Abstract
Highly abbreviated micro-dystrophin genes have been intensively studied for Duchenne muscular dystrophy (DMD) gene therapy. Following adeno-associated virus (AAV) gene transfer, robust microgene expression is achieved in murine DMD models in the absence of immune suppression. Interestingly, a recent study suggests that AAV gene transfer in dystrophic dogs may require up to 18 weeks' immune suppression using a combination of three different immune-suppressive drugs (cyclosporine, mycophenolate mofetil, and anti-dog thymocyte globulin). Continued immune suppression is not only costly but also may cause untoward reactions. Further, some of the drugs (such as anti-dog thymocyte globulin) are not readily available. To overcome these limitations, we developed a novel 5-week immune suppression scheme using only cyclosporine and mycophenolate mofetil. AAV vectors (either AV.RSV.AP that expresses the heat-resistant human alkaline phosphatase gene, or AV.CMV.μDys that expresses the canine R16-17/H3/ΔC microgene) at 2.85×1012 vg particles were injected into adult dystrophic dog limb muscles under the new immune suppression protocol. Sustained transduction was observed for nearly half year (the end of the study). The simplified immune suppression strategy described here may facilitate preclinical studies in the dog model.
Shin and colleagues develop and test a novel immune suppression scheme to allow for successful recombinant adeno-associated virus (AAV)-mediated microdystrophin gene transfer to dystrophic dogs. This shorter, simplified scheme uses readily available drugs such as cyclosporine and mycophenolate, administered over the course of 5 weeks. Adult dystrophic dog limb muscles injected with AAV encoding a canine dystrophin microgene under the new immune suppression protocol show sustained transduction for nearly half a year.
Introduction
Duchenne muscular dystrophy (DMD) is a lethal disease characterized by progressive muscle deterioration. Although clinical manifestation was documented as early as 1868 (Parent 2005; Rondot 2005), it remains an incurable disease today. The discovery of the dystrophin gene offers the hope of effectively managing DMD by dystrophin gene replacement therapy (Kunkel, 2005; Duan, 2011). Tremendous progress has been achieved over the last decade in DMD gene therapy (Duan, 2006a, 2006b, 2011). A particularly attractive regimen is to deliver an abbreviated, yet functional micro-dystrophin gene via adeno-associated virus (AAV).
AAV is a member of the parvovirus family. More than 100 different AAV serotypes have been reported (Gao et al., 2005). The wild-type virus carries a ∼4.8-kb single-stranded DNA genome. Interestingly, wild-type AAV is minimally associated with human diseases. Recent clinical trials suggest that recombinant AAV may represent a powerful tool in treating inherited genetic diseases (reviewed in Mingozzi and High, 2011b). Numerous investigators have evaluated AAV-mediated DMD gene therapy in the murine model (reviewed in Blankinship et al., 2006; Duan, 2011). Collectively, these data show robust transduction of dystrophic mouse muscles without the need of immune suppressive drugs.
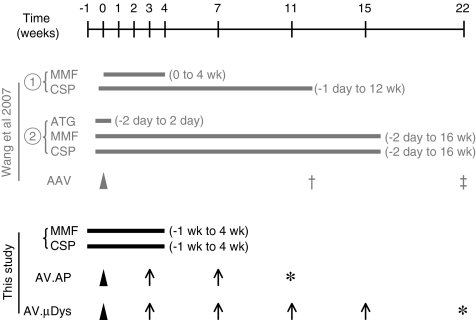
Dystrophin-deficient dogs are naturally occurring canine DMD models (reviewed in Duan, 2011). They show clinical symptoms and signs similar to those seen in human patients (Shelton and Engvall, 2005; Duan, 2011). Specifically, affected dogs display severe muscle degeneration, handicapped gait, cachexia, growth retardation, and premature death (Duan, 2011; Smith et al., 2011). Strategies to efficiently transfer AAV to dystrophic dog muscles will likely accelerate translation of AAV microgene therapy to patients. In contrast to mice, direct delivery of AAV (such as AAV-6 and AAV-9) to adult dog muscles is associated with considerable immune rejection (Wang et al., 2007a; Yue et al., 2008). Wang et al. (2007b) recently reported that administration of cyclosporine (CSP) and mycophenolate mofetil (MMF) effectively reduced AAV immune reaction in normal dog muscles (Fig. 1). Unfortunately, this method failed in golden retriever muscular dystrophy (GRMD) dogs (Wang et al., 2007b). To achieve persistent muscle gene transfer in dystrophic dogs, Wang et al. (2007b) were forced to extend CSP/MMF regimen to a total of 18 weeks. In addition, the authors had to add anti-dog thymocyte globulin (ATG), a customer-made T-cell–depleting reagent (Fig. 1). This not only adds to the cost, but more importantly, it increases potential side effects (Srinivas and Meier-Kriesche, 2008; Gaber et al., 2010; Schonder et al., 2010).
FIG. 1.
