Abstract
Inflammation and angiogenesis play a crucial role in the pathomechanism of diabetic nephropathy. Monocyte chemoattractant protein 1 (MCP) is a key regulator of the immune system in kidneys, and its inhibition with a dominant-negative mutant lacking the N-terminal amino acids 2–8 (7ND) reduces renal fibrosis. Angiomotin (Amot) is a novel angiogenesis modulator. We studied the effects of inhibition of Amot and MCP using DNA vaccination on incipient diabetic nephropathy in rats. Plasmid DNA (with either 7ND or human Amot) was electroporated twice into hind-limb muscles of rats with streptozotocin-induced diabetes mellitus. Sham-electroporated diabetic rats and healthy animals served as controls. After 4 months, renal histology and biochemical analyses were performed. In sham-electroporated diabetic rats, glomerular histology revealed pathological changes. 7ND and Amot treatments reduced glomerular hypertrophy and periodic acid–Schiff positivity. In both treated groups, the expression of profibrotic (transforming growth factor-β, collagen 1), proinflammatory (interleukin-6, tumor necrosis factor-α), and proangiogenic (vascular endothelial growth factor) genes in the renal cortex was lower than in the diabetic group without treatment. The mentioned renoprotective effects could be mediated via higher total antioxidant capacity and improved glycemic control. Anti-angiogenic and anti-inflammatory DNA vaccination ameliorates the progression of glomerular pathology in an animal model of diabetic nephropathy.
Monocyte chemoattractant protein-1 (MCP1) is a key regulator of the immune system in kidneys. Angiomotin (Amot) is a recently identified angiogenesis regulator. Both Amot and MCP1 are thought to play a role in diabetic nephropathy (DN). In this report, Celec and colleagues demonstrate that DNA vaccination against Amot or monocyte chemoattractant protein-1 (MCP1) retards the progression of DN in rats with streptozotocin-induced diabetes mellitus.
Introduction
Diabetic nephropathy (DN) is one of the main causes of end-stage renal disease. Based on the pathophysiology, it can be attributed to microangiopathy, a chronic complication of diabetes mellitus (Zent and Pozzi, 2007). Pathological changes in DN include glomerular hypertrophy, glomerulosclerosis, and proteinuria (Raptis and Viberti, 2001). Despite intensive research and tremendous improvement in the understanding of the pathogenesis of DN, effective treatment(s) halting completely the progression of the disease are still lacking. Thus, therapeutic targeting of pathophysiologically important molecular pathways not affected by the currently available therapeutic arsenal is to be considered (Shah et al., 2009).
Although there is a lack of agreement regarding the molecular pathogenesis of DN, based on recent clinical and experimental studies angiogenesis and inflammation seem to play a major role (Rivero et al., 2009; Vlassara et al., 2009). In obese Zucker rats, up-regulation of inflammatory mediators and activation of the respective pathways represent a crucial pathomechanism of DN (Xu et al., 2005). Inflammation induces and/or aggravates oxidative stress, which further potentiates the renal pathology (Chander et al., 2004). Recent studies revealed associations of DN with polymorphisms of the gene encoding interleukin-6 (IL-6) receptor (Wang et al., 2005) and with systemic markers of inflammation (Stehouwer et al., 2002; Saraheimo et al., 2003). In patients with DN, urinary levels of monocyte chemoattractant protein-1 (MCP) are higher (Tashiro et al., 2002). This is in line with the finding of increasing macrophage influx, correlating with the severity of DN (Lewis et al., 2008). Anti-inflammatory treatment substantially reduced renal pathology, and the excretion of MCP, in an animal model of DN (Awad et al., 2006). The up-regulation of MCP in DN was shown already a decade ago (Wada et al., 2000). It seems to be induced by both glucose and glycated proteins (Banba et al., 2000). The importance of MCP in the pathogenesis of DN has been clearly demonstrated in the model of streptozotocin-induced diabetes in MCP knockout mice (Chow et al., 2006). 7ND is a dominant-negative mutant form of MCP lacking the N-terminal amino acids 2–8 that effectively inhibits MCP. It has been used successfully in several experiments targeting MCP (Göser et al., 2005; Kanamori et al., 2007; Koga et al., 2008; Kaya et al., 2011). Thus, using 7ND provides a rationale for anti-inflammatory treatment based on macrophage influx inhibition.
Recently, Nakagawa et al. (2009) reviewed the role of angiogenesis in DN. Much effort has been put into the research on vascular endothelial growth factor (VEGF) and its role in the pathogenesis of DN, which might be similar to that in diabetic retinopathy (Chen and Ziyadeh, 2008). Genetic polymorphisms of the VEGF gene are associated with a higher risk of DN (Yang et al., 2003). Patients in an early stage of DN display higher VEGF concentrations in plasma and urine (Kim et al., 2004). In human DN, glomerular pathological changes correlated with glomerular VEGF expression (Kanesaki et al., 2005). Similar findings were reported also in animal models of DN (Cha et al., 2004). Except for VEGF, the main regulator of angiogenesis, several other angiogenic factors were explored regarding their role in tumor growth (Ferrara and Alitalo, 1999). However, their function in other pathologies is less clear. One of these emerging angiogenic factors is angiomotin (Amot) (Troyanovsky et al., 2001). It has been shown that DNA vaccination with Amot very effectively blocked tumor growth in a mouse model (Holmgren et al., 2006). The ability of Amot DNA vaccination to inhibit angiogenesis and the successful use of anti-angiogenic treatment in the prevention of DN represent a rationale for the use of Amot DNA vaccination in our study. In addition, experimental inhibition of Amot could help in the understanding of the complex interplay between various angiogenic factors in renal pathology. The use of Amot as a target for DNA vaccination represents a novel approach in the anti-angiogenic therapy of DN.
