Abstract
Interactions with extracellular matrices (ECM) shape the signaling and functions of many types of cells and receptors, and distinct ECM coatings have been used in a wide range of substrates for drug discovery processes. Here, we investigate the influence of ECM protein coatings on the signaling of endogenous purinergic 2Y (P2Y) receptors in human embryonic kidney HEK293 cells using dynamic mass redistribution (DMR) assays enabled by label-free optical biosensor. Results showed that ECM proteins had significant impacts on the DMR characteristics, potency, and efficacy of seven P2Y agonists. This study documents the importance of surface chemistry in regulating receptor signaling.
Introduction
Cell adhesion to extracellular matrix (ECM) is integral to the survival and functions of tissue cells. The ECM onto which cells are harbored is part of environmental cues for regulating the dynamic behaviors of cells.1 The cell surface integrins often bind to ligands in the ECM substratum and transduce signals through their intracellular domains, thus regulating functions as diverse as tissue maintenance, immune response, and development.2 The past decades have witnessed ever-increasing insights for how both compositions and organization of the ECM regulate cell functions, partly because of advances in material engineering including patterning to encourage and direct cell behavior ranging from cell adhesion to gene expression to phenotype3–7 and partly because of increasing resolution in assay technologies to decode the surface impacts on cell dynamics. Further, distinct ECM coatings have been used in a wide array of substrates for assaying and screening drug molecules in many types of cells during drug discovery process. Elucidating the impacts of surface chemistry on receptor biology and ligand pharmacology is important to improve the quality of screening assays and hits identified.
Purinergic 2Y (P2Y) receptors are a family of G protein-coupled receptors (GPCRs) that are activated by nucleotides including adenosine 5′-triphosphate (ATP), adenosine diphosphate (ADP), uridine triphosphate (UTP), uridine diphosphate (UDP), and UDP-glucose. To date, the P2Y family consists of at least eight family members (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14).8 P2Y receptors are found in most human tissues and have diverse physiological roles including regulation of platelet aggregation, muscle contraction, neurotransmission, and migration, which make them good receptor systems to study surface impacts. However, pharmacological characterization of endogenous P2Y receptors is difficult for obvious reasons. First, multiple family receptors are often coexpressed in a native cell including human embryonic kidney HEK293.9 HEK293 is the most widely used cell background for drug screening and receptor characterization studies. Second, many P2Y ligands often exhibit relatively poor selectivity among the family members. Third, activation of distinct P2Y receptors often triggers similar pathways,8 and conventional molecular characterization assays (e.g., Ca2+ mobilization, cAMP, ERK, arrestin) often have poor resolution to quantify and separate contributions from distinct receptors without cell manipulations.10 To increase the complexity is the potential influence of surfaces on receptor biology.
Dynamic mass redistribution (DMR) assays have attracted much interest in delineation of receptor biology, drug pharmacology, and cell biology in native cells in recent years.10–18 DMR assays utilize a label-free optical biosensor to noninvasively track in real time the dynamic redistribution of cellular matter within ∼150 nm of the sensor surface and convert it into a kinetic and integrated response (i.e., DMR signal) upon stimulation with a ligand.19 DMR assays are rich in texture and promise delineating receptor biology and ligand pharmacology with broad coverage of pathways downstream the receptor–ligand interaction.20 Thus, we set out to (1) characterize the signaling of endogenous P2Y receptors in HEK293 and (2) to study the influence of ECM coatings on receptor signaling using DMR assays.
Methods
Materials
Forskolin and MRS2179 were obtained from Tocris. ADP, ATP, 2-methylthio-ATP (2MeSATP), UDP, UTP, uridine 5-thiotriphosphate (UTPγS), collagen IV, fibronectin, and gelatin were obtained from Sigma. All nucleotides of the highest grade were ordered and used directly without further purification. All nucleotides were dissolved in water and freshly prepared for each experiment, whereas others were stocked in dimethyl sulfoxide. Cell culture-compatible (i.e., tissue-culture treated [TCT]) and fibronectin-coated Epic® 384well biosensor microplates were obtained from Corning, Inc. Both collagen- and gelatin-coated plates were freshly prepared by overnight evaporation of 10 μL of 20 μg/mL protein solution under nitrogen.
Cell Culture
HEK293 was obtained from American Type Cell Culture. The cell culture medium used was minimum essential medium having 2 mM glutamine, 4.5 g/L glucose, 2 mM glutamine, 10% fetal bovine serum, and antibiotics.
DMR Assays
Epic system (Corning, Inc.) used for whole-cell sensing is a resonant waveguide grating biosensor system that is designed for high-throughput screening using a 384-well footprint. This system consists of a temperature-control unit (26°C), an optical detection unit, and an on-board liquid handling unit with robotics. The detection unit is centered on integrated fiber optics to scan across each biosensor and enables kinetic measures of cellular responses with a temporal resolution of 15 s. The system reports a ligand-induced DMR signal as a shift in resonant wavelength (in picometers). The resultant DMR represents an averaged response of cells within the scanning path of the system.21 For DMR assays, cells were typically cultured in 50 μL of the serum-rich medium at 37°C under air/5% CO2 for overnight. The seeding density was twelve thousands of cells per well at the passage of 2 to 10. The cell confluency at the time of assays was ∼95%. After culture, cells were washed and maintained with 1× HBSS (Hanks' salt balanced buffer and 20 mM HEPES [pH 7.1]) and further incubated within Epic system for 1 h. Afterward, a 2-min baseline was established to ensure the cells reach a steady state. Compound solutions were then transferred, and the cellular responses were recorded.