Schematic outline of the experimental timeline. Gray, immune suppression protocol previously used to achieve adeno-associated virus (AAV) transduction in dystrophic dog muscles (Wang et al., 2007b). Two different methods were tested. The first method (cyclosporine [CSP] and mycophenolate mofetil [MMF]) failed to support AAV transduction in dystrophic dog muscles. Cross, the last time point of muscle biopsy used in the first method. The second method (anti-dog thymocyte globulin [ATG], CSP, and MMF) resulted efficient AAV transduction that lasted for up to 6 weeks after discontinuation of immune suppression. The second method includes two variances. The strategy depicted here was used in AAV-mediated human micro-dystrophin gene transfer. Double cross, the last time point of muscle biopsy used in human microgene study. A slightly different strategy (a total of CSP and MMF for 18 weeks and the last time point of AAV expression at 30 weeks after AAV injection) was used in AAV-mediated canine micro-dystrophin gene transfer (not shown here). Black, the experimental protocol used in the current study. In this protocol, immune suppression was started at 1 week before AAV injection and continued for additional 4 weeks after AAV injection. Arrowhead, the time of AAV injection; arrow, the time of muscle biopsy used in the current study; asterisk, the time of necropsy used in the current study.
In this study, we reported a simplified, less aggressive immune suppression protocol (Fig. 1). We applied CSP and MMF for a total of 5 weeks (starting from 1 week before AAV injection). We used Y445F tyrosine mutant AAV-6 vectors that express either a foreign reporter gene (human placental alkaline phosphatase gene, AP) or a therapeutic canine micro-dystrophin gene (Zhong et al., 2008; Qiao et al., 2010). AAV was injected into the extensor carpi radialis (ECR) and extensor carpi ulnaris (ECU) muscles of young adult dystrophic dogs. Sustained high-level expression (AP and micro-dystrophin) was observed through the end of the study (up to 22 weeks).
Materials and Methods
Animals
All animal experiments were approved by the Animal Care and Use Committee of the University of Missouri and were in accordance with National Institutes of Health guidelines. Dystrophin-deficient dogs were produced by inseminating GRMD carrier dogs with semen from affected male dogs as we described (Fine et al., 2011). Semen from three different dystrophic breeds was used including GRMD, labrador muscular dystrophy (LMD), and corgi muscular dystrophy dogs (Duan 2011; Fine et al., 2011; Smith et al., 2011). Affected dogs were identified by polymerase chain reaction (PCR) genotyping using the umbilical cord DNA (Fine et al., 2011; Smith et al., 2011; Yue et al., 2011). Diagnosis was further confirmed by measuring the serum creatine kinase level and muscle biopsy. Both male and female affected dogs were used in the study. At the beginning of the experiments, the age of dystrophic dogs was between 18 to 24 months.
Immune suppression
Experimental dogs were subjected to a 5-week immune suppression scheme using CSP (Neoral, 100 mg/mL; Novartis; NDC 0078-0274-22) and MMF (CellCept, 200 mg/mL; Genentech; NDC 0004-0261-29). CSP was administered orally at the dose of 10–20 mg/kg/d to achieve a whole blood trough level of 400–600 ng/mL (Gregorevic et al., 2009). The blood trough level was achieved usually at ∼6 days after starting CSP). MMF was administered orally twice a day at the dose of 20 mg/kg (40 mg/kg/d). Immune suppression was started at 1 week prior to AAV injection and continued for 4 weeks after AAV injection (Fig. 1).
Recombinant AAV vector
The proviral cis plasmid for the AP vector was published before (Yue et al., 2003, 2008). In this vector, the heat-resistant human placental AP gene was expressed under the transcriptional control of the Rous sarcoma virus (RSV) promoter and the simian virus 40 (SV40) polyadenylation signal. The proviral cis plasmid (YL196) for the R16-17/H3/ΔC μDys vector was based on the canine dystrophin gene. This microgene is modeled according to a previously described R16-17/ΔC micro-dystrophin gene (Lai et al., 2009; Banks et al., 2010; Li et al., 2011). It contains the dystrophin N-terminal domain, hinge 1, spectrin-like repeat 1, 16 and 17 (R1, R16, and R17), hinge 3, R24, hinge 4, and the cysteine-rich (CR) domain. The expression of the canine microgene is controlled by the cytomegalovirus (CMV) promoter, an engineered Kozak sequence and the SV40 polyadenylation signal. The Y445F tyrosine modified AAV-6 was purified through three rounds of cesium chloride isopycnic ultracentrifugation using our published protocol (Zhong et al., 2008; Qiao et al., 2010). The resulting viruses were termed AV.AP and AV.μDys. The titer for AV.AP virus was determined as described elsewhere (Yue et al., 2003, 2008). The titer for AV.μDys was determined by quantitative PCR using primers that amplify exons 69 and 70 in the dystrophin CR domain. The forward primer is 5′ TTTTCTGGTCGAGTTGCAAAAG. The reverse primer is 5′ CCATGTTGTCCCCCTCTAAGAC.
