Abstract
Emerging evidence identifies multiple roles for nucleoside-diphosphate-kinase in host-microbe interaction. We provide the first synopsis of utilization of this molecule by various microorganisms during colonization of host tissues. Additionally, we propose novel mechanisms this effector may participate in, which could be crucial for microbial adaptation in chronic host infection.
Keywords: nucleoside-diphoshate-kinase, Ndpk/nm23, persistent infection, Porphyromonas gingivalis, secreted effector, purinergic signaling, extracellular ATP, danger signal
1. Introduction
Nucleoside-diphosphate-kinase (Ndk) is an evolutionarily highly conserved enzyme that catalyzes γ-phosphate transfer between nucleoside triphosphates and diphosphates (NTPs/NDPs). Ndk enzymes are promiscuous, as they do not have a preferential NTP source and are capable of transferring phosphate to multiple NDP targets. Generation of high energy NTP molecules is crucial for synthesis of DNA and RNA for a variety of organisms, and evidence shows that Ndk-mediated NTP production is important for polysaccharide formation in opportunistic pathogens [1]. It seems that for microbe-associated Ndk, NTPase and NTP-generation capabilities may represent conserved enzyme functions that have allowed this kinase to take on additional roles during colonization of host tissues by microorganisms (Fig. 1).
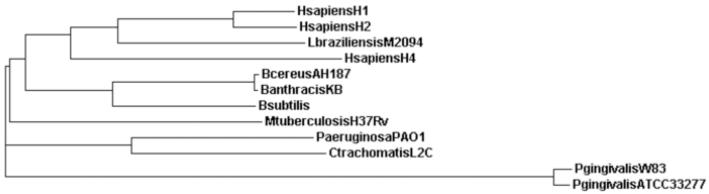
Fig. 1.
An unrooted neighbor-joining tree showing the evolutionary relationship between various organisms. Ndk from Homo sapiens isoform 1 (HsapiensH1), Homo sapiens isoform 2 (HsapiensH2), Homo sapiens isoform 4 (HsapiensH4), Leishmania braziliensis (LbraziliensisM2094), Bacillus cereus (BcereusAH187), Bacillus anthracis (BanthracisKB), Bacillus subtilis (Bsubtilis), Mycobacterium tuberculosis (MtuberculosisH37Rv), Pseudomonas aeruginosa (PaeruginosaPAO1), Chlamydia trachomatis (CtrachomatisL2C), Porphyromonas gingivalis strain W83 (PgingivalisW83) and Porphyromonas gingivalis strain ATCC 33277 (PgingivalisATCC33277).
Immune evasion is an important process in which microorganisms may directly modulate host-directed processes in response to the presence of potential pathogens. Included within the arsenal of numerous microbes are secreted effectors, which are oftentimes proteins that are able to enzymatically alter the host’s intra- or extracellular environment. While Ndk has been proposed to be a secreted effector m for some microorganisms, current understanding of the multi-functional attributes of this enzyme in the context of the host-microbe interaction is quite limited. Despite this, potential roles for Ndk during host tissue colonization have been indicated, which include modulation of host-derived small danger molecule signaling [2,3,4,5,6,7,8,9]. In addition to the immuno-modulation, there appear to be alternative functions that Ndk likely assumes during microbial colonization. Within this review we define potential interactions for microbe-derived Ndk that have yet to be explored. Due to the incomplete characterization of Ndk in microbial systems, we suggest novel mechanisms that may be utilized by Ndk-expressing host adapted microorganisms to facilitate extracellular delivery of this effector enzyme. Finally, we discuss limitations that have persisted within microbe-derived Ndk research and describe recent utilization of an opportunistic bacterium, Porphyromonas gingivalis, and human primary gingival epithelial cell (GEC) model for novel characterization of Ndk in dynamic host-microbe interaction.
2. Microbe-derived Ndk supports colonization and aids in subversion of immunity via purinergic signaling
Key immune processes mediated by extracellular ATP (eATP) P2X7 receptor signaling include cell death, cell motility, microbial phagocytosis, and processing of the release pro-inflammatory cytokines [10]. These specific host responses induced by eATP support the idea that this host-derived small signaling molecule can be used to control or limit pathogenic colonization of host tissues. In fact, recent advances in purinergic signaling network identify eATP release following infection as vital for innate immune response to a variety of pathogens such as Bacillus anthracis [11] and Mycobacterium tuberculosis [12]. Central to the eATP-induced immune response to pathogens is the activation of the inflammasome complex, which promotes cleavage of caspase-1 and subsequent processing and release of mature pro-inflammatory cytokines, most notably interleukin (IL)-1β and IL-18 [13]. In addition to stimulating secretion of pro-inflammatory cytokines via the inflammasome complex, eATP-P2X7 signaling activates production of reactive-oxygen-species (ROS). Oxidative stress induction is an upstream activator for the inflammasome complex in addition to a mechanism for direct control over intracellular pathogens, as seen in phagocyte oxidative burst. There is a large body of evidence linking microbial control over purinergic signaling with reduced oxidative stress within host tissues. Additionally, Ndk secreted from P. gingivalis has recently been proposed to participate in this effect during the infection of human gingival epithelium [14].
In both acute and chronic pathologies, invading microorganisms are highly dependent on the ability to protect themselves from host mediated defense systems. It appears that Ndk applies its evolutionarily-conserved NTPase capabilities to diminish the levels of host derived extracellular purine signaling molecules such as eATP, indicating that Ndk has assumed additional roles within microorganisms. The abundance of information available for Ndk-purinergic signaling relationships allows us to speculate that purinergic signaling may in fact be the primary target for Ndk in modulating host immune response, as ample findings support Ndk as an effector molecule capable of influencing this elaborate signaling network [2,3,4,5,6,7,8,9].
2.1. A potential link between intracellular pathogens and Ndk-mediated host protection
At first glance, there appears to be no definitive theme that explains what researchers have observed when studying Ndk in the context of host-pathogen interaction. The literature indicates that in certain cases microbial Ndk appears to be host-protective, while in others Ndk enhances host-cell destruction. For the past decade it has seemed as if these two host-cell fates were linked with the lifestyle of the microbial invaders. However, recent advances in Ndk research bring new information and questions to the table. Host immune subversion is a common theme for both intra- and extracellular pathogens, yet the role of Ndk in pathologies associated with the former group is much more certain than for the latter class of microorganisms. We provide evidence supporting the theory that Ndk is a host-protective effector molecule especially secreted by host-adapted intracellular pathogens, allowing these microorganisms to establish successful persistent infections in their target host tissues [14].