The aim of our study was to study the effects of Amot and MCP inhibition on the progression of incipient DN by using DNA vaccination in diabetic rats. A preventive approach before the onset of any functional changes was chosen to monitor potential effects on early morphological alterations with the focus on profibrotic factors and oxidative stress.
Materials and Methods
Animal model of diabetes
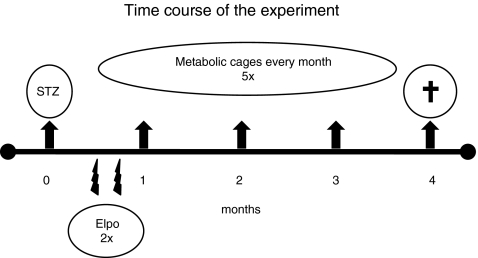
Ten male Wistar rats (Anlab, Prague, Czech Republic; 200 g) served as healthy controls (CTRL). In another 40 rats, diabetes was induced by the intraperitoneal injection of streptozotocin (50 mg/kg in 0.01 mol/L citrate buffer). One week later, the 30 rats displaying fasting glycemia of >15 mmol/L were randomly divided into three groups: DM, 7ND, and Amot (10 rats/group). Two and three weeks after the induction of diabetes, two groups of diabetic rats (7ND, Amot) were injected twice with 50 μg of plasmid DNA in a volume of 100 μl of Tris-EDTA (TE) buffer (pH 8) intramuscularly. Plasmids were injected obliquely 0.3 cm deep into the Tibialis anterior muscle of both hind limbs using an insulin syringe (2×100 μg cumulative dose). Rats in the CTRL and DM groups received the same volume of TE buffer. Plasmid construct pcDNA3-7ND was kindly provided by Dr. Kensuke Egashira (Egashira et al., 2000), and pcDNA3-Amot was kindly provided by Dr. Federica Cavallo (Holmgren et al., 2006). The expression of genes on these plasmids is driven by the constitutive eukaryotic cytomegalovirus (CMV) promoter. Plasmids were amplified in Escherichia coli DH5α cells and purified with a commercially available kit (Plasmid Maxi Prep; Qiagen, Hilden, Germany). After injection, in vivo electroporation (300 V/cm, 3×3 pulses, 1 Hz, 100 ms duration) was applied in all rats using a Grass S88 square pulse stimulator (Grass Technologies, West Warwick, RI). The hair on the hind-limb area was removed to ensure tight contact between electrodes and skin. The shape of the electric pulses was a square wave, meaning that the voltage remained constant during the pulse duration. Three series of three electric pulses were used, each series with opposite polarity. Once a month, rats were placed into metabolic cages for 24 hr of urine collection, and proteinuria and creatinine concentration were determined. After each collection, fasting blood samples were obtained from the tail vein. Plasma creatinine and glucose were measured, and creatinine clearance was calculated. Blood pressure was measured noninvasively by tail plethysmography once a month. Four months after the induction of diabetes, rats were sacrificed by exsanguination in general anesthesia. Kidneys were harvested for histological and biochemical analyses. The time course of the experiment is shown in Fig. 1.
FIG. 1.
Time course of the experiment. Two and three weeks after streptozotocin (STZ) injections, plasmid DNA was applied by intramuscular injections and in vivo electroporation (Elpo). Arrows show that every month rats were placed into metabolic cages for 24 hr. After 4 months, rats were sacrificed to collect blood and tissue samples.
The study was approved by the Ethics Committee of the Institute of Pathophysiology, Comenius University, Bratislava, Slovakia.
Biochemical analysis
Homogenates of renal cortex (10%) were prepared from samples frozen in liquid nitrogen by mechanical disruption in PBS. Homogenates were centrifuged (10,000 g, 5 min), and supernatants were collected. In the collected homogenates, supernatants, and plasma samples, markers of oxidative stress and proteins were determined. Data on markers of oxidative stress were corrected for protein levels, determined by using the Lowry method.
Malondialdehyde (MDA) was quantified spectrophotometrically by a modified version of the original assay (Ohkawa et al., 1979). In brief, 20 μl of homogenates or plasma was mixed with 20 μl of 0.67% thiobarbituric acid (TBA), 20 μl of glacial acetic acid, and 30 μl of distilled water. The mixture was heated to 95°C for 45 min. After cooling, the MDA-TBA adducts were extracted using n-butanol. Absorbance was measured at 532 nm. 1,1,3,3-Tetramethoxypropane was used for the calibration curve.
Fructosamine was determined spectrophotometrically by a modified protocol according to a previously published method (San-Gil et al., 1985). Samples were mixed with nitro blue tetrazolium solution in sodium carbonate buffer. After incubation (37°C, 15 min), absorbance was measured at 530 nm. 1-Deoxymorpholino-D-fructose was used for the calibration curve.