Gene delivery, muscle biopsy, and dog necropsy
AAV vectors were directly injected to the ECR and ECU muscles of anesthetized dogs. Briefly, dystrophic dogs were pretreated with atrophin sulfate (0.04 mg/kg, intramuscular injection). Anesthesia was induced with ketamine (6 mg/kg, intravenous [iv]) and diazepam (0.2 mg/kg, iv). Anesthesia was maintained with 2% sevoflurane using a vaporizer. The ECR and ECU muscles were identified by anatomic markers. A 2-cm-long skin incision was made to expose the muscle belly with the deep fascia remaining intact. A total of 2.85×1012 vg particles (500 μL) of AAV vectors were delivered to an area of muscle approximately ∼5 cm long with multiple injections. The four corners of the injected area were marked with a tattoo dye (beneath the fascia). After AAV injection, the skin was closed in a continuous pattern with 4-0 vicryl sutures.
For muscle biopsies, the experimental dogs were anesthetized as described for AAV vector injection, and a skin incision was made over the target muscle. The injected area was identified by the tattoo markings. A 1-cm3 sample of muscle was removed using a scalpel blade and the fascia was closed in a continuous pattern with 4-0 vicryl sutures. The skin was closed with a surgical staple or 3-0 nylon sutures. Whole body necropsy was performed as we described elsewhere (Yue et al., 2008).
Morphology studies
Expression of the human placental AP gene was determined by histochemical staining on 8-μm cryotissue sections according to previous publications (Ghosh et al., 2006; Yue et al., 2008). Dystrophin was examined with two epitope-specific antibodies including a mouse monoclonal antibody against spectrin-like repeat 16 (Mandys103, 1:20; a gift from Dr. Glenn Morris, The Robert Jones and Agnes Hunt Orthopaedic Hospital, UK) and a mouse monoclonal antibody against the C-terminal domain (Dys-2, 1:30; Novocastra) (Morris et al., 2011).
Blood chemistry
To determine the baseline, blood from each dystrophic dog was collected 1 day before immune-suppressive drugs were administered. Hematology, blood electrolytes, and serum chemistry were evaluated between 2 to 15 weeks after AAV injection (Table 1). All assays were performed at the UMC Vet Med Diagnostic Lab (Columbia, MO).
Table 1.
Blood Examination Results
| |
Pre-immunosuppression |
After AAV injection |
|
||
|---|---|---|---|---|---|
| Mean±SEM | n | Mean±SEM | n | Unit | |
| Hematologya | |||||
| Hct | 41.0±0.6 | 3 | 42.4±1.0 | 6 | % |
| Hgb | 14.9±0.2 | 3 | 15.6±0.4 | 6 | g/dL |
| Lymphocyte | 4.6±1.3 | 3 | 4.4±0.8 | 4 | ×103/μL |
| MCV | 70.7±0.3 | 3 | 70.7±0.2 | 6 | fL |
| Platelet count | 416.3±42.4 | 3 | 577.8±15.2 | 4 | ×103/μL |
| RBC | 5.8±0.1 | 3 | 6.0±0.2 | 6 | ×106/μL |
| WBC | 14.7±1.7 | 3 | 20.7±2.0 | 6 | ×103/μL |
| Electrolyte | |||||
| Calcium | 10.2±0.1 | 3 | 10.3±0.1 | 6 | mEq/L |
| Chloride | 106.7±0.7 | 3 | 105.3±2.2 | 6 | mEq/L |
| Potassium | 5.0±0.1 | 3 | 5.0±0.1 | 6 | mEq/L |
| Sodium | 146.7±0.9 | 3 | 146.2±1.1 | 6 | mEq/L |
| Serum chemistryb | |||||
| Albumin | 3.2±0.1 | 3 | 3.3±0.1 | 6 | g/dL |
| ALT | 545.3±79.5 | 3 | 415.2±74.9 | 6 | U/L |
| Cholesterol | 273.0±26.3 | 3 | 318.2±7.3 | 6 | mg/dL |
| CK | 19153±4037 | 3 | 7177±3464 | 4 | U/L |
| Creatinine | 0.4±0.0 | 3 | 0.4±0.0 | 6 | mg/dL |
| Glucose | 90.7±3.4 | 3 | 100.5±6.7 | 6 | mg/dL |
| Total bilirubin | 0.2±0.0 | 3 | 0.2±0.0 | 6 | mg/dL |
| Total protein | 6.4±0.2 | 3 | 6.4±0.1 | 6 | g/dL |
| Urea nitrogen* | 9.0±1.2 | 3 | 13.3±1.3 | 6 | mg/dL |
Hct, hematocrit; Hgb, hemoglobin; MCV, mean corpuscular volume; RBC, red blood cell; WBC, white blood cell.
ALT, alanine aminotransferase; CK, creatine kinase.
Significantly different (p=0.04).