The opportunistic periodontal pathogen P. gingivalis is a successful colonizer of oral mucosa that can invade and spread intercellularly in human gingival epithelium without alerting the immune system to its presence [15,16]. P. gingivalis possesses a variety of immune evasion strategies [17] and Ndk appears to be an emerging component of its arsenal of effector molecules. Given that local eATP levels participate in activating highly regulated immune processes to limit infection, preventing the initial eATP-mediated host responses seems to be essential for establishment of successful chronic infections by P. gingivalis [7]. We previously demonstrated that eATP-P2X7 receptor coupling induces GEC death and this damaging effect can be abrogated by the infection with P. gingivalis, whereas the ndk-deficient, mutant-infected GECs show much higher levels of cell death. Also, culture supernatants of ndk-deletion mutant P. gingivalis demonstrate reduced ATPase activity and the incubation of eATP-treated host cells with the purified Ndk substantially blocks the P2X7 associated membrane pore formation. Additionally, complementation of ndk-mutant P. gingivalis restores the observed host cell - protection seen for the wild-type phenotype. A recent review on human primary GECs infection model directly links the secreted P. gingivalis Ndk with the modulation and inhibition of eATP-mediated cellular ROS production via membrane bound NADPH-oxidase complex and mitochondrial signaling pathways for affluent bacterial intracellular life [14]. This new information indicates dual modes of action for the Ndk, which, appears to function at both intracellular and extracellular levels during host infection.
Burkholderia cepacia complex (BCC) is a grouping of several opportunistic species of Burkholderia (e.g. B. cenocepacia) that are associated with fatal pulmonary infections in cystic fibrosis (CF) patients. BCC member species are capable of invading host cells, and numerous studies demonstrate employment of immune evasion strategies such as arrest of phagosome maturation [18] or delayed NADPH-oxidase complex recruitment to phagosomes [19]. While Ndk also appears to participate in immune modulation during B. cepacia associated infections, the effects Ndk mediates seem to still be controversial. It has been shown that ATP-utilizing enzymes, including Ndk, from B. cepacia have differential effects on the host based on whether the microorganism belongs to clinical or environmental strains [4]. It is found that clinical strains secrete Ndk and other ATP-utilizing enzymes that contribute to macrophage and mast cell death, primarily through modification of eATP and generation of other adenine nucleoside molecules [4]. While the major activator of the P2X7 receptor is eATP, other forms of adenine nucleotide molecules (e.g. ADP, AMP) has been suggested to stimulate the receptor following initial ligation by eATP [20].
Additionally, other modifications made to ATP (e.g. Benzoyl-ATP) could more robustly activate P2X7, resulting in enhanced membrane instability and cell death. Coupled with the observed cytotoxicity induced by B. cepacia, it is implied that secreted products of this microorganism facilitate such modifications of eATP, contributing to host cell death. Interestingly, Ndk was found to be relatively inactive in clinical strains when compared to environmental strains [4]. In the latter case, infection did not result in enhanced cell death, suggesting that a more active Ndk may be the contributing factor that determines host-cell survival during environmentally derived B. cepacia infection. Environmental strains of B. cepacia are likely to not be as virulent as clinical strains in terms of early stage colonization. Thus, Ndk may help in the establishment of opportunistic infection by preventing eATP-mediated host tissue destruction.
Mycobacterial species have long been associated with severe debilitating pathologies and are known to target macrophages to support their intracellular replication. Accordingly, host-response subversion in Mycobacteria is extensively studied [21] and Ndk has been proposed to be one of the potential key contributors to Mycobacterial persistence in various mammalian hosts. Originally observed in 1999, Mycobacterium bovis bacille Calmette-Guérin (BCG) secreted filtrates containing Ndk were able to prevent eATP-mediated macrophage cell death [3]. In this model Ndk acted to prevent host-cell death, allowing phagocytes to survive, which is an advantageous strategy employed by the mycobacterial species’ favored stealth life style for successful long term carriage in host cells.
Much like for their bacterial counterparts, the eATP-P2X7 signaling network is an important host response to limit infection by eukaryotic pathogens. Trypanasoma cruzi, the causative agent of Chagas’ disease in humans, infects many tissue types such as macrophages, fibroblasts and the endothelium [22]. During acute infection with this microorganism, eATP-P2X7 signaling acts to suppress dissemination, and has been associated with lymphocyte population destruction resulting in splenic atrophy [23] ]. In another pathology caused by a eukaryotic pathogen, Toxoplasmosis, activation of P2X7 receptor by eATP mediates destruction of Toxoplasma gondii tachyzoites in macrophages [24]. On the other hand, for other eukaryotic opportunistic pathogens such as Leishmania amazonensis, recent research identifies a role for Ndk in the attenuation of eATP-P2X7-mediated host cell death, contributing to host and pathogen survival [8]. Leishmaniasis is a condition in which vector-borne pathogenic protozoans belonging to the Genus Leishmania cause chronic pathologies in vertebrate hosts. For certain species of Leishmania, such as L. amazonensis, evidence suggests a dependence on immunocompetence in host to maintain a viable infection [25]. This requirement for an active immune response is of great interest because it implies that pathogen-directed immune evasion strategies are necessary for growth of the microorganism once within host tissue. For L. amazonensis, Ndk participates in immune evasion by preventing eATP-mediated macrophage death [8]. Specifically, Leishmania-secreted Ndk was found to keep the host cell membrane integrity intact, and stabilized the mitochondrial membrane potential in macrophages, thus preventing host-cell death.
It seems that for intracellular pathogens there is a unifying theme of Ndk protecting host cells from eATP-mediated cell death. Nonetheless, for extracellular pathogens that do not require a similar host environment for their infectious cycle, Ndk may not be needed for the same purposes. For several years, the theory on the differential Ndk utilization by various microorganisms has been challenging for researchers to fully understand. Until very recently the role of Ndk in extracellular pathogens appeared to be damaging in nature, yet upon careful analyses of previous literature it seems that Ndk does not fully participate in this effect. We discuss below the limitations of previous studies that weakened the clarity of Ndk’s role in extracellular microorganism pathogenesis and cite a current model that has provided a new insight on this issue.
2.2. Does a different role for Ndk in infections caused by extracellular pathogens exist?
The extracellular pathogen Vibrio cholerae is associated with acute diarrheal conditions and evidence suggested a potential role for Ndk in the pathogenesis of this bacterium. In a 2000 study of V. cholerae, secreted factors enhanced macrophage and mast cell death upon stimulation with eATP [5]. Enzyme activities were assayed and Ndk was detected along with other ATP-modifying enzymes. The investigators treated macrophages and mast cells with the mixed supernatant fractions with and without eATP and found that in the latter condition, cell death and the associated morphological changes such as nuclear fragmentation and vacuolization occurred at higher rates. Additionally, oxidized ATP (an irreversible inhibitor of P2X7 receptor) treatment of host cells inhibited these effects, suggesting that V. cholerae supernatant fractions are able to stimulate P2X7 receptors in a manner that increases phagocytic cell death. In this study, the role of Ndk in the observed host cell death was debatable, with release of other ATP-modifying enzymes being attributed with enhanced P2X7 activation [20]. It is possible that a key limitation of the study was that only concentrated V. cholerae growth culture supernatant fractions were assayed, making it particularly difficult to directly link specific bacterial effectors with host-cell death or survival. This approach was though consistent with the previous bacterial Ndk studies which exclusively utilized isolated fractions and cell free extracts to study dynamic host-pathogen interactions.