DMR assays are noninvasive in nature, thus enabling multiple assay formats.22 DMR agonism assays measure the DMR signal arising from a ligand itself. DMR antagonist assays measure the impact of a receptor antagonist on the receptor agonist-induced DMR, wherein the antagonist was introduced before the agonist. DMR costimulation assays monitor cellular responses induced by a ligand in the presence of another compound. At least two independent sets of experiments, each with at least three replicates, were carried out for each measurement. The assay coefficient of variation was found to be <10%. All dose responses were analyzed using nonlinear regression with the Prism software based on the initial positive DMR (P-DMR) event induced by all P2Y agonists (Graph Pad).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from HEK293 cells using RNeasy mini kit (Qiagen, Cat. No. 74104). The cells were cultured on T75 TCT flask (Corning, Inc.) before harvest and RNA extraction. To eliminate genomic DNA contamination, on-column DNase digestion was performed using RNase-free DNase set (Qiagen, Cat. No. 79254). The concentration and quality of total RNA were determined using Nanodrop 8000 (Thermo Scientific). Customized polymerase chain reaction (PCR) array plates for 352 GPCR genes and reagents were ordered from SABiosciences. About 1 μg total RNA was used for a 96-well PCR-array. The PCR array was performed on an ABI 7300 Real-Time PCR System following manufacturer's instructions.
Results
mRNA Expression of P2Y Receptors in HEK293
We first examined the expression of P2Y receptors in HEK293 at mRNA level using quantitative real-time PCR (RT-PCR), as there is great variability in expression pattern of endogenous P2Y receptors in HEK293 reported in literatures.23–25 Results showed that HEK293 expresses mRNA of P2Y1, P2Y2, and P2Y11, whose threshold cycle (Ct) value was found to be 26.4, 27.6, and 27.3, respectively. No mRNA was detected for P2Y8 and 2Y10, whereas P2Y4, P2Y6, P2Y12, P2Y13, and P2Y14 gave rise to a Ct value greater than 32. The Ct values were 20.0 and 17.2 for two control genes, hypoxanthine phosphoribosyltransferase 1 and β-actin, respectively. This result suggests that HEK293 expresses multiple P2Y receptors including P2Y1, P2Y2, and P2Y11.
DMR Profiles of P2Y Agonists in HEK293 Cultured on TCT Biosensor Surfaces
As P2Y agonists often display relatively poor specificity, we first examined the DMR signals induced by a panel of P2Y agonists, including ADP, ATP, ATPγS, 2-MeSATP, UDP, and UTP. ADP potentially activates P2Y1, P2Y12, and P2Y13 with almost equal potency. ATP is an agonist preferring for P2Y2 and P2Y11 and also acts as a weak agonist for P2Y1 and P2Y13. UTP and UTPγS are selective agonists for P2Y2 and P2Y4. UDP is an agonist for P2Y6.
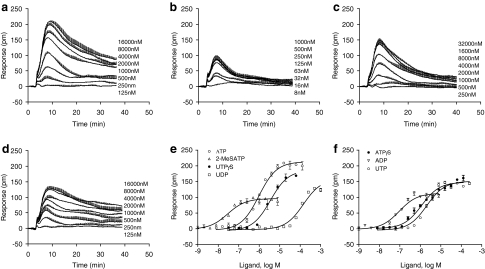
DMR assays using HEK293 cells cultured on TCT biosensor plates showed that all seven P2Y agonists led to a dose-dependent and saturable DMR signal (Fig. 1). Examining the DMR induced by the agonists in details revealed the following interesting aspects. First, all DMR shared similar characteristics in dynamics and consisted of a rapid increased signal (P-DMR) and a succeeding decreased signal (negative-DMR [N-DMR]). Second, nonlinear regression analysis suggests that all agonists gave rise to a single sigmoidal dose response, leading to an EC50 of 31±4, 84±6, 1025±127, 2142±151, 3327±325, 1145±93, and 113,000±13,000 nM, (n=4 for all) for 2-MeSATP, ADP, ATP, UTP, UTPγS, ATPγS, and UDP, respectively. Third, the agonists differed greatly in efficacy to trigger DMR (Fig. 1 and Table 1). The efficacy, based on the P-DMR amplitude, was found to be in the following order: ATP (213±21 pm)>UTPγS (174±13 pm)>UDP (154±15)∼ATPγS (148±9 pm)∼UTP (149±11 pm)>ADP (106±10 pm)∼2-MeSATP (98±7 pm) (n=6). Fourth, the agonists examined were found to be divergent in the late DMR response (e.g., amplitudes at 45 min poststimulation). 2-MeSATP at the saturating doses led to a P-DMR that eventually decayed back to the lowest evaluated level, which was close to the baseline (Fig. 1b). Together, these results suggest that the agonists examined exhibit complicated pharmacology and may reflect their different abilities to activate distinct P2Y receptors in HEK293 cells.