Results
Experimental overview
To determine whether a simplified immune suppression regimen could support persistent AAV transduction in 18- to 24-month-old dystrophic dogs, we co-administered calcineurin inhibitor CSP and anti-proliferative agent MMF. Immune-suppressive drugs were started 1 week before intramuscular AAV injection and continued for a total of 5 weeks (Fig. 1). To facilitate administration, both drugs were in liquid form and were administered orally with a 10-mL syringe (no needle attached). The dosage of CSP was adjusted every 3 days until the trough level was reached (Gregorevic et al., 2009). The health of the experimental subjects was carefully monitored daily by a veterinarian. No adverse reactions were observed. There was also no significant change in the complete blood count (Table 1). The comprehensive metabolic panels were essentially unremarkable except for a mild increase of blood urea nitrogen (BUN) (Table 1) (Gupta et al., 1991; Wissmann et al., 1996). Nevertheless, post-AAV infection BUN was still within the normal range (<28 mg/dL).
AAV vectors were packaged in a modified AAV-6 capsid in which the tyrosine residue at the position 445 was replaced by phenylalanine (Zhong et al., 2008; Qiao et al., 2010). Two different AAV vectors were tested. In both vectors, transgene expression was driven by a strong ubiquitous viral promoter (RSV and CMV for AV.AP and AV.μDys, respectively). AV.AP expressed a human placental AP gene and AV.μDys expressed the R16-17/H3/ΔC canine micro-dystrophin gene. The micro-dystrophin gene encoded the dystrophin N-terminal domain, an abbreviated central domain and the CR domain. The abbreviated central domain consisted of three hinges (H1, H3, and H4) and four spectrin-like repeats (R1, R16, R17, and R24) (Lai et al., 2009).
At the end of the first week immune suppression, 500 μL AAV (2.85×1012 vg particles) was injected into the belly of the ECR and ECU muscles of young adult dystrophic dogs. Subjects that received the AV.AP vector were surgically biopsied at 3 and 7 weeks post-injection and necropsy was performed at 11 weeks post-injection (Fig. 1). In AV.μDys infected dogs, micro-dystrophin expression was evaluated at 3, 7, 11, and 15 weeks post-injection by surgical biopsy and at 22 weeks post-injection by necropsy (Fig. 1).
Robust expression of the human AP reporter gene in dystrophic dogs
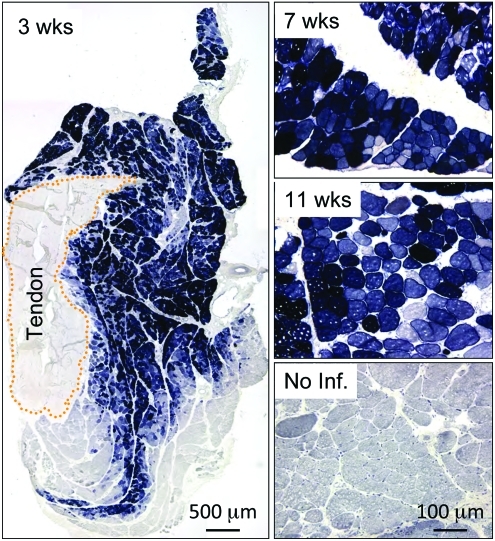
To test whether our modified immune suppression protocol was sufficient to support persistent AAV gene transfer in dystrophic dog muscle, we first injected AV.AP to the ECR muscle. The heat-resistant human placental AP gene was selected because the dog does not carry a similar gene (dog placental AP is heat labile) (Moak and Harris, 1979). Further, AP is one of the most sensitive reporter genes and has been frequently used in evaluating AAV gene transfer in dogs (Bell et al., 2005; Yue et al., 2008; Gregorevic et al., 2009). Biopsy at the 3-week time point showed widespread AP expression in at least 70% of myofibers (Fig. 2). Robust AP expression was seen at 7 and 11 weeks post-injection (3 and 7 weeks after stopping immune suppression, respectively) (Fig. 2). AP expression was not detected in uninjected nearby muscles or internal organs (data not shown).
FIG. 2.