Total antioxidant capacity (TAC) was measured according to Erel (2004), by mixing the samples with acetate buffer, and 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS reagent) with hydrogen peroxide. The color reaction was monitored spectrophotometrically at 660 nm. Trolox was used as the standard.
Ferric reducing ability of plasma (FRAP) or tissue (FRAT) was analyzed as described previously (Benzie and Strain, 1996). Fe3+ was reduced to Fe2+ in the presence of 2,4,6-tripyridyl-s-triazine (TPTZ reagent) by mixing the prewarmed FRAP solution (37°C) with the samples. Absorbance was read after 4 min at 593 nm. FeSO4 was used for the calibration curve.
Hydroxyproline (OH-Pro) was measured in the homogenates after acidic hydrolysis (24 hr, 120°C). Samples were incubated for 30 min in acetate/citrate buffer with chloramine-T. After incubation with Ehrlich's reagent (65°C, 30 min), absorbance at 550 nm was recorded (Sisson et al., 1999). Synthetic OH-Pro was used as the standard.
Creatinine and urea were determined (Vitros 250; Johnson & Johnson, Rochester, NY). Proteinuria was analyzed using the pyrogallol red method. Glycemia was measured using a standard blood glucose meter (Abbott Diabetes Care, Alameda, CA).
RNA was isolated from homogenized samples of the renal cortex using the TRI Reagent (MRC, Cambridge, UK). The quantity and quality of RNA were checked spectrophotometrically. Expression of IL-6, tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β1), collagen 1 (COL-1), and VEGF was analyzed using real-time RT-PCR using SYBR Green (QuantiFast SYBR Green One Step RT PCR kit; Qiagen). The expression was quantified using the ΔΔCt method against the average Ct value of housekeeping genes β-actin, glyceraldehyde-3-phosphate dehydrogenase, and cyclophilin A, and presented in arbitrary units.
Histological analysis
Harvested renal cortical samples were fixed in formalin and embedded in paraffin. Hematoxylin–eosin and periodic acid–Schiff (PAS) stained sections were subjected to morphometric analysis of glomeruli as described previously (Boor et al., 2009). Glomerular tuft area and PAS positivity as a marker of glomerulosclerosis were determined using the Image Tool version 3 software. At least 50 consecutive glomeruli per kidney slice were evaluated in a blinded manner by a single observer (M.P.).
Statistical analysis
Data were analyzed using one-way ANOVA with the least significant difference post hoc test. P values less than 0.05 were considered significant. Microsoft Excel 2007 and XL Statistics 5 were used for calculations and statistical testing. Data are presented as means+SEM.
Results
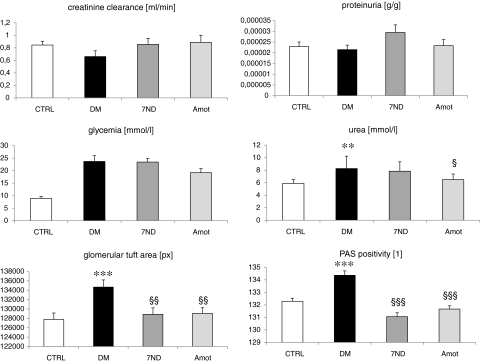
All diabetic rats had higher glycemia levels in comparison with the CTRL group (23.7+6.9 mmol/L vs. 8.9+1.2 mmol/L; p<0.001). Rats in the Amot group had slightly lower glycemia levels than other diabetic groups, but significance was not reached. Plasma urea concentrations were higher in the DM group (∼116%; p<0.01). In our study, we did not measure the production of Amot and 7ND proteins due to lack of access to corresponding antibodies. We have confirmed the production of both transgenes using real-time RT-PCR of muscle samples. The PCR was positive only in the corresponding groups, as Amot and 7ND are not expressed in normal healthy muscle tissue. Data on renal function and morphometric parameters of glomeruli are shown in Fig. 2. Creatinine clearance was lower in diabetic rats than in control rats; however, due to interindividual variability, this difference (∼21%) did not reach the level of significance. Both treated groups had creatinine clearance similar to control rats. We have found no significant differences between groups in proteinuria. In both treatment groups, plasma urea levels were lower in comparison with that in the DM group. Renal histology revealed a larger glomerular tuft area (by 5.5%; p<0.001) and a higher PAS positivity (by 1.5%; p<0.001) in the diabetic rats (DM) when compared with the controls. DNA vaccination with 7ND and Amot attenuated these changes in glomerular tuft area (by 4% in both groups; p<0.01) and in PAS positivity (by 2% in both groups; p<0.001). No significant differences in systolic blood pressure were recorded between the groups (data not shown).
FIG. 2.
Parameters of renal function and histology. No significant differences between groups were found in creatinine clearance, plasma creatinine, and proteinuria. Plasma urea levels, glomerular size, and PAS positivity were higher in diabetic rats. Therapeutic interventions lowered the parameters significantly, except for urea in the 7ND group. **p<0.01 vs. CTRL; ***p<0.001 vs. CTRL; §p<0.05 vs. DM; §§p<0.01 vs. DM; §§§p<0.001 vs. DM.