Fig. 1.
Dose-dependent DMR signals induced by P2Y agonists. (a) ATP; (b) 2-MeSATP; (c) UTP; (d) ATPγS; (e, f ) the P-DMR amplitudes of P2Y agonists as a function of agonist concentration. HEK293 cells were confluent on the TCT biosensor plate at the time of assays. The data presented are means±SEM of four replicate wells (n=4). 2MeSATP, 2-methylthio-ATP; DMR, dynamic mass redistribution; P2Y, purinergic 2Y; P-DMR; positive DMR; TCT, tissue culture-treated; UTP, uridine triphosphate.
Table 1.
The Comparison of Potency and Efficacy of Purinergic 2Y Agonists to Trigger Dynamic Mass Redistribution in Confluent HEK293 Cultured on Four Different Surfaces
| Surface |
ATP |
ATPγS |
2-MeSATP |
ADP |
UTP |
UTPγS |
UDP |
|---|---|---|---|---|---|---|---|
| (a) Potency (nM) | |||||||
| TCT | 1025±127 | 1145±93 | 31±4 | 84±6 | 2142±151 | 3327±325 | 113,000±13,000 |
| Fibronectin | 654±31 | 605±33 | 19±3 | 43±5 | 1076±97 | 2793±411 | 41,241±5639 |
| Collagen IV | 1322±192 | 1303±114 | 26±2 | 68±7 | 1364±159 | 3104±276 | 64,811±7100 |
| Gelatin | 973±74 | 1000±81 | 27±5 | 69±3 | 1818±198 | 3049±301 | 114,000±15,000 |
| (b) Efficacy (the peak response in picometer) | |||||||
|---|---|---|---|---|---|---|---|
| TCT | 213±21 | 148±9 | 98±7 | 106±10 | 149±11 | 174±13 | 154±15 |
| Fibronectin | 244±19 | 168±10 | 113±6 | 116±7 | 188±13 | 187±14 | 267±23 |
| Collagen IV | 256±23 | 154±9 | 84±5 | 99±5 | 160±12 | 193±11 | 265±21 |
| Gelatin | 242±24 | 175±11 | 123±11 | 128±6 | 178±12 | 210±19 | 106±13 |
2MeSATP, 2-methylthio-ATP; ADP, adenosine diphosphate; ATP, adenosine 5′-triphosphate; TCT, tissue culture-treated; UDP, uridine diphosphate; UTP, uridine triphosphate; UTPγS; uridine 5-thiotriphosphate.
Differential Ability of P2Y1 Antagonist MRS2179 to Block the DMR Induced by Distinct Agonists
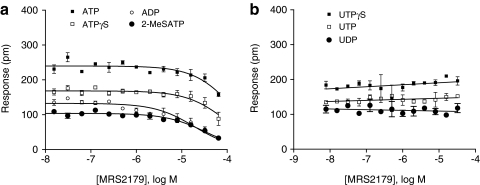
We next examined the receptor specificity of distinct agonist-induced DMR signals using P2Y1-specific antagonist MRS2179. DMR agonism assays showed that MRS2179 up to 32 μM did not result in any detectable DMR in HEK293 cells cultured on the TCT biosensor plates (data not shown). DMR antagonism assays showed that MRS2179 dose-dependently attenuated both 2-MeSATP and ADP DMR signals with almost identical potency (Fig. 2a). However, MRS2179 only slightly attenuated both ATP and ATPγS DMR (Fig. 2a), but had no effect on the DMR induced by UDP, UTP, or UPTγS (Fig. 2b). The percentage inhibition by MRS2179 at 32 μM was 74%, 67%, 31%, and 45% for ADP, 2-MeSATP, ATP, and ATPγS, respectively. In this antagonism assay, the cells were pretreated with MRS2179 at different doses, followed by stimulation with respective agonist at a fixed dose (1× EC100). These results suggest that both ADP and 2-MeSATP DMRs are mostly specific to P2Y1, whereas both ATP and ATPγS DMRs are partially due to the activation of P2Y1, and the UDP-, UTP-, and UTPγS-induced DMRs are not related to P2Y1.
Fig. 2.
The dose-dependent inhibition of P2Y agonists by P2Y1 antagonist MRS2179. The P-DMR amplitudes of each agonist as a function of MRS2179 dose were plotted. (a) ATP, 2-MeSATP, ATPγS, and ADP; (b) UDP, UTP, and UTPγS. The concentration was 2, 2, 100, 10, 16, 16, and 16 μM for 2-MeSATP, ADP, UDP, UTPγS, ATPγS, ATP, and UTP, respectively. Data presented are means±SEM of triplicate wells (n=3). ADP, adenosine diphosphate; ATP, adenosine 5′-triphosphate; UDP, uridine diphosphate; UTPγS; uridine 5-thiotriphosphate.