Another extracellular pathogen, Pseudomonas aeruginosa, has been suggested to employ Ndk within its arsenal of host-destructive effector molecules [2]. In that study only mucoid P. aeruginosa strains secreted Ndk and exogenous ATP mixed with supernatant from mucoid strain P. aeruginosa 8821M demonstrated significantly enhanced cell death in macrophages in comparison to nonmucoid strain P. aeruginosa PAO1. More importantly, it was found that Ndk-free supernatant fractions were responsible for the observed macrophage death. The authors attribute results showing reduced levels of host cell death in fractions containing Ndk to the enzyme’s ability to cleave eATP, effectively consuming the signal responsible for activating P2X7 receptor. In a subsequent study of nonmucoid P. aeruginosa strain 808, the hypothesis that Ndk is not involved in eATP-mediated cell death of phagocytes was further confirmed. Results from this investigation show that Ndk is a component of the secretome of this P. aeruginosa strain. However it is much less active than other ATP-modifying enzymes [6].
Thus, these findings suggest that for both P. aeruginosa and V. cholerae, Ndk is likely not involved with immune cell death and it is not linked to cytotoxicity. In fact, Ndk has been shown to have cyto-protective characteristics in other pathogens [3,7,8]. It appears that Ndk plays a more specific role for opportunistic pathogens that have the ability to successfully co-exist and persist in their target host cells. Extracellular pathogens such as V. cholerae and P. aeruginosa do not require host cell survival for the establishment or maintenance of infection, and so host-protective enzymes such as Ndk have not been evolutionarily designed to perform the same roles as they have been for facultative or obligate intracellular pathogens. It is interesting to note that extracellular opportunistic commensals do not appear to encode this highly conserved enzyme within their genomes including Fusobacterium nucleatum [26]
A very recent study has further solidified the importance of cyto-protective attributes of Ndk for intracellular pathogens and has provided new information regarding the alleged functional distinctions between Ndk homologs existing in both intra- and extracellular microorganisms. Concentrated cell free supernatants from intracellular M. tuberculosis and Salmonella typhimurium prevented J774 macrophage cell death induced by eATP treatment [9]. The treatment of J774 cells with the bacterial growth media secreted products of ndk-mutant S. typhimurium in the presence of eATP resulted in cell death. In order to analyze the potential functional differences between Ndk in intra- or extracellular pathogens, the study deleted the Ndk-gene from S. typhimurium and replaced it with the homolog from extracellular V. cholerae. The complemented gene product showed exact biochemical properties (autophosphorylation and phosphotransfer activities) as the wild-type S. typhimurium Ndk. More intriguingly, experiments using V. cholerae transformed Ndk-mutant S. typhimurium concentrated bacterial supernatants still displayed host-cell protective phenotype [9]. Moreover, the ndk-mutant of V. cholerae growth culture supernatants induced significantly higher number of host cell death than the wild strain. The combined results of this study suggest that regardless of the intra- or extracellular lifestyle of the microorganism, biochemical activity and host-cell protection mediated by Ndk is conserved.
What once began as a possible functional division in the utilization of Ndk by these two groups of pathogenic microorganisms now seems to be an outdated theory. We can argue that Ndk, within the intracellular pathogen secretome, serves to facilitate infection by targeting of eATP-P2X7 signaling via complex inside-out signaling, while extracellular pathogens favor the utilization of other ATP-modifying enzymes which exclusively function on the outside of host cell membrane (there is evidence of other enzymes performing similar roles of Ndk during microbial colonization of host tissues [27], however Ndk may be of greater importance during infection due to the multiple immune-evasion strategies it may participate in).
It seems that a potentially new division has emerged between intra- and extracellular microorganisms based on the use of different types of ATP-modifying enzymes during colonization. As we have seen in several studies, non-Ndk ATP-modifying enzymes inducing pro-apoptotic phenotypes are likely important components of the pathogenic nature of various extracellular microorganisms. At the same time, these enzymes appear to be repressed or overshadowed through host-protective enzymes such as Ndk secreted by host-adapted intracellular microorganisms. The fact that Ndk is conserved by both of these groups implies that there are likely conserved regulatory mechanisms utilized to control expression and use of ATP-modifying enzymes, though investigation into this line of thinking remains unexplored. Another area of Ndk research that has eluded researchers concerns the mechanisms of secretion of Ndk from prokaryotic microbes as well as high organisms including human cells. The remainder of this article will discuss potential routes of secretion from both microbes and host cells by pointing to potential modes of interactions between the two systems in which Ndk plays a central role. Thus, we propose plausible exploitation of host secretion systems for delivery of microbe-derived Ndk to the extracellular environment and for targeting of specific cellular machineries for successful host infection.
3. Ndk in human biology: A potential target for microbial host manipulation?
Conservation of Ndk is of great interest for evolutionary biologists due to its “housekeeping” role in nucleotide pool maintenance. Recent studies suggests a pre-Metazoan origin for Ndk within eukaryotes [28]. A recent review by Boissan, et al. divides the ten human Ndk species-namely, “Nme” genes-into two groups, with the first containing the more closely related human-Ndk isoforms 1-4 and the second group bearing the more divergent isoforms [29]. It also is worth noting that the former group contains the isoforms in which Ndk activity has been definitely demonstrated. Human-Ndk isoform 1 is famous for its identification as the first metastasis suppressor gene, [30] and disruption of this protein has been attributed to enhanced metastatic infiltration of tumor cells in breast cancer [31]. Emerging evidence also suggests that human-Ndk isoform 1 is selectively targeted by multiple oncogenic viruses and that viral proteins are responsible for disruption of the enzyme’s functions, resulting in enhanced metastatic potential of infected cells [32].
3.1. Exploitation of human Nme (Ndk) may allow microbe-mediated transformation
Several viral pathogens, including Epstein-Barr virus (EBV), Human Papillomavirus (HPV) and Kaposi’s Sarcoma-associated herpesvirus (KSHV), are associated with host cell transformation, resulting in severe malignancies of host tissues. EBV, HPV and KSHV have all been shown to directly interact with human-Ndk isoform 1[33,34,35], resulting in functional suppression of the enzyme. For EBV, EBV nuclear antigens (EBNA) 3C and 1 appear to be very important for virus-mediated inhibition of human-Ndk isoform 1. EBNA3C inhibits the ability of this human-Ndk isoform to prevent migration of Burkitt lymphoma and breast cancer cells [36] and lymphoblastoid cells [37]. This interaction of EBNAs with human-Ndk isoform 1 is mediated by a specific contact region within the EBNA protein and it appears that EBNA3C can influence transcription-activating properties associated with malignant transformation [38].