Efficient transduction of dystrophic dog muscle with tyrosine-modified AAV-6. A brief course of immune suppression was applied to dystrophic dogs as described in method section. Y445F tyrosine mutant AAV-6 AV.AP (2.85×1012 vg particles) was directly injected into the extensor carpi radials muscle of DMD dogs. Photomicrographs are representative AP histochemical staining images at 3, 7, and 11 weeks after AAV injection. An uninfected DMD dog muscle was used as no infection control. Color images available online at www.liebertonline.com/hum
Long-term expression of a therapeutic dystrophin gene in DMD dog muscles
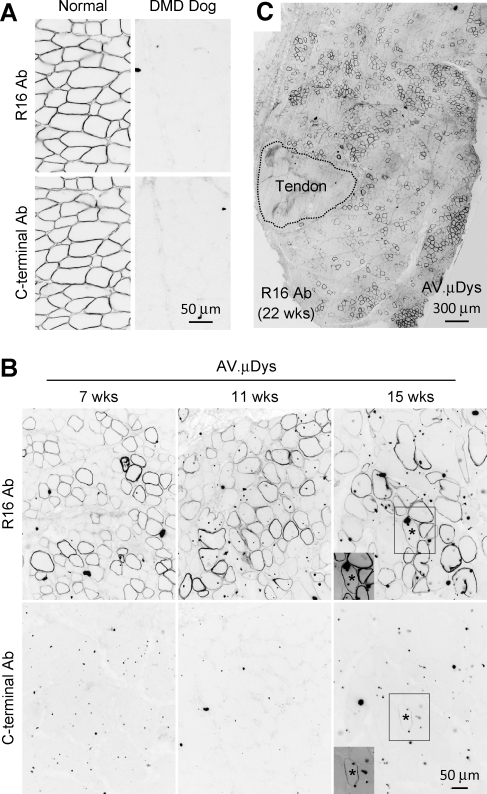
To determine whether our approach can be used in a therapeutic context, we used a canine micro-dystrophin AAV vector (AV.μDys). This microgene carries dystrophin spectrin-like repeat R16 but it does not contain the C-terminal domain (Lai et al., 2009). To evaluate microgene expression, we used R16-specific and C-terminal specific antibodies in our study. These antibodies effectively detected dystrophin in normal dog muscles (Fig. 3A). As expected, they did not stain dystrophic dog muscles (Fig. 3A, data not shown). The specificity of AAV-mediated expression was determined in serial muscle sections by positive staining with the R16, but not the C-terminal antibody (Fig. 3B). Rarely occurring revertant fibers were recognized by both R16 and C-terminal antibodies (Fig. 3B, asterisk). Consistent with the results seen with the AV.AP vector (Fig. 2), strong dystrophin expression was observed at 3, 7, and 11 weeks after gene transfer (Fig. 3B and data not shown). To determine whether we could achieve longer AAV transduction, we extended the study to a total of 22 weeks (Fig. 3B and 3C). Biopsy at the 15-week time point revealed excellent transduction (Fig. 3B). Robust expression persisted for almost half year without any sign of diminution (Fig. 3C). Examination at necropsy revealed the absence of AAV-mediated expression in nearby muscles and internal organs (data not shown).
FIG. 3.
Direct intramuscular injection of tyrosine modified AAV-6 leads to robust and persistent micro-dystrophin expression in DMD dog limb muscle. (A) Representative immunostaining photomicrographs of normal and untreated DMD dog limb muscles. Dystrophin expression in normal dog muscle was revealed by an antibody that recognizes dystrophin spectrin-like repeat R16 (R16 antibody) and an antibody that recognizes the C-terminal domain. Dystrophin expression was not detected in DMD dog muscle. (B) Representative dystrophin immunostaining at 7, 11, and 15 weeks after intramuscular injection of the AAV micro-dystrophin vector. Top panel, immunostaining with the R16 specific antibody; bottom panel, immunostaining with the C-terminal specific antibody. Asterisk, an endogenous revertant myofiber stained positive by both the R16 and C-terminal antibodies. Insert, the long exposure image of the boxed region in the same photomicrograph. (C) A representative cross-sectional image of the extensor carpi radialis muscle stained with the R16 specific antibody at 22 weeks after intramuscular AAV injection. Dotted area marks the tendon.
Discussion
The highly truncated micro-dystrophin gene holds great potential for treating DMD. The concept of using an abbreviated gene for DMD gene therapy derives from early clinical observations. It was noted that an in-frame deletion of 46% of the dystrophin gene was associated with very mild disease (England et al., 1990). However, it is not clear whether dystrophin protein with a larger deletion (for example, a deletion of more than 60% of the amino acid sequence) can still protect muscle from degeneration in human patients (or in any large mammals). This question was partially answered by studies performed in various mouse DMD models including dystrophin-null, dystrophin/utrophin double knockout, and dystrophin/myoD double knockout mice (Wang et al., 2000; Harper et al., 2002; Gregorevic et al., 2004; Lai et al., 2009). Collectively, these studies suggest that micro-dystrophin can effectively mitigate muscle disease in dystrophic mice. DMD dogs share many similarities to human patients. Additional evidence from dystrophic dogs may provide critical support to human trials.
A notorious barrier in testing AAV gene transfer in dog muscle is immune rejection. In the absence of immune suppression, AAV transduced myofibers are rapidly eliminated by cellular immune responses even in normal adult dogs following direct muscle injection (Wang et al., 2007a; Yue et al., 2008, 2011). Experimenting with different gene delivery routes (such as systemic gene transfer) may alter (or possibly minimize) T-cell response (Vandenberghe and Wilson 2007; Mingozzi and High, 2011a). However, the huge dose of vectors needed for systemic delivery renders it not cost-effective for most vectors at the early stage of gene therapy development. Currently, direct muscle injection remains one of the most appealing approaches to compare the pros and cons of various configurations of a muscle gene therapy vector.