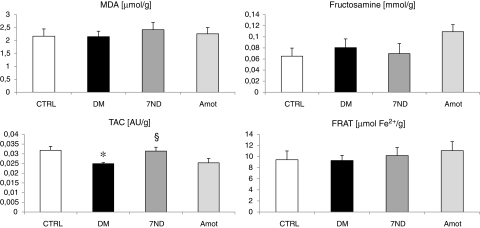
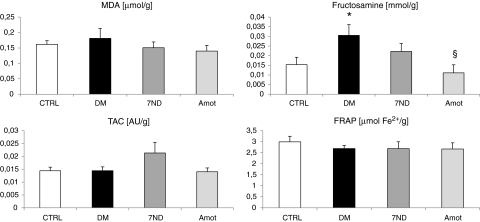
Markers of oxidative stress in the renal cortex and in plasma are shown in Figs. 3 and 4, respectively. MDA levels in plasma were slightly higher in the DM group (by 12%). Both treatments reduced the MDA levels even below the levels of the CTRL group (by 17% and 23%, respectively), without reaching significance. Similarly, plasma fructosamine was higher in the DM group in comparison with the CTRL group (approximately twofold higher; p<0.05). However, in the renal cortex, fructosamine content was comparable. DNA vaccination with 7ND resulted in lower fructosamine (by 27%, not significant), whereas in the Amot group plasma fructosamine was lower than that in the DM group by 64% (p<0.05). TAC was the single parameter displaying significant differences between groups in the renal cortex. Diabetic rats had similar TAC in plasma, but lower TAC in the renal cortex, than control rats (by 22%; p<0.05). 7ND treatment resulted in higher TAC in the renal cortex if compared with the DM group. None of the treatments affected FRAP in plasma or FRAT in the renal cortex.
FIG. 3.
Oxidative stress parameters in renal cortex. No differences between groups were found in MDA, fructosamine, and FRAT. Diabetic rats had significantly lower TAC of the renal cortex. Treatment with 7ND increased TAC to normal values. *p<0.05 vs. CTRL; §p<0.05 vs. DM.
FIG. 4.
Oxidative stress parameters in plasma. No differences between groups were found in MDA, TAC, and FRAP. Diabetic rats had significantly higher plasma fructosamine. Treatment with Amot attenuated the increase of fructosamine. *p<0.05 vs. CTRL; §p<0.05 vs. DM.
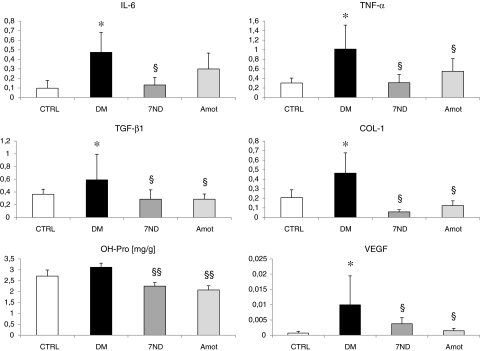
Results from the analysis of gene expression and OH-Pro as a marker of fibrosis are shown in Fig. 5. OH-Pro in the renal cortex was higher in the DM group in comparison with the CTRL group, but significance was not reached (15%). DNA vaccination with 7ND or with Amot reduced the OH-Pro levels significantly when compared with the DM group (28% and 33% for 7ND and Amot, respectively; both p<0.01). The expression of all analyzed genes in the renal cortex was higher in diabetic rats than in control rats. 7ND and Amot lowered the expression of these genes significantly (p<0.05), except for IL-6 in the Amot group (Fig. 4).
FIG. 5.
OH-Pro and expression of genes related to inflammation, fibrosis, and angiogenesis in the renal cortex. The expression of all analyzed genes was higher in diabetic rats. Treatment with 7ND or Amot attenuated the increase, except for IL-6 in the Amot group. OH-Pro was lower in both treatment groups in comparison with the DM group. *p<0.05 vs. CTRL; §p<0.05 vs. DM; §§p<0.01 vs. DM.
Discussion
The DNA vaccination was initially used in the treatment and/or prevention of AIDS (Barouch et al., 2000), tuberculosis (Lowrie et al., 1999), and malaria (Schneider et al., 1998). Later studies focused on noninfectious diseases, especially cancer (Prud'homme, 2005). A dramatic improvement in the efficiency of this approach was achieved by the introduction of in vivo electroporation (Buchan et al., 2005). Experimental autoimmune diabetes mellitus was prevented by DNA vaccination with glutamate decarboxylase, however, only with concurrent application of expression plasmid encoding the anti-inflammatory IL-4 (Tisch et al., 2001). Treatment with TGF-β or intercellular adhesion molecule-1 DNA resulted in increased pancreatic expression of IL-10 and in the modulated activity of immunocompetent cells preventing insulitis in diabetes-prone NOD (nonobese diabetic) mice (van den Engel et al., 2002). Immunomodulatory DNA vaccination prevented the diabetes induction in NOD mice (Herrera et al., 2006; Goudy et al., 2008). According to our knowledge, DNA vaccination has not been tested yet in the therapy/prevention of diabetic complications including nephropathy on an established diabetes model.