During infection of host tissues with KSHV, latency-associated nuclear antigen (LANA) encoded by the virus mediated nuclear translocation of human-Ndk isoform 1. This interaction resulted in the activation of mitogen-associated protein kinase pathways, secretion of pro-migratory factors and invasive cell phenotype [35]. In another study, HPV E7 protein-expressing keratinocytes were demonstrated to have reduced human-Ndk isoforms 1 and 2 expression, resulting in the acquisition of metastatic characteristics [33]. Thus, it seems that interactions between viral proteins and host-Ndk isoforms may coordinately regulate transcription and metastatic capabilities of virus-infected cells. Perhaps certain effectors possessed by colonizing microorganisms could also potentiate suppression of human-Ndk isoforms, inducing transformation of host cells and contributing to malignancies. Indeed, certain opportunistic bacteria, such as Helicobacter pylori, are able to induce carcinogenesis in host tissues via secretion of effector molecules that interact with oncogenic pathways [39]. Interestingly, cyclooxygenase-2, an important enzyme involved with inflammation and metastasis, is affected by both H. pylori cag-pathogenicity island and EBNA3C-human-Ndk isoform-1 interactions, suggesting a conservation of host metastasis suppressor interference across evolution. It is tempting to speculate that selective colonizing bacteria could possess adaptive Ndk-mediated mechanisms that result in enhanced host-cell metastatic potential.
3.2. Coupling of host energetics and membrane trafficking via Ndk may support persistent infection
While human-Ndk isoform 1 has been primarily attributed with suppression of tumor metastasis, isoform 2 has been shown to be a major participant in endoplasmic reticulum (ER) and other membrane-associated processes. Assembly of cargo vesicles within the ER is a highly ordered process that involves utilization of high-energy GTP molecules for recruitment and activity of coat proteins [40]. These coat proteins facilitate membrane budding of the ER, resulting in the formation of coat protein complexes such as coat protein complex II (COPII) on the periphery of an ER-derived vesicle. During later stages of vesicle maturation and trafficking, COPII coats are disassembled and shed from cargo vesicles via activity of GTPase activating proteins (GAPs) in a highly ordered process [41].
Human-Ndk isoform 2 has been shown to be vital for COPII complex assembly and ER export [42] and it has also been shown to interact with lipid phosphotidylinositols (PtdIns) 4P and 4,5P2 [43]. PtdIns allow binding of lipids and proteins, in addition to interacting with vesicle receptors that mediate fusion with membranes [44]. Interestingly, human-Ndk isoform 2 was shown to possess specific surface domains that allow interaction with anionic liposomes, resulting in cross-linking of the lipid vesicles that formed membrane-like networks [43]. Other studies show additional interactions between human-Ndk isoform 2 and membrane components, implicating this human protein with endocytic caveolae stability and complex formation with G-proteins [45].
Mitochondria are responsible for producing ATP via oxidative phosphorylation, a complex system located within the inner mitochondrial membrane. Another important component of the inner mitochondrial membrane is cardiolipin, a negatively charged lipid that participates in membrane dynamics associated with mitochondrial fusion and fission [46]. In addition to providing energy, the mitochondrion is a major contributor to cell fate by promoting apoptosis in response to exogenous signals, including eATP. Intriguingly, human-Ndk isoform 4 has been shown to localize to the inner mitochondrial membrane and interacts predominantly with cardiolipin [47]. This protein utilizes surface-associated electrostatic interactions to bind cardiolipin, resulting in membrane network formation that was also observed between PtdIns and human-Ndk isoform 2 [43]. It is thought that human-Ndk isoform 4 could provide the contact points between the inner and outer mitochondrial membranes, facilitating lipid transfer between these subcellular locations [48]. The fact that two human-Ndk isoforms can directly bind lipids opens the possibility that this could be a conserved feature that may be found in Ndk of other organisms.
Based on the previously indicated human-Ndk associations with membrane components of the ER and mitochondria, it is logical to suggest that Ndk from colonizing microorganisms may also localize to the similar subcellular compartments to support their survival. A recent review article by Mehta et al. proposes that human-Ndk isoforms may act in concert to provide a cellular phosphorelay network by bridging the mitochondria with the ER and cell membrane [49]. Additionally, it appears that human-Ndk could provide cytoskeletal-like stability to such a network, as monomers of isoform 2 self-assemble into filamentous structures in vitro when NTP sources are provided [50]. With isoform-4 and cardiolipin serving as a mitochondrial anchor, it is feasible that isoform-2 and lipid/ER interactions could provide a means for energy within ATP to be directly or indirectly transferred to the cell membrane. Tokarska-Schlattner et al. also showed that human-Ndk isoform 4 is involved with coupling of mitochondrial respiration and nucleotide transfer [47]. This could represent an emerging paradigm for microbial exploitation of host energetics that enhances intracellular replication or proliferation. If microorganisms could find a way to intercept or participate with human-Ndk energy networks, they would have a ready supply of energy at their disposal that could permit expansion of colonization.
For certain host-adapted microorganisms, such as P. gingivalis, there is strong evidence providing a direct link between infection and interaction with mitochondria and the ER. Intracellular infection of human epithelial cells with this opportunistic bacterium has been shown to result in interference with intrinsic mitochondrial-cell death pathways [51], inhibited recruitment of pro-apoptotic factors [52], attenuated cytochrome c release and stabilization of mitochondrial membrane potential [53]. Additionally, more recent evidence suggests that intracellular P. gingivalis co-localizes with the ER network [54] and that P. gingivalis infection results in suppressed mitochondrial-ROS production via secretion of bacterial Ndk [manuscript in submission]. The significant amount of data suggesting a direct interaction between P. gingivalis and the mitochondrion and ER leads us to speculate that there is likely a role(s) for Ndk in processes mediated by these cellular components. For other potential pathogens, similar evidence for microbe-derived Ndk interactions with host-cell processes may be waiting to be uncovered.
3.3. Potential functional mimicry of host Ndk by microbes
We have provided evidence supporting the potential for microbial targeting of specific processes influenced by human host Nm23 isoforms. When searching for additional host processes that could be directly affected by microbe-derived Ndk, one does not have to search far in order to recognize potential infection-related targets.
As we have mentioned, GAPs are important components of ER-derived cargo vesicle formation and trafficking. An interesting study of Mycobacteria Ndk suggests that this effector can inhibit phagosome maturation by assuming GAP roles [55]. Specifically, the study utilizes recombinant M. tuberculosis Ndk which exerts GAP activity towards host cell small GTPases Rab5 and Rab7, resulting in failure to recruit downstream effectors of these molecules. Both of these proteins are temporal markers of phagosome maturation, with Rab5 associating at early stages and mediating transition to late forms and Rab7 mediating fusion with late endosomes and lysosomes [56]. GTPases must be bound to GTP to remain in an active form, thus M. tuberculosis Ndk may convert GTP to GDP, essentially inactivating these phagosome-associated components. This remains the only study in which microbe-derived Ndk has been shown to adopt GAP activity, while this could be a conserved mechanism as Ndk from other microorganisms retains the underlying NTPase activity suggested for the observed phagosome maturation inhibition. Nevertheless, the lack of genetically tractable M. tuberculosis systems in general poses a significant impediment in functional characterization of Ndk in dynamic pathogen-host interaction.