Costimulation DMR Profiles of Different Agonist Combinations
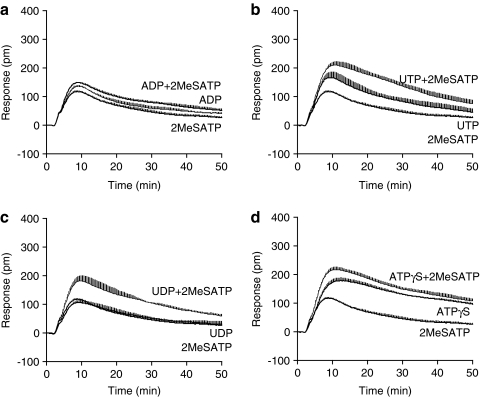
Compartmentalized signaling is known to be central in GPCR signaling.26,27 As the biosensor measures an integrative cellular response upon stimulation, the costimulation profiles with two agonists would offer alternative means to study the specificity of ligand–receptor interactions. It is expected that the activation of a single target by two full agonists, each at its saturating dose, would result in a DMR almost identical to those induced by either agonist alone, but the activation of two distinct targets by two agonists would result in a DMR that is close to the sum of the two agonist-induced DMR signals, given that the two agonists do not saturate the cell system for the same pathway being activated.28 Thus, we examined the DMR upon costimulation with two agonists, each at its saturating dose. DMR costimulation assays showed that the DMR induced by costimulation with ADP and 2-MeSATP was similar to that induced by ADP or 2-MeSATP alone (Fig. 3a), suggesting that both agonists mostly activate the same receptor (i.e., P2Y1). The costimulation with 2-MeSATP and UTP led to a DMR that was close to the sum of the two agonist-induced DMR (Fig. 3b), suggesting that, instead of P2Y1, UTP activates another endogenous P2Y receptor in HEK293. Based on the expression of P2Y receptors in HEK293 as well as the known pharmacology of UTP, the UTP DMR is largely due to the activation of P2Y2. Similar results were obtained for costimulation of cells with 2-MeSATP and UDP or UTPγS (Fig. 3c), suggesting that UDP and UTPγS also activate P2Y2 receptor. However, the costimulation with ATPγS and 2-MeSATP led to a DMR that is similar to that induced by ATPγS alone, suggesting that ATPγS activates other receptors beside P2Y1. Further, stimulation of cells with ATP in the presence of either one of the six other agonists led to a DMR similar to that induced by ATP alone (data not shown), suggesting that ATP activates all three endogenous receptors. Together, these results further suggest that distinct agonists activate different receptor(s); both ADP and 2-MeSATP activate P2Y1, whereas UTP, UTPγS, and UDP preferentially activate P2Y2, and ATP and ATPγS activates more than one P2Y receptor.
Fig. 3.
The costimulation DMR signals, in comparison with the DMR induced by individual agonist. (a) The DMR induced by 2-MeSATP, ADP, and 2-MeSATP+ADP; (b) the DMR induced by 2-MeSATP, UTP, and 2-MeSATP+UTP; (c) the DMR induced by 2-MeSATP, UDP, and 2-MeSATP+UDP; and (d) the DMR induced by 2-MeSATP, ATPγS, and 2-MeSATP+APTγS. The concentration was 2, 2, 16, 100, and 16 μM for 2-MeSATP, ADP, UTP, UDP, and ATPγS, respectively. Data presented are means±SEM of eight replicate wells (n=8).
Different Profiles of Forskolin to Modulate Distinct Agonist-Induced DMR Signals
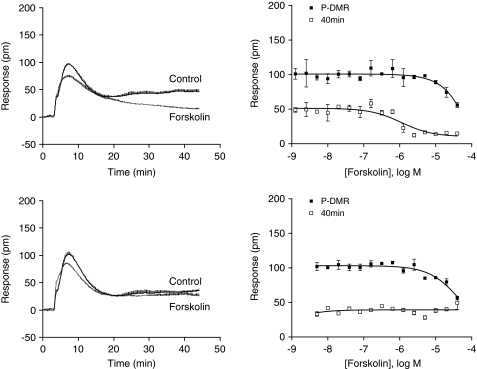
ATP was found to differ greatly from all other ligands examined from several perspectives. First, the maximal DMR induced by ATP is the greatest in amplitude among all agonists examined. Second, MRS2179 only partially attenuated the DMR induced by ATP at the saturating doses. Third, the ATP DMR led to the most sustainable P-DMR (i.e., the highest amplitude at 45 min poststimulation). As ATP is capable of activating all three endogenous P2Y receptors in HEK293 and the three receptors are primarily coupled to Gq pathway, but only P2Y11 is also Gs-coupled, we examined the impact of forskolin on all agonist-induced DMR. Forskolin is a well-known adenylate cyclase activator, and the cAMP-PKA pathway plays an important role in the integration of GPCR signaling. DMR assays showed that the pretreatment of cells with forskolin dose-dependently attenuated both early and late P-DMR induced by ATP (Fig. 4a, b). However, forskolin at high doses completely blocked the late response with an apparent IC50 of 1.12±0.10 μM (n=4), whereas forskolin only partially attenuated the early response with a much lower potency (>20 μM). Similar results were obtained for ATPγS (data not shown). However, only the early P-DMR, but not the late P-DMR, induced by 2-MeSATP or ADP was partially attenuated by the pretreatment with forskolin (Fig. 4c, d). Previously, we had showed that forskolin can cause complete desensitization to the activation of Gs-coupled receptors, but partially attenuate the Gq-mediated DMR.29 Thus, these results suggest that the late ATP response is largely due to the activation of Gs pathway, but the early ATP response is possibly due to the activation of Gq pathway; however, both 2-MeSATP and ADP DMR are mostly due to the activation of Gq pathway.