The ability of both plasmid constructs to block angiogenesis and inflammation has already been shown in vivo. Amot was successfully used in a xenograft model of breast cancer to inhibit angiogenesis and tumor growth (Holmgren et al., 2006). 7ND, beyond other applications, has been effective in the prevention of renal fibrosis in unilateral ureteral obstruction (Wada et al., 2004). In our study, both constructs were used for the first time in a model of diabetes mellitus.
In a model of DN, we have investigated the potential preventive effects of anti-inflammatory and anti-angiogenic DNA vaccination on early morphological changes associated with incipient DN. Our results show that treatment with both 7ND and Amot DNA resulted in attenuation of diabetes-induced glomerular hypertrophy and glomerulosclerosis. These effects were not dependent on blood pressure. In line with the known slow progression of DN in this model, most of the functional parameters were affected by neither diabetes nor the treatment (Tesch and Allen, 2007). Analysis of markers of oxidative stress points toward potential mechanisms of action: by higher TAC at least by 7ND treatment and by lower fructosamine production at least by Amot treatment. Higher TAC in rats treated with 7ND might be the cause of the local anti-inflammatory effect of 7ND. Lower production of MCP-induced production of reactive oxygen species might lead to increased TAC (Volk et al., 2000). This explanation is, however, speculative and requires further research. The altered oxygen metabolism in DN leading to oxidative and carbonyl stress has been reviewed recently (Miyata and de Strihou, 2009). Hypoxia in the renal cortex might be both the cause and the consequence of dysregulated angiogenesis. It can be supposed that improved intracellular metabolism of glucose leading to lower concentrations of glycating agents might be the cause of the observed lower fructosamine levels in the Amot group.
Anti-angiogenic and anti-inflammatory therapeutic approaches have been proved experimentally in animal models of DN in the past (Zent and Pozzi, 2006; Tesch, 2008). Interventions include applications of anti-angiogenic peptides like endostatin (Ichinose et al., 2005), tumstatin (Yamamoto et al., 2004), or antibodies against VEGF (De Vriese et al., 2001; Flyvbjerg et al., 2002). Recently, the renoprotective effects of anti-angiogenic adenoviral mediated gene therapy were reported in streptozotocin-induced diabetes using vasohibin-1 (Nasu et al., 2009). Inhibition of inflammation associated with DN was achieved by anti-inflammatory agents, such as mycophenolate mofetil (Utimura et al., 2003), methotrexate (Yozai et al., 2005), or statins (Usui et al., 2003), which are used clinically for other indications. On the other hand, the effects of some drugs already used in clinical practice to treat DN were revealed to be mediated by anti-inflammatory mechanisms [spironolactone (Han et al., 2006), thiazolidinedione (Ohga et al., 2007)]. Interestingly, experimental studies indicate that both mechanisms (angiogenesis and inflammation) are highly interconnected, and alterations in one of the pathways induce changes in the other one (Wang et al., 2008; Mu et al., 2009).
DNA vaccination has several important advantages to peptide application. The preparation of peptides is expensive and must be repeated. The expression of target proteins by host cells ensures correct folding. Our study has, on the other hand, several limitations. The preventive effect of DNA vaccination could not be shown on functional renal parameters, as in our experiment they were not changed by 4 months of untreated diabetes. The use of plasmid vector with a constitutive promoter prevents any possible regulation of expression. Moreover, a bacterial delivery approach might be more effective in the activation of the immune system. The promoter that drives the expression from the plasmid vectors (CMV promoter) is an early strong promoter providing the highest level of expression among several different eukaryotic promoters at day 5 after gene electroporation into the muscle (Durieux et al., 2005). Strong expression of the transgene is crucial for the presentation of foreign antigen to immunocompetent cells and efficient vaccination. After plasmid electroporation into the muscle, the transgene is being expressed and the product secreted into the bloodstream (Maruyama et al., 2000). Thus, it is easily accessible to antigen-presenting cells in sufficient amounts to trigger the systemic immune response.
Electroporation protocols may vary substantially between research groups, depending on specific application needs and the techniques used. The electroporation conditions used in our study were based on data from relevant research articles on rat muscle electroporation. We used a total of 100 μg of DNA in an injection volume of 100 μl (Durieux et al., 2005), a voltage of 300 V/cm (Cukjati et al., 2007), and multiple DNA injections along with bidirectional application of electric pulses, which was previously optimized and known to provide a safe and highly efficient method for therapeutic gene delivery into skeletal muscle (Maruyama et al., 2000; Saito et al., 2006). It is also known that electroporation-related muscle damage increases with transfection efficiency, i.e., with the amount of DNA injected, as reported previously by other research groups (Durieux et al., 2004). However, we did not analyze the extent of muscle damage in our study.
Based on data available from the literature, the proposed mechanism of action of the tested treatment is DNA vaccination, i.e., the immune reaction against the product of the transgenes expressed in host mammalian cells. Although we cannot definitely confirm this mechanism, because the presence of neither cellular nor humoral immune response was analyzed in our study, we have chosen constructs and protocols established and proved successful in other published studies (Egashira et al., 2000; Holmgren et al., 2006; Koga et al., 2008).
In conclusion, our data show that DNA vaccination with anti-angiogenic and anti-inflammatory agents retards the progression of DN in streptozotocin-induced diabetes in rats. Attenuation of oxidative and carbonyl stress might at least partially explain the mechanism of action. Whether a combination of both treatments has any potential synergism remains to be solved in future studies, especially in those focused on the therapeutic effects in established DN.