In the context of microbial colonization, we have presented eATP-P2X7 coupling as a direct target for modulation via microbial-secreted Ndk. However, other indirect mechanisms are likely to exist. The ability of human-Ndk isoform 2 to bind PtdIns molecules may be of interest for researchers studying Ndk in microbial colonization, especially if microbe-derived Ndk could be demonstrated to perform a similar role. Ndk-mediated inhibition of purinergic signaling during microbial colonization may be of relevance to this because recent evidence shows that PtdIns molecules interact with P2X receptors [57]. In fact, eATP-induced cell death via P2X7 has been shown to be dependent on receptor interaction with PtdIns molecules in various tissues, including macrophages and primary T-lymphocytes [58]. Thus, if microbe-secreted Ndk could cleave eATP while simultaneously interacting with intracellular PtdIns, P2X7-mediated responses could be more highly orchestrated, and perhaps enabling for enhanced persistence of microorganisms (Table 1).
Utilization of Ndk in host-microbe interface
| Organism | Classification of Cell Wall |
Preferred lifestyle in host cells |
Proposed functional roles | Reference |
|---|---|---|---|---|
| Porphyromonas gingivalis | Gram -negative | Intracellular | Modulation of host purinergic signaling; attenuation of ROS production; protection against mitochondrial cell-death pathways(a) |
[7]; [unpublished] |
|
Salmonella enterica
typhimurium |
Gram -negative | Intracellular | Modulation of host purinergic signaling |
[9] |
| Pseudomonas aeruginosa | Gram -negative | Extracellular | Modulation of host purinergic signaling |
[3, 6] |
| Vibrio cholerae | Gram -negative | Extracellular | Modulation of host purinergic signaling |
[5,9] |
| Chlamydia trachomatis | Gram -negative | Intracellular | Unknown | [67](b) |
| Bacillus anthracis | Gram -positive | Intracellular | Unknown; spore formation(a) | [63] |
| Mycobacteria spp. | Other | Intracellular | Modulation of host purinergic signaling; inhibition of phagosome maturation |
[2, 53] |
| Leishmania amazonensis | N/A | Intracellular | Modulation of host purinergic signaling |
[8] |
| Homo sapiens H1 | N/A | N/A | Metastasis suppressor; target for oncogenic viruses |
[28, 31-36] |
| Homo sapiens H2 | N/A | N/A | Inter-membrane connections (ER); target for functional mimicry(a) |
[41] |
| Homo sapiens H4 | N/A | N/A | Inter-membrane connections (Mitochondria); target for functional mimicry(a) |
[45] |
Footnotes: Unpublished results leading to proposed functional roles for microbe-derived Ndk.
No known role for Ndk in Chlamydia spp. has been established, though it is present within its genome.
4. Non-classical secretion pathways may allow microbial Ndk to be transported outside host cells
As the enzyme does not bear any known leader signal sequence, secretion of Ndk in both human hosts and colonizing microorganisms is poorly understood. Despite the lack of the conventional section motif, the possibility for secretion via the ER and Golgi cannot be ruled out. In fact, it has been proposed that Ndk relies on C-terminal motifs for Type-I secretion in P. aeruginosa. However, the residues implicated for this activity appear to not fulfill the specific properties that the study suggests [59]. Additionally, the fact that there is no obvious secretion marker suggests that this enzyme may not have originally been intended for secretion, but rather that its sole purpose was maintenance of intracellular nucleotide maintenance. Though this is debatable, there is room for speculation that multiple host cell and microbial secretion strategies may be involved in the secretion and delivery of this enzyme. Mechanisms of non-classical protein secretion in eukaryotic cells include direct translocation across the cell membrane, flip-flop of proteins from the inner membrane to the outer membrane, plasma membrane transporters and membrane blebbing resulting in released cargo proteins from shed vesicles [60]. It would be advantageous for intracellular microorganisms to be able to utilize more than one of these cellular mechanisms in order to deliver their Ndk outside of host cells. The direct evidence of secretion of Ndk outside of host cells has not been demonstrated by any microorganism with exception of an initial study by P. gingivalis infection in human primary GECs [7]. Below, we propose novel pathways that may be utilized by intracellular microorganisms for effective extracellular delivery of Ndk.
4.1. Bacterial OMVs bearing Ndk may regulate entrance/exit of the enzyme from host cell
Assembly of OMVs incorporates proteins within the Gram-negative outer membrane as its own membrane and can include periplasm-associated proteins in the lumen of the OMV [61]. If Ndk is targeted to either the periplasmic space or the outer membrane of Gram-negative microorganisms, it could become incorporated into OMVs and may be subsequently delivered to host cells. Perhaps more importantly, Gram-negatives secreting OMVs during intracellular colonization may allow Ndk to associate with the host-cell plasma membrane, potentially allowing release of Ndk into the extracellular environment. Delivery of secreted effectors via OMVs has been proposed to occur for certain colonizing microorganisms [62] and we propose that such a mechanism may occur for many Gram-negatives. Other mechanisms such as utilization of IL-1β secretory machineries may be exploited by Gram-positives or other types of microorganisms, potentially allowing translocation of effector Ndk molecules outside the host cell.
4.2. Exploitation of the inflammasome and IL-1β secretion pathway
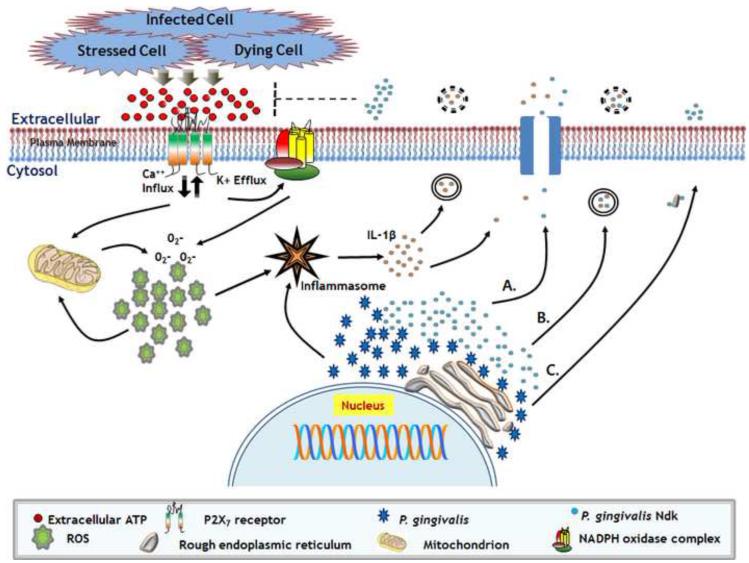
Nonclassical secretion pathways are important for host-derived molecules, including pro-inflammatory cytokine IL-1β. This cytokine does not bear a secretory signal peptide and is not suggested to be exported via classical ER-Golgi-mediated exocytosis. Although there is incomplete evidence, secretory lysosomes, plasma membrane-shed microvesicles, transporters, and even multivesicular bodies containing exosomes have been proposed to facilitate IL-1β release via caspase-1 activation upon eATP stimulation [63]. Additionally, IL-1β has been proposed to enter the lumen of vesicles via ATP-binding cassette (ABC) transporters [60]. ABC transporters are found within the cell membrane of Gram-negative microorganisms and mediate lipoprotein dynamics in the inner membrane, periplasm and outer membrane [64]. Bacterial Ndk has been found within supernatant and membrane fractions of bacterial cultures, thus bacterial-ABC transporters could operate in a fashion similar to those of the host and may allow membrane bound Ndk to be translocated into IL-1β-containing vesicles. It is conceivable that ~16 kDa Ndk can shed together with vesicles containing IL-1β which was also shown to be substantially secreted upon activation of P2X7 receptors by eATP during P. gingivalis infection in human primary GECs (Fig. 2) [65,66].