Fig. 4.
The impact of forskolin pretreatment on P2Y agonist-induced DMR. (a) The ATP DMR in the absence (Control) and presence of 5 μM forskolin. (b) The dose-dependent modulation of the ATP DMR by forskolin. Both early and late ATP P-DMR amplitudes were plotted as a function of forskolin. (c) The 2-MeSATP DMR in the absence (Control) and presence of 5 μM forskolin. (d) The dose-dependent modulation of the 2-MeSATP DMR by forskolin. Both early and late 2-MeSATP P-DMR amplitudes were plotted as a function of forskolin. The concentration was 0.4 μM and 3.2 μM for 2-MeSATP and ATP, respectively. Data presented are means±SEM of four replicate wells (n=4).
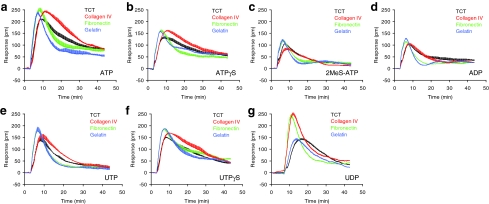
Surface Influence on P2Y Agonist-Mediated DMR Signals
The ECM protein coatings have been widely used for cell culture as well as regulation of dynamic behavior of cells. To examine the surface impact on the signaling of endogenous P2Y receptors in HEK293, the TCT biosensor surface were further coated with three distinct ECM proteins including fibronectin, collagen IV, and gelatin. Examining the characteristics of DMR signals induced by the panel of P2Y agonists, each at a saturating dose, led to several interesting observations (Fig. 5). First, the ECM coating altered the DMR kinetics. A general trend was that fibronectin and gelatin coatings significantly shortened the time to reach the peak, but collagen IV coating slowed down the pace to reach the peak, compared with those on the TCT surface. A similar trend was observed for the decaying kinetics of the N-DMR events—both fibronectin and gelatin coatings accelerated the N-DMR kinetics, whereas collagen IV decreased the N-DMR kinetics. An exception was that UDP on collagen IV-coated surfaces led to faster P-DMR and N-DMR events than those on TCT (Fig. 5g). Second, the ECM coatings altered the signal amplitudes. Generally, all three ECM coatings increased the signaling amplitudes induced by all agonists, but the degree of which is ECM dependent. Third, the sensitivity of DMR signals to distinct ECM coatings was clearly ligand dependent. For ADP and 2-MeSATP, the collagen coating had little impact on their characteristics (Fig. 5c, d), suggesting that P2Y1 signaling is less sensitive to collagen IV coating. However, the UDP DMR was more sensitive to collagen IV than gelatin. Collagen IV greatly increased both amplitude and kinetics of the UDP P-DMR event, whereas gelatin only slightly decreased its P-DMR kinetics but had little impact on its amplitude (Fig. 5g). Fourth, all agonists, except for UDP, gave rise to almost identical DMR on fibronectin- and gelatin-coated surfaces. This is less expected, because gelatin is an irreversibly and partially hydrolyzed form of collagen, and its chemical composition is close to that of its parent collagen. As gelatin is more flexible mechanically than collagen, it is possible that P2Y signaling is sensitive to the mechanical property, rather than chemical composition, of collagen coating, and P2Y1 signaling activated by 2-MeSATP or ADP behaves opposite from the P2Y2 signaling activated by UDP. It has been postulated that cells sense elevated ECM rigidity through integrins and respond with modified signaling.30
Fig. 5.
The comparison of DMR signals induced by P2Y agonists, each at its EC100, of confluent HEK293 cells cultured on four different surfaces (TCT and fibronectin-, collagen IV-, and gelatin-coated biosensor surfaces). (a) ATP; (b) 2-MeSATP; (c) ADP; (d) ATPγS; (e) UTP; (f ) UTPγS; and (g) UDP. The concentration was 2, 2, 100, 10, 16, 16, and 16 μM for 2-MeSATP, ADP, UDP, UTPγS, ATPγS, ATP, and UTP, respectively. Data presented are means±SEM of eight replicate wells (n=3).