To achieve successful AAV transduction in adult dog muscle, Wang et al. (2007b) applied CSP and MMF. Initially, the authors started CSP (oral) at 1 day before AAV-6 injection and continued for 12 weeks. MMF (subcutaneous injection) was initiated on the day of AAV injection but only continued for 4 weeks (Wang et al., 2007b). In wild-type dogs, this immune suppression regimen allowed robust AAV-6 expression for 4 weeks but expression dropped to minimal levels after discontinuation of MMF. Importantly, this immune suppression regimen failed in dystrophic dogs (Wang et al., 2007b). To achieve AAV transduction in dystrophic muscle, Wang et al. (2007b) switched to a more aggressive immune suppression protocol. They applied a 5-day T-cell depletion protocol with anti-dog thymocyte globulin (1 mg/kg/d, subcutaneous injection). The drug was given at 2 days before AAV injection and continued for two more days after AAV injection. Besides T-cell depletion, they also co-administered CSP and MMF starting from 2 days before AAV delivery and continued until 16 weeks after gene transfer (Wang et al., 2007b). This protocol resulted in strong expression of a human microgene. However, when immune suppression was discontinued, expression was lost. To obtain long-term expression, Wang et al. (2007b) replaced the human microgene with the canine microgene and continued combined CSP and MMF administration for additional two more weeks (for a total of 18 weeks after AAV injection). This strategy allowed persistent dystrophin expression for 12 weeks after stopping immune suppression (30 weeks after AAV injection) (Wang et al., 2007b).
Immune-suppressive drugs, especially the T cell–depleting drugs, are associated with a broad range of side effects such as fever, headache, tremor, hematological abnormalities, liver and renal toxicity, and opportunistic infection (Srinivas and Meier-Kriesche, 2008; Gaber et al., 2010; Schonder et al., 2010). For these reasons, minimized immunosuppression has been strongly promoted in the field of organ transplantation (Vincenti, 2003). We reasoned that a milder immune suppression scheme might help reduce untoward responses. To develop a less aggressive immune suppression protocol for dystrophic dogs, we only administered commercially available CSP and MMF for a total of 5 weeks (Fig. 1). Clinical evaluation and blood chemistry test revealed excellent tolerance in all experimental subjects (Table 1). The only notable change was a mild increase of BUN. However, as indicated in the product information sheet “It is not unusual for serum creatine and BUN levels to be elevated during cyclosporine therapy” (NOVARTIS T2009-104). As a matter of fact, BUN elevation has been well documented in the literature (Gupta et al., 1991; Wissmann et al., 1996). Here, we would also like to emphasize the following point. Although the change (between pre-immune suppression and after AAV injection) appeared statistically significant (from 9.0±1.2 mg/dL to 13.3±1.3 mg/dL) (Table 1), these values were all within the normal range (<28 mg/dL).
The most important goal of the current study was to establish a platform to allow direct testing of various AAV gene therapy strategies in dystrophic dog muscle without the need for aggressive immune suppression. To this end, we have achieved long-term transduction in dystrophic dog muscles using two different AAV vectors (one expressing a non-canine reporter gene and one expressing a canine micro-dystrophin gene) (Figs. 2 and 3). In the case of canine microgene AAV vector, robust expression lasted for nearly a half year (18 weeks after discontinuation of immune suppression, the end of the study) (Fig. 3).
It is currently not completely clear why our modified immune suppression protocol has resulted in persistent AAV transduction in dystrophic muscle (Table 2). There are several possible reasons. First, the dystrophic dogs used in this study were generated by crossing GRMD carriers with males carrying one of three different dystrophin gene mutations (Cooper et al., 1988; Duan 2011; Fine et al., 2011; Smith et al., 2011). However, the dogs used by Wang et al. (2007b) only had the GRMD mutation (Cooper et al., 1988; Duan, 2011). Because several thousand different dystrophin gene mutations have been observed in human DMD patients, it is unlikely that mutations in experimental dogs have dramatically affected our study (Flanigan et al., 2009). The second possibility is the difference of the drugs (such as regimen and dosage) (Table 2). We omitted the T cell–depleting drug ATG in our protocol. This should have weakened, rather than enhanced immune suppression. Wang et al. (2007b) used subcutaneous injection of MMF (7.5 mg/kg, twice a day), but we used oral administration of MMF (20 mg/kg, twice a day). It is unclear whether subcutaneous and oral forms of MMF display a different pharmacokinetic profile. It is possible (although unlikely) that the change in MMF may have contributed to our observation. Nevertheless, since CSP is already used as an oral drug (in our protocol and Wang et al. (2007b) protocol) we believe our method should be more convenient (both CSP and MMF as oral drugs). In terms of CSP, Wang et al. (2011) targeted a CSP trough level of 100 to 350 ng/mL using the dose of 10 mg/kg/d. In our study, we used a higher dose of 10–20 mg/kg/d to reach a higher CSP trough level of 400 ng/mL. The trough level used in our study has been used by others in dogs (Gregorevic et al., 2009). Despite the slight difference of the trough level, neither our study nor the study by Gregorevic et al. (2009) has indicated any untoward reaction (Table 1). Another drug-related issue is the timing of immune suppression initiation. We applied a preemptive strategy by starting immune suppression at 1 week before AAV injection (Fig. 1). Wang et al. (2007b) started immune suppression at 2 days before AAV injection. Thirdly, it is possible that the AAV serotype used in our study and that of Wang et al. (2007b) may have contributed to the difference. Wang and colleagues used the original AAV-6, but in our study we used a newly described Y445F tyrosine mutant AAV-6 (Zhong et al., 2008; Qiao et al., 2010). We recently found that Y445F-mutated AAV-6 resulted in higher transduction in murine muscle than the original AAV-6 (Qiao et al., 2010). Although future studies are needed to determine whether Y445F tyrosine mutant AAV-6 is stronger and/or less immunogenic in dystrophic dog muscles, robust transduction we observed with both AV.AP and AV.μDys vectors suggests that this modified AAV-6 is quite suitable for testing AAV gene therapy in DMD dog models (Figs. 2 and 3). Lastly, although the canine micro-dystrophin gene was used in both studies, the exact configuration of the microgene was different. In our study, we included the newly defined nNOS-targeting domain (Lai et al., 2009).