Acknowledgments
This study was supported by Slovak Research and Development Agency grant APVV-0754-10. The publication charges were paid by Biomedox, Inc.
Author Disclosure Statement
No competing financial interests exist.
References
- Awad A.S. Huang L.P. Ye H., et al. Adenosine A(2A) receptor activation attenuates inflammation and injury in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- Banba N. Nakamura T. Matsumura M., et al. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000;58:684–690. doi: 10.1046/j.1523-1755.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- Barouch D.H. Santra S. Schmitz J.E., et al. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- Benzie I.F. Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Boor P. Celec P. Behuliak M., et al. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism. 2009;58:1669–1677. doi: 10.1016/j.metabol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Buchan S. Gronevik E. Mathiesen I., et al. Electroporation as a “prime/boost” strategy for naked DNA vaccination against a tumor antigen. J. Immunol. 2005;174:6292–6298. doi: 10.4049/jimmunol.174.10.6292. [DOI] [PubMed] [Google Scholar]
- Cha D.R. Kang Y.S. Han S.Y., et al. Vascular endothelial growth factor is increased during early stage of diabetic nephropathy in type II diabetic rats. J. Endocrinol. 2004;183:183–194. doi: 10.1677/joe.1.05647. [DOI] [PubMed] [Google Scholar]
- Chander P.N. Gealekman O. Brodsky S.V., et al. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: prevention by chronic therapy with a peroxynitrite scavenger ebselen. J. Am. Soc. Nephrol. 2004;15:2391–2403. doi: 10.1097/01.ASN.0000135971.88164.2C. [DOI] [PubMed] [Google Scholar]
- Chen S. Ziyadeh F.N. Vascular endothelial growth factor and diabetic nephropathy. Curr. Diabetes Rep. 2008;8:470–476. doi: 10.1007/s11892-008-0081-3. [DOI] [PubMed] [Google Scholar]
- Chow F.Y. Nikolic-Paterson D.J. Ozols E., et al. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- Cukjati D. Batiuskaite D. Andre F., et al. Real time electroporation control for accurate and safe in vivo non-viral gene therapy. Bioelectrochemistry. 2007;70:501–507. doi: 10.1016/j.bioelechem.2006.11.001. [DOI] [PubMed] [Google Scholar]
- De Vriese A. Tilton R.G. Elger M., et al. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J. Am. Soc. Nephrol. 2001;12:993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- Durieux A.C. Bonnefoy R. Busso T. Freyssenet D. In vivo gene electrotransfer into skeletal muscle: effects of plasmid DNA on the occurrence and extent of muscle damage. J. Gene Med. 2004;6:809–816. doi: 10.1002/jgm.534. [DOI] [PubMed] [Google Scholar]
- Durieux A.C. Bonnefoy R. Freyssenet D. Kinetic of transgene expression after electrotransfer into skeletal muscle: importance of promoter origin/strength. Biochim. Biophys. Acta. 2005;1725:403–409. doi: 10.1016/j.bbagen.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Egashira K. Koyanagi M. Kitamoto S., et al. Anti-monocyte chemoattractant protein-1 gene therapy inhibits vascular remodeling in rats: blockade of MCP-1 activity after intramuscular transfer of a mutant gene inhibits vascular remodeling induced by chronic blockade of NO synthesis. FASEB J. 2000;14:1974–1978. doi: 10.1096/fj.00-0141com. [DOI] [PubMed] [Google Scholar]
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat. Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A. Dagnaes-Hansen F. De Vriese A.S., et al. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090–3094. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- Göser S. Ottl R. Brodner A., et al. Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1α in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation. 2005;112:3400–3407. doi: 10.1161/CIRCULATIONAHA.105.572396. [DOI] [PubMed] [Google Scholar]
- Goudy K.S. Wang B. Tisch R. Gene gun-mediated DNA vaccination enhances antigen-specific immunotherapy at a late preclinical stage of type 1 diabetes in nonobese diabetic mice. Clin. Immunol. 2008;129:49–57. doi: 10.1016/j.clim.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.Y. Kim C.H. Kim H.S., et al. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J. Am. Soc. Nephrol. 2006;17:1362–1372. doi: 10.1681/ASN.2005111196. [DOI] [PubMed] [Google Scholar]
- Herrera J.L. Fernandez-Montesinos R. Gonzalez-Rey E., et al. Protective role for plasmid DNA-mediated VIP gene transfer in non-obese diabetic mice. In: Vaudry H., editor; Laburthe M., editor. Vip, Pacap, and Related Peptides: From Gene to Therapy. Blackwell/New York Academy of Sciences; Boston, MA: 2006. pp. 337–341. [DOI] [PubMed] [Google Scholar]