Fig. 2.
Schematic overview of eATP-P2X7 receptor signaling in potential exploitation by opportunistic pathogens for extracellular release of Ndk outside of host cells. Proposed utilization of IL-1β cytokine secretion system by P. gingivalis for delivery of Ndk enzyme outside the human gingival epithelial cells. (A) Movement of secreted Ndk through host cell membrane-spanning channels concurrently with IL-1β to extracellular environment. (B) Simultaneous incorporation of IL-1β and microbe-derived Ndk within secretory vesicles that fuse with host cell membrane and release contents extracellularly. (C) Association of microbe-derived Ndk with host ER-network and subsequent export via classical host-cell secretion pathways.
4.3. Potential association of microbe-Ndk with host lipids could facilitate translocation
As we discussed previously, strong evidence supports the association of human-Ndk isoforms with the ER and cardiolipin within mitochondria. If a similar interaction occurs with microbe-derived Ndk and these host components, it could be possible that Ndk secreted into the cytosol of a host cell is capable of intracellular trafficking, perhaps even to the cell membrane. The properties of human-Ndk that allow close association with lipid membrane components, such as PtdIns, can be traced to specific surface residues that mediate electrostatic interactions. Specifically, human-Ndk isoform 4 relies on Arg-Arg-Lys located at residues 89-91, with Arg-90 being highly conserved in vertebrate orthologues [47]. Similar interactions were observed for human-Ndk isoform 2 [43] and in both situations, the residues proposed to mediate lipid interaction are located on either face of the hexameric enzyme structure. Ndk found in numerous organisms, including B. anthracis [67] and T. cruzi [68] assemble to form hexamers according to x-ray crystallography studies. While there have not been investigations into determining the presence of the aforementioned surface residues within microbe-secreted Ndks, it could be possible that a motif performing functions similar to human-Ndk exists. If such a surface region on microbial Ndk is present, it would suggest a conserved mechanism to allow modulation of host membrane dynamics by host-adapted microorganisms. A potential possibility of intra-host-cell microbe-derived Ndk movement could be found to lie within the inner membrane of the mitochondrion. Upon induction of death-receptor mediated apoptosis, cardiolipin and its derivative metabolites have been observed to translocate to sites other than the mitochondria, including the cell membrane [69]. If microbe-derived Ndk associates with cardiolipin, it could be possible that it assumes a “hitchhiker” role within the cell and is redistributed to the ER or cell membrane during external signal-mediated apoptosis.
5. Conclusions
We have introduced Ndk as a multifunctional molecule that is under investigation by multiple disciplines, including cell biology and bacterial pathogenesis. It is likely that a large connection between host and microbe-derived Ndks exists, in part due to information that highlights the multi-faceted properties of this interesting enzyme coming to light more frequently. Because of the inability to identify a traditional mode of Ndk secretion from microorganisms, it could be that secretory Ndk is a relatively new aspect of the enzyme’s evolutionary history, further supporting the notion that this molecule is a marker of host-adaptation by invading microorganisms. Indeed, the recent studies performed with live P. gingivalis and its ndk deletion mutants in human GECs provide a unique model demonstrating direct novel physiological functions for Ndk that are likely to be key modulators in successful host colonization by opportunistic pathogens. Despite the limited knowledge of Ndk the scientific community currently possesses, it seems that future characterizations into this microbial effector are essential to answering emerging questions that may have a profound impact on the way we understand pathogen adaptation in host tissues inflicted by chronic infections.
Acknowledgements
This work is supported by the NIH-NIDCR grants R01DE016593 and R01DE019444. Authors thank their laboratory members, Dr. Chul Hee Choi and Mr. Jefferson V DeGuzman for their invaluable support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chakrabarty AM. Nucleoside diphosphate kinase: role in bacterial growth, virulence, cell signalling and polysaccharide synthesis. Mol. Microbiol. 1998;28:875–882. doi: 10.1046/j.1365-2958.1998.00846.x. [DOI] [PubMed] [Google Scholar]
- [2].Zaborina O, Misra N, Kostal J, Kamath S, Kapatral V, El-Idrissi ME, Prabhakar BS, Chakrabarty AM. P2Z-Independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect. Immun. 1999;67:5231–5242. doi: 10.1128/iai.67.10.5231-5242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty AM. Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol. Microbiol. 1999;31:1333–1343. doi: 10.1046/j.1365-2958.1999.01240.x. [DOI] [PubMed] [Google Scholar]
- [4].Melnikov A, Zaborina O, Dhiman N, Prabhakar BS, Chakrabarty AM, Hendrickson W. Clinical and environmental isolates of Burkholderia cepacia exhibit differential cytotoxicity towards macrophages and mast cells. Mol. Microbiol. 2000;36:1481–1493. doi: 10.1046/j.1365-2958.2000.01976.x. [DOI] [PubMed] [Google Scholar]
- [5].Punj V, Zaborina O, Dhiman N, Falzari K, Bagdasarian M, Chakrabarty AM. Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect. Immun. 2000;68:4930–4937. doi: 10.1128/iai.68.9.4930-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zaborina O, Dhiman N, Ling Chen M, Kostal J, Holder IA, Chakrabarty AM. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiology. 2000;146:2521–2530. doi: 10.1099/00221287-146-10-2521. [DOI] [PubMed] [Google Scholar]
- [7].Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, Lamont RJ, Ojcius DM. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell. Microbiol. 2008;10:863–875. doi: 10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kolli BK, Kostal J, Zaborina O, Chakrabarty AM, Chang KP. Leishmania-released nucleoside diphosphate kinase prevents ATP-mediated cytolysis of macrophages. Mol. Biochem. Parasitol. 2008;158:163–175. doi: 10.1016/j.molbiopara.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dar HH, Prasad D, Varshney GC, Chakraborti PK. Secretory Nucleoside Diphosphate Kinase from both the Intra- and Extra-cellular Pathogenic Bacteria are functionally indistinguishable. Microbiology. 2011 doi: 10.1099/mic.0.049221-0. [DOI] [PubMed] [Google Scholar]