Table 2.
Protocol Comparison
| Wang et al. (2007b)* | This study | |
|---|---|---|
| AAV-micro-dystrophin | ||
| Construct | Canine ΔR4-R23/ΔC | Canine R16-17/H3/ΔC |
| Promoter | CMV | CMV |
| AAV serotype | Type 6 | Type 6, Y445F tyrosine mutant |
| Viral dose (per muscle) | 1×1011 v.g. | 2.85×1012 v.g. |
| Cyclosporin | ||
| Dose | 10 mg/kg/d, oral | 10–20 mg/kg/d, oral |
| Blood level | 100–350 ng/mL | 400–600 ng/mL |
| Duration | −2 day to 18 weeks | −7 day to 4 weeks |
| Mycophenolate | ||
| Dose | 15 mg/kg/d, subcutaneous | 40 mg/kg/d, oral |
| Duration | −2 day to 18 weeks | −7 day to 4 weeks |
| Anti-thymocyte globulin | ||
| Dose | 1 mg/kg/d, subcutaneous | Not used |
| Duration | −2 day to 2 days | |
| Longest time point | ||
| After AAV injection | 30 weeks | 22 weeks |
| After cessation of immune suppression | 12 weeks | 18 weeks |
This is different from the protocol used for human micro-dystrophin vector (depicted in Fig 1).
In summary, we have demonstrated for the first time that a less aggressive immunosuppression scheme is effective in supporting persistent AAV transduction in dystrophic dog muscles. Future application of our method may facilitate translational gene therapy studies in the canine model of muscular dystrophy.
Acknowledgment
This work was supported by grants from the National Institutes of Health (AR-49419, DD), Muscular Dystrophy Association (DD), and Jesse's Journey, The Foundation for Gene and Cell Therapy (DD). We thank Glenn Morris (The Robert Jones and Agnes Hunt Orthopaedic Hospital, UK) for providing dystrophin monoclonal antibodies. We would also like to acknowledge the Gene Therapy Resource Program (GTRP) of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Author Disclosure Statement
No competing financial interest exist.
References
- Banks G.B. Judge L.M. Allen J.M. Chamberlain J.S. The polyproline site in hinge 2 influences the functional capacity of truncated dystrophins. PLoS Genet. 2010;6:e1000958. doi: 10.1371/journal.pgen.1000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P. Limberis M. Gao G., et al. An optimized protocol for detection of E. coli beta-galactosidase in lung tissue following gene transfer. Histochem. Cell Biol. 2005;124:77–85. doi: 10.1007/s00418-005-0793-2. [DOI] [PubMed] [Google Scholar]
- Blankinship M.J. Gregorevic P. Chamberlain J.S. Gene therapy strategies for Duchenne muscular dystrophy utilizing recombinant adeno-associated viral vectors. Mol. Ther. 2006;13:241–249. doi: 10.1016/j.ymthe.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Cooper B.J. Winand N.J. Stedman H., et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- Duan D. Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum. Mol. Genet. 2006a;15(Spec No 2):R253–261. doi: 10.1093/hmg/ddl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D. From the smallest virus to the biggest gene: marching towards gene therapy for Duchenne muscular dystrophy. Discov. Med. 2006b;6:103–108. [PMC free article] [PubMed] [Google Scholar]
- Duan D. Duchenne muscular dystrophy gene therapy: lost in translation? Res. Rep. Biol. 2011;2:31–42. doi: 10.2147/RRB.S13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England S.B. Nicholson L.V. Johnson M.A., et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- Fine D.M. Shin J.H. Yue Y., et al. Age-matched comparison reveals early electrocardiography and echocardiography changes in dystrophin-deficient dogs. Neuromuscul Disord. 2011;21:453–461. doi: 10.1016/j.nmd.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan K.M. Dunn D.M. Von Niederhausern A., et al. Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Hum. Mutat. 2009;30:1657–1666. doi: 10.1002/humu.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber A.O. Knight R.J. Patel S. Gaber L.W. A review of the evidence for use of thymoglobulin induction in renal transplantation. Transplant Proc. 2010;42:1395–1400. doi: 10.1016/j.transproceed.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Gao G. Vandenberghe L.H. Wilson J.M. New recombinant serotypes of AAV vectors. Curr. Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Ghosh A. Yue Y. Duan D. Viral serotype and the transgene sequence influence overlapping adeno-associated viral (AAV) vector-mediated gene transfer in skeletal muscle. J. Gene Med. 2006;8:298–305. doi: 10.1002/jgm.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P. Blankinship M.J. Allen J.M., et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P. Schultz B.R. Allen J.M., et al. Evaluation of vascular delivery methodologies to enhance rAAV6-mediated gene transfer to canine striated musculature. Mol. Ther. 2009;17:1427–1433. doi: 10.1038/mt.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A.K. Rocher L.L. Schmaltz S.P., et al. Short-term changes in renal function, blood pressure, and electrolyte levels in patients receiving cyclosporine for dermatologic disorders. Arch. Intern. Med. 1991;151:356–362. [PubMed] [Google Scholar]