- Holmgren L. Ambrosino E. Birot O., et al. A DNA vaccine targeting angiomotin inhibits angiogenesis and suppresses tumor growth. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9208–9213. doi: 10.1073/pnas.0603110103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose K. Maeshima Y. Yamamoto Y., et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes. 2005;54:2891–2903. doi: 10.2337/diabetes.54.10.2891. [DOI] [PubMed] [Google Scholar]
- Kanamori H. Matsubara T. Mima A., et al. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem. Biophys. Res. Commun. 2007;360:772–777. doi: 10.1016/j.bbrc.2007.06.148. [DOI] [PubMed] [Google Scholar]
- Kanesaki Y. Suzuki D. Uehara G., et al. Vascular endothelial growth factor gene expression is correlated with glomerular neovascularization in human diabetic nephropathy. Am. J. Kidney Dis. 2005;45:288–294. doi: 10.1053/j.ajkd.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Kaya Z. Leib C. Werfel S., et al. Comparison of IL-10 and MCP-1-7ND gene transfer with AAV9 vectors for protection from murine autoimmune myocarditis. Cardiovasc. Res. 2011;91:116–123. doi: 10.1093/cvr/cvr063. [DOI] [PubMed] [Google Scholar]
- Kim N.H. Kim K.B. Kim D.L., et al. Plasma and urinary vascular endothelial growth factor and diabetic nephropathy in type 2 diabetes mellitus. Diabet. Med. 2004;21:545–551. doi: 10.1111/j.1464-5491.2004.01200.x. [DOI] [PubMed] [Google Scholar]
- Koga M. Kai H. Egami K., et al. Mutant MCP-1 therapy inhibits tumor angiogenesis and growth of malignant melanoma in mice. Biochem. Biophys. Res. Commun. 2008;365:279–284. doi: 10.1016/j.bbrc.2007.10.182. [DOI] [PubMed] [Google Scholar]
- Lewis A. Steadman R. Manley P., et al. Diabetic nephropathy, inflammation, hyaluronan and interstitial fibrosis. Histol. Histopathol. 2008;23:731–739. doi: 10.14670/HH-23.731. [DOI] [PubMed] [Google Scholar]
- Lowrie D.B. Tascon R.E. Bonato V.L.D,, et al. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–271. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- Maruyama H. Sugawa M. Moriguchi Y., et al. Continuous erythropoietin delivery by muscle-targeted gene transfer using in vivo electroporation. Hum. Gene Ther. 2000;11:429–437. doi: 10.1089/10430340050015897. [DOI] [PubMed] [Google Scholar]
- Miyata T. de Strihou C.V. Translation of basic science into clinical medicine: novel targets for diabetic nephropathy. Nephrol. Dial. Transplant. 2009;24:1373–1377. doi: 10.1093/ndt/gfp028. [DOI] [PubMed] [Google Scholar]
- Mu W. Long D.A. Ouyang X., et al. Angiostatin overexpression is associated with an improvement in chronic kidney injury by an anti-inflammatory mechanism. Am. J. Physiol. Renal Physiol. 2009;296:F145–F152. doi: 10.1152/ajprenal.90430.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T. Kosugi T. Haneda M., et al. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58:1471–1478. doi: 10.2337/db09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasu T. Maeshima Y. Kinomura M., et al. Vasohibin-1, a negative feedback regulator of angiogenesis, ameliorates renal alterations in a mouse model of diabetic nephropathy. Diabetes. 2009;58:2365–2375. doi: 10.2337/db08-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga S. Shikata K. Yozai K., et al. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-κB activation. Am. J. Physiol. Renal Physiol. 2007;292:F1141–F1150. doi: 10.1152/ajprenal.00288.2005. [DOI] [PubMed] [Google Scholar]
- Ohkawa H. Ohishi N. Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Prud'homme G.J. DNA vaccination against tumors. J. Gene Med. 2005;7:3–17. doi: 10.1002/jgm.669. [DOI] [PubMed] [Google Scholar]
- Raptis A.E. Viberti G. Pathogenesis of diabetic nephropathy. Exp. Clin. Endocrinol. Diabetes. 2001;109:S424–S437. doi: 10.1055/s-2001-18600. [DOI] [PubMed] [Google Scholar]
- Rivero A. Mora C. Muros M., et al. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin. Sci. 2009;116:479–492. doi: 10.1042/CS20080394. [DOI] [PubMed] [Google Scholar]
- Saito K. Lehar M. Li Z.B., et al. High efficiency gene delivery into laryngeal muscle with bidirectional electroporation. Otolaryngol. Head Neck Surg. 2006;135:209–214. doi: 10.1016/j.otohns.2006.04.003. [DOI] [PubMed] [Google Scholar]
- San-Gil F. Schier G.M. Moses R.G. Gan I.E. Improved estimation of fructosamine, as a measure of glycated serum protein, with the Technicon RA-1000 analyzer. Clin. Chem. 1985;31:2005–2006. [PubMed] [Google Scholar]
- Saraheimo M. Teppo A.M. Forsblom C., et al. Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia. 2003;46:1402–1407. doi: 10.1007/s00125-003-1194-5. [DOI] [PubMed] [Google Scholar]
- Schneider J. Gilbert S.C. Blanchard T.J., et al. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 1998;4:397–402. doi: 10.1038/nm0498-397. [DOI] [PubMed] [Google Scholar]
- Shah I.M. Mackay S.P. McKay G.A. Therapeutic strategies in the treatment of diabetic nephropathy—a translational medicine approach. Curr. Med. Chem. 2009;16:997–1016. doi: 10.2174/092986709787581897. [DOI] [PubMed] [Google Scholar]