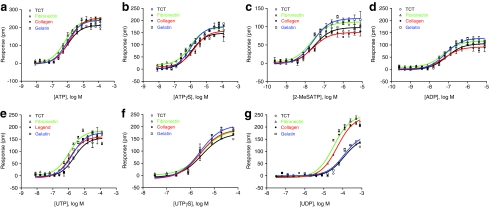
Finally, we examined the potency of all seven agonists on the four different surfaces. Results showed that distinct agonists gave rise to different sensitivity to ECM coatings. Generally, the fibronectin coating increased the potency of all agonists, whereas the gelatin coating had little impact on the potency of all agonists, compared with those on TCT surface (Fig .6 and Table 1). The collagen coating had a more significant impact on the UDP potency than gelatin did. Together, these results clearly suggest that the signaling of endogenous P2Y receptors in HEK293 is sensitive to surface chemistry, and DMR assays are capable of decoding the surface influence on receptor signaling.
Fig. 6.
The comparison of the potency of P2Y agonists to trigger DMR in HEK293 cultured on four different surfaces (TCT and fibronectin-, collagen IV-, and gelatin-coated biosensor surfaces). (a) ATP; (b) ATPγS; (c) 2-MeSATP; (d) ADP; (e) UTP; (f ) UTPγS; and (g) UDP. The P-DMR amplitudes of all P2Y agonists were plotted as a function of agonist concentration. Data presented are means±SEM of triplicate wells (n=3).
Discussion
HEK293 cells express multiple P2Y receptors. However, the expression pattern of P2Y receptors in HEK293 cells is variable in literature, possibly because of difference in subculture. An early RT-PCR study showed that HEK293 expresses P2Y1 and P2Y2, but not P2Y4,23 whereas several RT-PCR studies indicated that HEK293 expresses P2Y1 and P2Y4, but not P2Y2.24,25 HEK293 was also shown to expresses P2Y1124 and possibly P2Y13.9 Our quantitative real-time PCR showed that HEK293 expresses P2Y1, P2Y2, and P2Y11, but not other five P2Y receptors at mRNA level. HEK293 cells lack expression of P2X1–7.31
Lacking highly specific pharmacological tools makes it difficult to characterize receptor biology and ligand pharmacology. Here, we applied DMR assays afforded by label-free optical biosensors to examine the pharmacology of a panel of P2Y agonists including ADP, 2-MeSATP, ATP, ATPγS, UTP, UTPγS, and UDP and the influence of ECM coatings on ligand pharmacology. To characterize the ligand pharmacology, we relied on four different approaches. First, we used DMR agonism assays to examine the ability of ligands to trigger DMR signals. Results showed that all ligands examined led to a dose-dependent and saturable DMR, resulting in a single EC50. The DMR signals induced by the panel of agonists shared similar dynamics, but differed greatly in amplitudes (early and late responses) and kinetics. Further, different agonists exhibited different potency. Second, we used DMR antagonism assays to investigate the ability of known P2Y1 antagonist MRS2179 to block all agonist-induced DMR signals. The resultant modulation profiles indicate that both ADP and 2-MeSATP preferentially activate P2Y1 receptors, whereas the DMRs induced by UDP, UTP, and UTPγS are not related to the activation of P2Y1 receptor, and ATP and ATPγS activate more receptor(s) beside P2Y1 receptor. Third, we used DMR costimulation assays to further differentiate the receptor specificity. Results showed that both ADP and 2-MeSATP activate the same receptor, whereas UTP and UTPγS preferentially activate a receptor different from P2Y1, and ATP and ATPγS activate all three receptors. As the high potency of ADP to activate P2Y1 and ATP may contain trace amount of ADP, we cannot rule out the possibility that instead of ATP itself the contaminant ADP causes the activation of P2Y1. Fourth, we used the forskolin modulation profiles to further differentiate ligand pharmacology. The modulation profiles of different agonist-induced DMR by forskolin further suggest that the late response of the ATP DMR is mostly due to the activation of Gs pathway. However, we cannot rule out the possibility that the divergent late responses induced by different agonists may reflect the differences in the propensity of each ligand to promote receptor desensitization. Nonetheless, these results indicate that HEK293 expresses functional P2Y1, P2Y2, and P2Y11 receptors (Fig. 7). The activation of all three receptors in HEK293 was known to mediate signaling primarily via Gq pathway.23,24 The activation of Gq-coupled P2Y receptors causes sequential activation of trimeric G proteins and phospholipase C (PLC). The PLC then hydrolyzes the membrane lipid phosphatidylinositol bisphosphate, producing inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to and opens a calcium channel in the endoplasmic reticulum, leading to calcium mobilization. Both DAG and calcium interact with and activate protein kinase C (PKC). The activated PKC phosphorylates many different protein targets including small GTPase Rho, leading to the remodeling of cytoskeletal structure. We also found that the activation of P2Y receptors, possibly P2Y11, by ATP and ATPγS also led to Gs-mediated signaling. The activation of P2Y receptor(s) by different ligands results in distinct patterns of protein trafficking, cytoskeletal remodeling, and alterations in cell adhesion, thus leading to distinct DMR signatures for these ligands.