- Harper S.Q. Hauser M.A. Dellorusso C., et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat. Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- Kunkel L.M. 2004 William Allan Award address. Cloning of the DMD gene. Am. J. Hum. Genet. 2005;76:205–214. doi: 10.1086/428143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y. Thomas G.D. Yue Y., et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. Yue Y. Lai Y., et al. Nitrosative stress elicited by nNOSmicro delocalization inhibits muscle force in dystrophin-null mice. J. Pathol. 2011;223:88–98. doi: 10.1002/path.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F. High K.A. Immune responses to AAV in clinical trials. Curr. Gene Ther. 2011a;11:321–330. doi: 10.2174/156652311796150354. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. High K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 2011b;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- Moak G. Harris H. Lack of homology between dog and human placental alkaline phosphatases. Proc. Natl. Acad. Sci. U. S. A. 1979;76:1948–1951. doi: 10.1073/pnas.76.4.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. Man N.T. Sewry C.A. Monitoring duchenne muscular dystrophy gene therapy with epitope-specific monoclonal antibodies. Methods Mol. Biol. 2011;709:39–61. doi: 10.1007/978-1-61737-982-6_3. [DOI] [PubMed] [Google Scholar]
- Parent A. Duchenne De Boulogne: a pioneer in neurology and medical photography. Can. J. Neurol. Sci. 2005;32:369–377. doi: 10.1017/s0317167100004315. [DOI] [PubMed] [Google Scholar]
- Qiao C. Zhang W. Yuan Z., et al. AAV6 capsid tyrosine to phenylalanine mutations improve gene transfer to skeletal muscle. Hum. Gene. Ther. 2010;21:1343–1348. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondot P. G. B. A. Duchenne de Boulogne (1806–1875) J. Neurol. 2005;252:866–867. doi: 10.1007/s00415-005-0874-0. [DOI] [PubMed] [Google Scholar]
- Schonder K.S. Mazariegos G.V. Weber R.J. Adverse effects of immunosuppression in pediatric solid organ transplantation. Paediatr. Drugs. 2010;12:35–49. doi: 10.2165/11316180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Shelton G.D. Engvall E. Canine and feline models of human inherited muscle diseases. Neuromuscul. Disord. 2005;15:127–138. doi: 10.1016/j.nmd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Smith B.F. Yue Y. Woods P.R., et al. An intronic LINE-1 element insertion in the dystrophin gene aborts dystrophin expression and results in Duchenne-like muscular dystrophy in the corgi breed. Lab. Invest. 2011;91:216–231. doi: 10.1038/labinvest.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas T.R. Meier-Kriesche H.U. Minimizing immunosuppression, an alternative approach to reducing side effects: objectives and interim result. Clin. J. Am. Soc. Nephrol. 2008;3(Suppl 2):S101–116. doi: 10.2215/CJN.03510807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H. Wilson J.M. AAV as an immunogen. Curr. Gene Ther. 2007;7:325–333. doi: 10.2174/156652307782151416. [DOI] [PubMed] [Google Scholar]
- Vincenti F. Immunosuppression minimization: current and future trends in transplant immunosuppression. J. Am. Soc. Nephrol. 2003;14:1940–1948. doi: 10.1097/01.asn.0000076844.59963.cf. [DOI] [PubMed] [Google Scholar]
- Wang B. Li J. Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Allen J.M. Riddell S.R., et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum. Gene Ther. 2007a;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Wang Z. Kuhr C.S. Allen J.M., et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol. Ther. 2007b;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- Wang Z. Tapscott S.J. Storb R. Local gene delivery and methods to control immune responses in muscles of normal and dystrophic dogs. Methods Mol. Biol. 2011;709:265–275. doi: 10.1007/978-1-61737-982-6_17. [DOI] [PubMed] [Google Scholar]
- Wissmann C. Frey F.J. Ferrari P. Uehlinger D.E. Acute cyclosporine-induced nephrotoxicity in renal transplant recipients: the role of the transplanted kidney. J. Am. Soc. Nephrol. 1996;7:2677–2681. doi: 10.1681/ASN.V7122677. [DOI] [PubMed] [Google Scholar]
- Yue Y. Li Z. Harper S.Q., et al. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation. 2003;108:1626–1632. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y. Ghosh A. Long C., et al. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol. Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y. Shin J.-H. Duan D. Whole body skeletal muscle transduction in neonatal dogs with AAV-9. Methods Mol. Biol. 2011;709:313–329. doi: 10.1007/978-1-61737-982-6_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. Li B. Mah C.S., et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]