- [10].Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- [11].Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax Toxin Induces Macrophage Death by p38 MAPK Inhibition but Leads to Inflammasome Activation via ATP Leakage. Immunity. 2011;35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu K, Boom WH, Dubyak GR, Harding CV. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing major histocompatibility complex class II molecules capable of antigen presentation. Infect. Immun. 2010;78:5116–5125. doi: 10.1128/IAI.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr. Opin. Immunol. 2009;21:10–16. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spooner R, Yilmaz O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int. J. Mol. Sci. 2011;12:334–352. doi: 10.3390/ijms12010334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yilmaz O, Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect. Immun. 2006;74:703–710. doi: 10.1128/IAI.74.1.703-710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yilmaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology. 2008;154:2897–2903. doi: 10.1099/mic.0.2008/021220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes. Infect. 2009;11:637–645. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huynh KK, Plumb JD, Downey GP, Valvano MA, Grinstein S. Inactivation of macrophage Rab7 by Burkholderia cenocepacia. J. Innate Immun. 2010;2:522–533. doi: 10.1159/000319864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Keith KE, Hynes DW, Sholdice JE, Valvano MA. Delayed association of the NADPH oxidase complex with macrophage vacuoles containing the opportunistic pathogen Burkholderia cenocepacia. Microbiology. 2009;155:1004–1015. doi: 10.1099/mic.0.026781-0. [DOI] [PubMed] [Google Scholar]
- [20].North RA. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- [21].Meena LS. Rajni, Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS. J. 2010;277:2416–2427. doi: 10.1111/j.1742-4658.2010.07666.x. [DOI] [PubMed] [Google Scholar]
- [22].Epting CL, Coates BM, Engman DM. Molecular mechanisms of host cell invasion by Trypanosoma cruzi. Exp. Parasitol. 2010;126:283–291. doi: 10.1016/j.exppara.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mantuano-Barradas M, Henriques-Pons A, Araujo-Jorge TC, Di Virgilio F, Coutinho-Silva R, Persechini PM. Extracellular ATP induces cell death in CD4+/CD8+ double-positive thymocytes in mice infected with Trypanosoma cruzi. Microbes. Infect. 2003;5:1363–1371. doi: 10.1016/j.micinf.2003.09.017. [DOI] [PubMed] [Google Scholar]
- [24].Correa G, Marques da Silva C, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R. Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes. Infect. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- [25].Colmenares M, Kar S, Goldsmith-Pestana K, McMahon-Pratt D. Mechanisms of pathogenesis: differences amongst Leishmania species. Trans. R. Soc. Trop. Med. Hyg. 2002;96(Suppl 1):S3–7. doi: 10.1016/s0035-9203(02)90044-1. [DOI] [PubMed] [Google Scholar]
- [26].Wolf M, Muller T, Dandekar T, Pollack JD. Phylogeny of Firmicutes with special reference to Mycoplasma (Mollicutes) as inferred from phosphoglycerate kinase amino acid sequence data. Int. J. Syst. Evol. Microbiol. 2004;54:871–875. doi: 10.1099/ijs.0.02868-0. [DOI] [PubMed] [Google Scholar]
- [27].Sansom FM, Robson SC, Hartland EL. Possible effects of microbial ecto-nucleoside triphosphate diphosphohydrolases on host-pathogen interactions. Microbiol. Mol. Biol. Rev. 2008;72:765–781. doi: 10.1128/MMBR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Desvignes T, Pontarotti P, Bobe J. Nme gene family evolutionary history reveals pre-metazoan origins and high conservation between humans and the sea anemone, Nematostella vectensis. PLoS One. 2010;5:e15506. doi: 10.1371/journal.pone.0015506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Boissan M, Dabernat S, Peuchant E, Schlattner U, Lascu I, Lacombe ML. The mammalian Nm23/NDPK family: from metastasis control to cilia movement. Mol. Cell. Biochem. 2009;329:51–62. doi: 10.1007/s11010-009-0120-7. [DOI] [PubMed] [Google Scholar]
- [30].Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA, Sobel ME. Evidence for a novel gene associated with low tumor metastatic potential. J. Natl. Cancer. Inst. 1988;80:200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- [31].Steeg PS, de la Rosa A, Flatow U, MacDonald NJ, Benedict M, Leone A. Nm23 and breast cancer metastasis. Breast Cancer Res. Treat. 1993;25:175–187. doi: 10.1007/BF00662142. [DOI] [PubMed] [Google Scholar]
- [32].Saha A, Robertson ES. Functional modulation of the metastatic suppressor Nm23-H1 by oncogenic viruses. FEBS. Lett. 2011 doi: 10.1016/j.febslet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mileo AM, Piombino E, Severino A, Tritarelli A, Paggi MG, Lombardi D. Multiple interference of the human papillomavirus-16 E7 oncoprotein with the functional role of the metastasis suppressor Nm23-H1 protein. J. Bioenerg. Biomembr. 2006;38:215–225. doi: 10.1007/s10863-006-9037-y. [DOI] [PubMed] [Google Scholar]
- [34].Murakami M, Kaul R, Kumar P, Robertson ES. Nucleoside diphosphate kinase/Nm23 and Epstein-Barr virus. Mol. Cell. Biochem. 2009;329:131–139. doi: 10.1007/s11010-009-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Qin Z, Dai L, Toole B, Robertson E, Parsons C. Regulation of Nm23-H1 and cell invasiveness by Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2011;85:3596–3606. doi: 10.1128/JVI.01596-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Subramanian C, Cotter MA, 2nd, Robertson ES. Epstein-Barr virus nuclear protein EBNA-3C interacts with the human metastatic suppressor Nm23-H1: a molecular link to cancer metastasis. Nat. Med. 2001;7:350–355. doi: 10.1038/85499. [DOI] [PubMed] [Google Scholar]
- [37].Murakami M, Lan K, Subramanian C, Robertson ES. Epstein-Barr virus nuclear antigen 1 interacts with Nm23-H1 in lymphoblastoid cell lines and inhibits its ability to suppress cell migration. J. Virol. 2005;79:1559–1568. doi: 10.1128/JVI.79.3.1559-1568.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Subramanian C, Robertson ES. The metastatic suppressor Nm23-H1 interacts with EBNA3C at sequences located between the glutamine- and proline-rich domains and can cooperate in activation of transcription. J. Virol. 2002;76:8702–8709. doi: 10.1128/JVI.76.17.8702-8709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ding SZ, Goldberg JB, Hatakeyama M. Helicobacter pylori infection, oncogenic pathways and epigenetic mechanisms in gastric carcinogenesis. Future Oncol. 2010;6:851–862. doi: 10.2217/fon.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jensen D, Schekman R. COPII-mediated vesicle formation at a glance. J Cell Sci. 2011;124:1–4. doi: 10.1242/jcs.069773. [DOI] [PubMed] [Google Scholar]