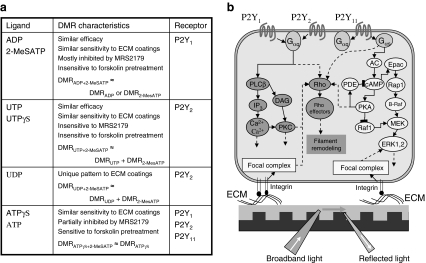
Fig. 7.
Ligands and their DMR characteristics and signaling. (a) A table summarizing key DMR characteristics of each ligand. (b) A schematic illustrating the influence of ECM coatings on the signaling of endogenous P2Y receptors, as recorded using resonant waveguide grating biosensor-based DMR assays. ECM, extracellular matrix.
The influence of ECM coatings on the ligand pharmacology was also examined using DMR assays. First, the surface coating impacted the potency of P2Y agonists in an ECM-dependent manner. Compared with those on the TCT surfaces, fibronectin coating increased the potency of all agonists, whereas gelatin had little impact on the potency of all agonists. Second, the surface coating impacted the efficacy of P2Y agonists. All three ECM coatings generally increased the DMR amplitudes of all agonists. Third, the surface coating impacted the characteristics of P2Y agonist-induced DMR signals, particularly the kinetics. A notable finding was that the P2Y1 signaling induced by 2-MeATP or ADP is more sensitive to gelatin than collagen IV, but an opposite sensitivity was observed for the UDP response. Together, these results suggest that the ECM coating has impact on the signaling of endogenous P2Y receptors. A possible cellular mechanism for the surface influence on receptor signaling is that cell surface integrins bind to the ECM proteins to form adhesion complexes, which in turn lead to activation of the Rho kinase pathway and/or ERK30 (Fig. 7b). The distinct interactions of HEK293 cells with different ECM coatings determine how the cells are adherent on the surface and how the adhesion complexes are formed and organized, which in turn shapes receptor signaling.
In conclusion, we have used DMR assays to study the ligand pharmacology and signaling of a panel of P2Y agonists acting on endogenous P2Y receptors in HEK293 cells cultured on four distinct surfaces. Multiple assay formats not only differentiate the origin of distinct agonist-induced DMR signals, but also decode the surface influence on the signaling of endogenous P2Y receptors in HEK293 cells. Given that distinct types of cells inhabit different environments in vivo, it is reasonable to speculate that the influence of surface chemistry on receptor signaling would be dependent on both cell backgrounds and receptors. Further investigations of different model systems including primary cells and stem cells will offer insights about the roles and mechanisms of surfaces in regulating cell biology. Nonetheless, the data presented here clearly document the rich texture of DMR assays for receptor biology studies.
Abbreviations
- 2MeSATP
2-methylthio-ATP
- ADP
adenosine diphosphate
- ATP
adenosine 5′-triphosphate
- DAG
diacylglycerol
- DMR
dynamic mass redistribution
- ECM
extracellular matrix
- GPCR
G protein-coupled receptor
- IP3
inositol triphosphate
- N-DMR
negative-DMR
- P2Y
Purinergic 2Y
- PCR
polymerase chain reaction
- P-DMR
positive DMR
- PKC
protein kinase C
- PLC
phospholipase C
- RT-PCR
real time PCR
- TCT
tissue culture-treated
- UDP
uridine diphosphate
- UTP
uridine triphosphate
- UTPγS
uridine 5-thiotriphosphate
Acknowledgment
This work was supported partially by National Institutes of Health (Grant No. 5U54MH084691).
Disclosure Statement
E.T., H.S., and Y.F. are employees and shareholders of Corning, Inc.
References
- 1.Engler AJ. Sen S. Sweeney HL. Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Giancotti FG. Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 3.Mrksich M. What can surface chemistry do for cell biology? Curr Opin Chem Biol. 2002;6:794–797. doi: 10.1016/s1367-5931(02)00362-9. [DOI] [PubMed] [Google Scholar]
- 4.Discher DE. Janmey P. Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 5.Groves JT. Learning the chemical language of cell-surface interactions. Sci STKE. 2005;301:pe45. doi: 10.1126/stke.3012005pe45. [DOI] [PubMed] [Google Scholar]
- 6.Stevens MM. George JH. Exploring and engineering the cell surface interface. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 7.Millon-Fremillon A. Bouvard D. Grichine A. Manet-Dupe S. Block MR. Albiges-Rizo C. Cell adaptive response to extracellular matrix density is controlled by ICAP-1-dependent β1-integrin affinity. J Cell Biol. 2008;180:427–441. doi: 10.1083/jcb.200707142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbracchio MP. Burnstock G. Boeynaems JM. Barnard EA. Boyer JL. Kennedy C. Knight GE. Fumagalli M. Gachet C. Jacobson KA. Weisman GA. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirkner K. Schweigel J. Gerevich Z. Franke H. Allgaier C. Barsoumian EL. Draheim H. Illes P. Adenine nucleotides inhibit recombinant N-type calcium channels via G protein-coupled mechanisms in HEK 293 cells: involvement of the P2Y13 receptor-type. Br J Pharmacol. 2004;141:141–151. doi: 10.1038/sj.bjp.0705588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galandrin S. Oligny-Longpre G. Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;8:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Fang Y. Ferrie AM. Fontaine NH. Yuen PK. Characteristics of dynamic mass redistribution of EGF receptor signaling in living cells measured with label free optical biosensors. Anal Chem. 2005;77:5720–5725. doi: 10.1021/ac050887n. [DOI] [PubMed] [Google Scholar]
