Armah G, Aryeetey M, Yartey J, Kamiya H, Sakurai M. Enteric pathogens in severe forms of acute gastroenteritis in Ghanaian children. Acta Paediatr Jpn. 1996;38:672–6. doi: 10.1111/j.1442-200x.1996.tb03729.x. [DOI] [PubMed] [Google Scholar]
- 56.Davenport V, Groves E, Hobbs CG, Williams NA, Heyderman RS. Regulation of Th-1 T cell-dominated immunity to Neisseria meningitidis within the human mucosa. Cell Microbiol. 2007;9:1050–61. doi: 10.1111/j.1462-5822.2006.00851.x. [DOI] [PubMed] [Google Scholar]
- 57.Beswick EJ, Pinchuk IV, Das S, Powell DW, Reyes VE. Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect Immun. 2007;75:4334–41. doi: 10.1128/IAI.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Todryk SM, Bejon P, Mwangi T, Plebanski M, Urban B, Marsh K, Hill AV, Flanagan KL. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE. 2008;3:e2027. doi: 10.1371/journal.pone.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen DS, Schofield L. Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog. 2010;6:e1000771. doi: 10.1371/journal.ppat.1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alonso JM, Guiyoule A, Zarantonelli ML, et al. A model of meningococcal bacteremia after respiratory superinfection in influenza A virus-infected mice. FEMS Microbiol Lett. 2003;222:99–106. doi: 10.1016/S0378-1097(03)00252-0. [DOI] [PubMed] [Google Scholar]
- 61.van der Sluijs KF, Nijhuis M, Levels JH, Florquin S, Mellor AL, Jansen HM, van der Poll T, Lutter R. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J Infect Dis. 2006;193:214–22. doi: 10.1086/498911. [DOI] [PubMed] [Google Scholar]
- 62.Hartgers FC, Obeng BB, Kruize YC, et al. Responses to malarial antigens are altered in helminth-infected children. J Infect Dis. 2009;199:1528–35. doi: 10.1086/598687. [DOI] [PubMed] [Google Scholar]
- 63.Goulding J, Snelgrove R, Saldana J, et al. Respiratory infections: do we ever recover? Proc Am Thorac Soc. 2007;4:618–25. doi: 10.1513/pats.200706-066TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNamee LA, Harmsen AG. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun. 2006;74:6707–21. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pittet LA, Hall-Stoodley L, Rutkowski MR, Harmsen AG. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2010;42:450–60. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ackermann LW, Wollenweber LA, Denning GM. IL-4 and IFN-gamma increase steady state levels of polymeric Ig receptor mRNA in human airway and intestinal epithelial cells. J Immunol. 1999;162:5112–8. [PubMed] [Google Scholar]
- 68.Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med. 2008;14:558–64. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 69.Venter M, Lassauniere R, Kresfelder TL, Westerberg Y, Visser A. Contribution of common and recently described respiratory viruses to annual hospitalizations in children in South Africa. J Med Virol. 2011;83:1458–68. doi: 10.1002/jmv.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zampoli M, Morrow B, Hsiao NY, Whitelaw A, Zar HJ. Prevalence and outcome of cytomegalovirus-associated pneumonia in relation to human immunodeficiency virus infection. Pediatr Infect Dis J. 2011;30:413–7. doi: 10.1097/INF.0b013e3182065197. [DOI] [PubMed] [Google Scholar]
- 71.Moss P. The emerging role of cytomegalovirus in driving immune senescence: a novel therapeutic opportunity for improving health in the elderly. Curr Opin Immunol. 2010;22:529–34. doi: 10.1016/j.coi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Sauce D, Larsen M, Fastenackels S, et al. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119:3070–8. doi: 10.1172/JCI39269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lloyd-Evans N, Drasar BS, Tomkins AM. A comparison of the prevalence of campylobacter, Shigellae and Salmonellae in faeces of malnourished and well nourished children in The Gambia and Northern Nigeria. Trans R Soc Trop Med Hyg. 1983;77:245–7. doi: 10.1016/0035-9203(83)90082-2. [DOI] [PubMed] [Google Scholar]
- 74.Ge KY, Chang SY. Definition and measurement of child malnutrition. Biomed Environ Sci. 2001;14:283–91. [PubMed] [Google Scholar]
- 75.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 76.Rink L, Haase H. Zinc homeostasis and immunity. Trends Immunol. 2007;28:1–4. doi: 10.1016/j.it.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 77.Hughes SM, Amadi B, Mwiya M, Nkamba H, Tomkins A, Goldblatt D. Dendritic cell anergy results from endotoxemia in severe malnutrition. J Immunol. 2009;183:2818–26. doi: 10.4049/jimmunol.0803518. [DOI] [PubMed] [Google Scholar]
- 78.McMurray DN, Bartow RA, Mintzer CL, Hernandez-Frontera E. Micronutrient status and immune function in tuberculosis. Ann N Y Acad Sci. 1990;587:59–69. doi: 10.1111/j.1749-6632.1990.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 79.Rink L, Gabriel P. Zinc and the immune system. Proc Nutr Soc. 2000;59:541–52. doi: 10.1017/s0029665100000781. [DOI] [PubMed] [Google Scholar]
- 80.Ndagije F, Baribwira C, Coulter JB. Micronutrients and T-cell subsets: a comparison between HIV-infected and uninfected, severely malnourished Rwandan children. Ann Trop Paediatr. 2007;27:269–75. doi: 10.1179/146532807X245652. [DOI] [PubMed] [Google Scholar]
- 81.Saba I, Kosan C, Vassen L, Moroy T. IL-7R-dependent survival and differentiation of early T-lineage progenitors is regulated by the BTB/POZ domain transcription factor Miz-1. Blood. 2011;117:3370–81. doi: 10.1182/blood-2010-09-310680. [DOI] [PubMed] [Google Scholar]
- 82.Mbugi EV, Meijerink M, Veenemans J, et al. Alterations in early cytokine-mediated immune responses to Plasmodium falciparum infection in Tanzanian children with mineral element deficiencies: a cross-sectional survey. Malar J. 2010;9:130. doi: 10.1186/1475-2875-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wassmer SC, Cianciolo GJ, Combes V, Grau GE. Inhibition of endothelial activation: a new way to treat cerebral malaria? PLoS Med. 2005;2:e245. doi: 10.1371/journal.pmed.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Phillips DI, Cooper C, Fall C, Prentice L, Osmond C, Barker DJ, Rees Smith B. Fetal growth and autoimmune thyroid disease. Q J Med. 1993;86:247–53. [PubMed] [Google Scholar]
- 85.Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G, Yunus M, Moore SE, Fuchs G. Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. Am J Clin Nutr. 2007;85:845–52. doi: 10.1093/ajcn/85.3.845. [DOI] [PubMed] [Google Scholar]
- 86.Desai S, Ronquillo R, Usuga X, Martinson J, Adeyemi O, Tenorio A, Landay A. Conference on Retroviruses and Opportunistic Infections. Montreal, Canada: 2009. Immune senescence, activation and abnormal T cell homeostasis despite effective HAART, a hallmark of early aging in HIV Disease. [Google Scholar]
- 87.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–8. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]