- Sisson T.H. Hattori N. Xu Y. Simon R.H. Treatment of bleomycin-induced pulmonary fibrosis by transfer of urokinase-type plasminogen activator genes. Hum. Gene Ther. 1999;10:2315–2323. doi: 10.1089/10430349950016960. [DOI] [PubMed] [Google Scholar]
- Stehouwer C.D.A. Gall M.A. Twisk J.W.R., et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- Tashiro K. Koyanagi I. Saitoh A., et al. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J. Clin. Lab. Anal. 2002;16:1–4. doi: 10.1002/jcla.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch G.H. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2008;294:F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- Tesch G.H. Allen T.J. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 2007;12:261–266. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- Tisch R. Wang B. Weaver D.J., et al. Antigen-specific mediated suppression of β cell autoimmunity by plasmid DNA vaccination. J. Immunol. 2001;166:2122–2132. doi: 10.4049/jimmunol.166.3.2122. [DOI] [PubMed] [Google Scholar]
- Troyanovsky B. Levchenko T. Mansson G., et al. Angiomotin: an angiostatin binding protein that regulates endothelial cell migration and tube formation. J. Cell Biol. 2001;152:1247–1254. doi: 10.1083/jcb.152.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui H. Shikata K. Matsuda M., et al. HMG-CoA reductase inhibitor ameliorates diabetic nephropathy by its pleiotropic effects in rats. Nephrol. Dial. Transplant. 2003;18:265–272. doi: 10.1093/ndt/18.2.265. [DOI] [PubMed] [Google Scholar]
- Utimura R. Fujihara C.K. Mattar A.L., et al. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int. 2003;63:209–216. doi: 10.1046/j.1523-1755.2003.00736.x. [DOI] [PubMed] [Google Scholar]
- van den Engel N.K. Haack M.A. Martin S. Kolb H. Oral DNA vaccination with a plasmid encoding soluble ICAM-1 modulates cytokine expression profiles in nonobese diabetic mice. J. Mol. Med. (Berl.) 2002;80:301–308. doi: 10.1007/s00109-002-0324-8. [DOI] [PubMed] [Google Scholar]
- Vlassara H. Torreggiani M. Post J.B., et al. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int. 2009;76:S3–S11. doi: 10.1038/ki.2009.401. [DOI] [PubMed] [Google Scholar]
- Volk T. Hensel M. Schuster H. Kox W.J. Secretion of MCP-1 and IL-6 by cytokine stimulated production of reactive oxygen species in endothelial cells. Mol. Cell. Biochem. 2000;206:105–112. doi: 10.1023/a:1007059616914. [DOI] [PubMed] [Google Scholar]
- Wada T. Furuichi K. Sakai N., et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492–1499. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- Wada T. Furuichi K. Sakai N., et al. Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J. Am. Soc. Nephrol. 2004;15:940–948. doi: 10.1097/01.asn.0000120371.09769.80. [DOI] [PubMed] [Google Scholar]
- Wang H. Zhang Z.X. Chu W., et al. Molecular screening and association analyses of the interleukin 6 receptor gene variants with type 2 diabetes, diabetic nephropathy, and insulin sensitivity. J. Clin. Endocrinol. Metab. 2005;90:1123–1129. doi: 10.1210/jc.2004-1606. [DOI] [PubMed] [Google Scholar]
- Wang J.J. Zhang S.X. Mott R., et al. Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2008;294:F1166–F1173. doi: 10.1152/ajprenal.00375.2007. [DOI] [PubMed] [Google Scholar]
- Xu Z.G. Lanting L. Vaziri N.D., et al. Upregulation of angiotensin II type 1 receptor, inflammatory mediators, and enzymes of arachidonate metabolism in obese Zucker rat kidney: reversal by angiotensin II type 1 receptor blockade. Circulation. 2005;111:1962–1969. doi: 10.1161/01.CIR.0000161831.07637.63. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y. Maeshima Y. Kitayama H., et al. Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes. 2004;53:1831–1840. doi: 10.2337/diabetes.53.7.1831. [DOI] [PubMed] [Google Scholar]
- Yang B.M. Cross D.F. Ollerenshaw M., et al. Polymorphisms of the vascular endothelial growth factor and susceptibility to diabetic microvascular complications in patients with type I diabetes mellitus. J. Diabetes Complications. 2003;17:1–6. doi: 10.1016/s1056-8727(02)00181-2. [DOI] [PubMed] [Google Scholar]
- Yozai K. Shikata K. Sasaki M., et al. Methotrexate prevents renal injury in experimental diabetic rats via anti-inflammatory actions. J. Am. Soc. Nephrol. 2005;16:3326–3338. doi: 10.1681/ASN.2004111011. [DOI] [PubMed] [Google Scholar]
- Zent R. Pozzi A. Antiangiogenic therapy in diabetic nephropathy. J. Am. Soc. Nephrol. 2006;17:325–327. doi: 10.1681/ASN.2005121290. [DOI] [PubMed] [Google Scholar]
- Zent R. Pozzi A. Angiogenesis in diabetic nephropathy. Semin. Nephrol. 2007;27:161–171. doi: 10.1016/j.semnephrol.2007.01.007. [DOI] [PubMed] [Google Scholar]