- 12.Fang Y. Li G. Peng J. Optical biosensor provides insights for bradykinin B2 receptor signaling in A431 cells. FEBS Lett. 2005;579:6365–6374. doi: 10.1016/j.febslet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Fang Y. Label-free cell-based assays with optical biosensors in drug discovery. Assay Drug Dev Technol. 2006;4:583–595. doi: 10.1089/adt.2006.4.583. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y. Li G. Ferrie AM. Non-invasive optical biosensor for assaying endogenous G protein-coupled receptors in adherent cells. J Pharmacol Toxicol Methods. 2007;55:314–322. doi: 10.1016/j.vascn.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Lee PH. Gao A. van Staden C. Ly J. Salon J. Xu A. Fang Y. Verkleeren R. Evaluation of dynamic mass redistribution technology for pharmacological studies of recombinant and endogenously expressed G protein-coupled receptors. Assay Drug Dev Technol. 2008;6:83–93. doi: 10.1089/adt.2007.126. [DOI] [PubMed] [Google Scholar]
- 16.Kenakin T. Cellular assays as portals to seven-transmembrane receptor-based drug discovery. Nat Rev Drug Discov. 2009;8:617–626. doi: 10.1038/nrd2838. [DOI] [PubMed] [Google Scholar]
- 17.Schröder R. Janssen N. Schmidt J. Kebig A. Merten N. Hennen S. Müller A. Blättermann S. Mohr-Andrä M. Zahn S. Wenzel J. Smith NJ. Gomeza J. Drewke C. Milligan G. Mohr K. Kostenis E. Deconvolution of complex G protein-coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat Biotechnol. 2010;28:943–949. doi: 10.1038/nbt.1671. [DOI] [PubMed] [Google Scholar]
- 18.Codd EE. Mobus JR. Murray BS. Zhang SP. Flores CM. Dynamic mass redistribution as a means to measure and differentiate signaling via opioid and cannabinoid receptors. Assay Drug Dev Technol. 2011;9:362–372. doi: 10.1089/adt.2010.0347. [DOI] [PubMed] [Google Scholar]
- 19.Fang Y. Ferrie AM. Fontaine NH. Mauro J. Balakrishnan J. Resonant waveguide grating biosensor for living cell sensing. Biophys J. 2006;91:1925–1940. doi: 10.1529/biophysj.105.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang Y. Ferrie AM. Label-free optical biosensor for ligand-directed functional selectivity acting on β2 adrenoceptor in living cells. FEBS Lett. 2008;582:558–564. doi: 10.1016/j.febslet.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Fang Y. Non-invasive optical biosensor for probing cell signaling. Sensors. 2007;7:2316–2329. doi: 10.3390/s7102316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang Y. Label-free receptor assays. Drug Discov Today Technol. 2010;7:e5–e11. doi: 10.1016/j.ddtec.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schachter LB. Sromek SM. Nicholas RA. Harden TK. HEK 293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology. 1997;36:1181–1187. doi: 10.1016/s0028-3908(97)00138-x. [DOI] [PubMed] [Google Scholar]
- 24.Moore DJ. Chambers JK. Wahlin JP. Tan KB. Moore GB. Jankins O. Emson PC. Murdock RP. Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription–polymerase chain reaction study. Biochem Biophys Acta. 2001;1521:107–119. doi: 10.1016/s0167-4781(01)00291-3. [DOI] [PubMed] [Google Scholar]
- 25.Fischer W. Wirkner K. Weber M. Eberts C. Koles L. Reinhardt R. Franke H. Allgaier C. Gillen C. Illes P. Characterization of P2X3, P2Y1 and P2Y4 receptors in cultured HEK 293-hP2X3 cells and their inhibition by ethanol and trichloroethanol. J Neurochem. 2003;85:779–790. doi: 10.1046/j.1471-4159.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- 26.Houslay MD. Milligan G. Tailoring cAMP-signalling responses through isoforms multiplicity. Trends Biochem Sci. 1997;22:217–244. doi: 10.1016/s0968-0004(97)01050-5. [DOI] [PubMed] [Google Scholar]
- 27.Willoughby D. Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 28.Tran E. Fang Y. Duplexed label-free G protein-coupled receptor assays for high throughput screening. J Biomol Screen. 2008;13:975–985. doi: 10.1177/1087057108326141. [DOI] [PubMed] [Google Scholar]
- 29.Tran E. Fang Y. Label-free optical biosensor for probing integrative role of adenylyl cyclase in G protein-coupled receptor signaling. J Recept Signal Transduct. 2009;29:154–162. doi: 10.1080/10799890903052544. [DOI] [PubMed] [Google Scholar]
- 30.Larsen M. Artym VV. Green JA. Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Humphreys BD. Virginio C. Surprenant A. Rice J. Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]