- [41].Lee MC, Miller EA. Molecular mechanisms of COPII vesicle formation. Semin. Cell. Dev. Biol. 2007;18:424–434. doi: 10.1016/j.semcdb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- [42].Kapetanovich L, Baughman C, Lee TH. Nm23H2 facilitates coat protein complex II assembly and endoplasmic reticulum export in mammalian cells. Mol. Biol. Cell. 2005;16:835–848. doi: 10.1091/mbc.E04-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Baughman C, Morin-Leisk J, Lee T. Nucleoside diphosphate kinase B (NDKB) scaffolds endoplasmic reticulum membranes in vitro. Exp. Cell. Res. 2008;314:2702–2714. doi: 10.1016/j.yexcr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- [44].Chasserot-Golaz S, Coorssen JR, Meunier FA, Vitale N. Lipid dynamics in exocytosis. Cell. Mol. Neurobiol. 2010;30:1335–1342. doi: 10.1007/s10571-010-9577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hippe HJ, Wolf NM, Abu-Taha HI, Lutz S, Le Lay S, Just S, Rottbauer W, Katus HA, Wieland T. Nucleoside diphosphate kinase B is required for the formation of heterotrimeric G protein containing caveolae. Naunyn Schmiedebergs Arch. Pharmacol. 2011 doi: 10.1007/s00210-011-0618-x. [DOI] [PubMed] [Google Scholar]
- [46].Huang H, Frohman MA. Lipid signaling on the mitochondrial surface. Biochim. Biophys. Acta. 2009;1791:839–844. doi: 10.1016/j.bbalip.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tokarska-Schlattner M, Boissan M, Munier A, Borot C, Mailleau C, Speer O, Schlattner U, Lacombe ML. The nucleoside diphosphate kinase D (NM23-H4) binds the inner mitochondrial membrane with high affinity to cardiolipin and couples nucleotide transfer with respiration. J. Biol. Chem. 2008;283:26198–26207. doi: 10.1074/jbc.M803132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Epand RF, Schlattner U, Wallimann T, Lacombe ML, Epand RM. Novel lipid transfer property of two mitochondrial proteins that bridge the inner and outer membranes. Biophys. J. 2007;92:126–137. doi: 10.1529/biophysj.106.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mehta A, Orchard S. Nucleoside diphosphate kinase (NDPK, NM23, AWD): recent regulatory advances in endocytosis, metastasis, psoriasis, insulin release, fetal erythroid lineage and heart failure; translational medicine exemplified. Mol. Cell. Biochem. 2009;329:3–15. doi: 10.1007/s11010-009-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Morin-Leisk J, Lee TH. Nucleotide-dependent self-assembly of Nucleoside Diphosphate Kinase (NDPK) in vitro. Biochim. Biophys. Acta. 2008;1784:2045–2051. doi: 10.1016/j.bbapap.2008.07.011. [DOI] [PubMed] [Google Scholar]
- [51].Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, Stavropoulos MF, Yilmaz O, Lamont RJ. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell. Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yao L, Jermanus C, Barbetta B, Choi C, Verbeke P, Ojcius DM, Yilmaz O. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral. Microbiol. 2010;25:89–101. doi: 10.1111/j.2041-1014.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yilmaz O, Jungas T, Verbeke P, Ojcius DM. Activation of the phosphatidylinositol 3-kinase/Akt pathway contributes to survival of primary epithelial cells infected with the periodontal pathogen Porphyromonas gingivalis. Infect. Immun. 2004;72:3743–3751. doi: 10.1128/IAI.72.7.3743-3751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Choi CH, DeGuzman JV, Lamont RJ, Yilmaz O. Genetic transformation of an obligate anaerobe, P. gingivalis for FMN-green fluorescent protein expression in studying host-microbe interaction. PLoS One. 2011;6:e18499. doi: 10.1371/journal.pone.0018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sun J, Wang X, Lau A, Liao TY, Bucci C, Hmama Z. Mycobacterial nucleoside diphosphate kinase blocks phagosome maturation in murine RAW 264.7 macrophages. PLoS One. 2010;5:e8769. doi: 10.1371/journal.pone.0008769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Bohdanowicz M, Grinstein S. Vesicular traffic: a Rab SANDwich. Curr. Biol. 2010;20:R311–314. doi: 10.1016/j.cub.2010.02.030. [DOI] [PubMed] [Google Scholar]
- [57].Zhao Q, Logothetis DE, Seguela P. Regulation of ATP-gated P2X receptors by phosphoinositides. Pflugers Arch. 2007;455:181–185. doi: 10.1007/s00424-007-0271-x. [DOI] [PubMed] [Google Scholar]
- [58].Zhao Q, Yang M, Ting AT, Logothetis DE. PIP(2) regulates the ionic current of P2X receptors and P2X(7) receptor-mediated cell death. Channels (Austin) 2007;1:46–55. [PubMed] [Google Scholar]
- [59].Kamath S, Chen ML, Chakrabarty AM. Secretion of nucleoside diphosphate kinase by mucoid Pseudomonas aeruginosa 8821: involvement of a carboxy-terminal motif in secretion. J. Bacteriol. 2000;182:3826–3831. doi: 10.1128/jb.182.13.3826-3831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur. J. Biochem. 2003;270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- [61].Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes. Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- [62].Furuta N, Tsuda K, Omori H, Yoshimori T, Yoshimura F, Amano A. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect. Immun. 2009;77:4187–4196. doi: 10.1128/IAI.00009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bauernfeind F, Ablasser A, Bartok E, Kim S, Schmid-Burgk J, Cavlar T, Hornung V. Inflammasomes: current understanding and open questions. Cell. Mol. Life Sci. 2011;68:765–783. doi: 10.1007/s00018-010-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Narita S. ABC transporters involved in the biogenesis of the outer membrane in gram-negative bacteria. Biosci. Biotechnol. Biochem. 2011;75:1044–1054. doi: 10.1271/bbb.110115. [DOI] [PubMed] [Google Scholar]
- [65].Abdul-Sater AA, Said-Sadier N, Ojcius DM, Yilmaz O, Kelly KA. Inflammasomes bridge signaling between pathogen identification and the immune response. Drugs Today (Barc) 2009;45(Suppl B):105–112. [PMC free article] [PubMed] [Google Scholar]
- [66].Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell. Microbiol. 2010;12:188–198. doi: 10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Misra G, Aggarwal A, Dube D, Zaman MS, Singh Y, Ramachandran R. Crystal structure of the Bacillus anthracis nucleoside diphosphate kinase and its characterization reveals an enzyme adapted to perform under stress conditions. Proteins. 2009;76:496–506. doi: 10.1002/prot.22364. [DOI] [PubMed] [Google Scholar]
- [68].Souza TA, Trindade DM, Tonoli CC, Santos CR, Ward RJ, Arni RK, Oliveira AH, Murakami MT. Molecular adaptability of nucleoside diphosphate kinase b from trypanosomatid parasites: stability, oligomerization and structural determinants of nucleotide binding. Mol. Biosyst. 2011;7:2189–2195. doi: 10.1039/c0mb00307g. [DOI] [PubMed] [Google Scholar]
- [69].Sorice M, Circella A, Cristea IM, Garofalo T, Di Renzo L, Alessandri C, Valesini G, Esposti MD. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell. Death Differ. 2004;11:1133–1145. doi: 10.1038/sj.cdd.4401457. [DOI] [PubMed] [Google Scholar]