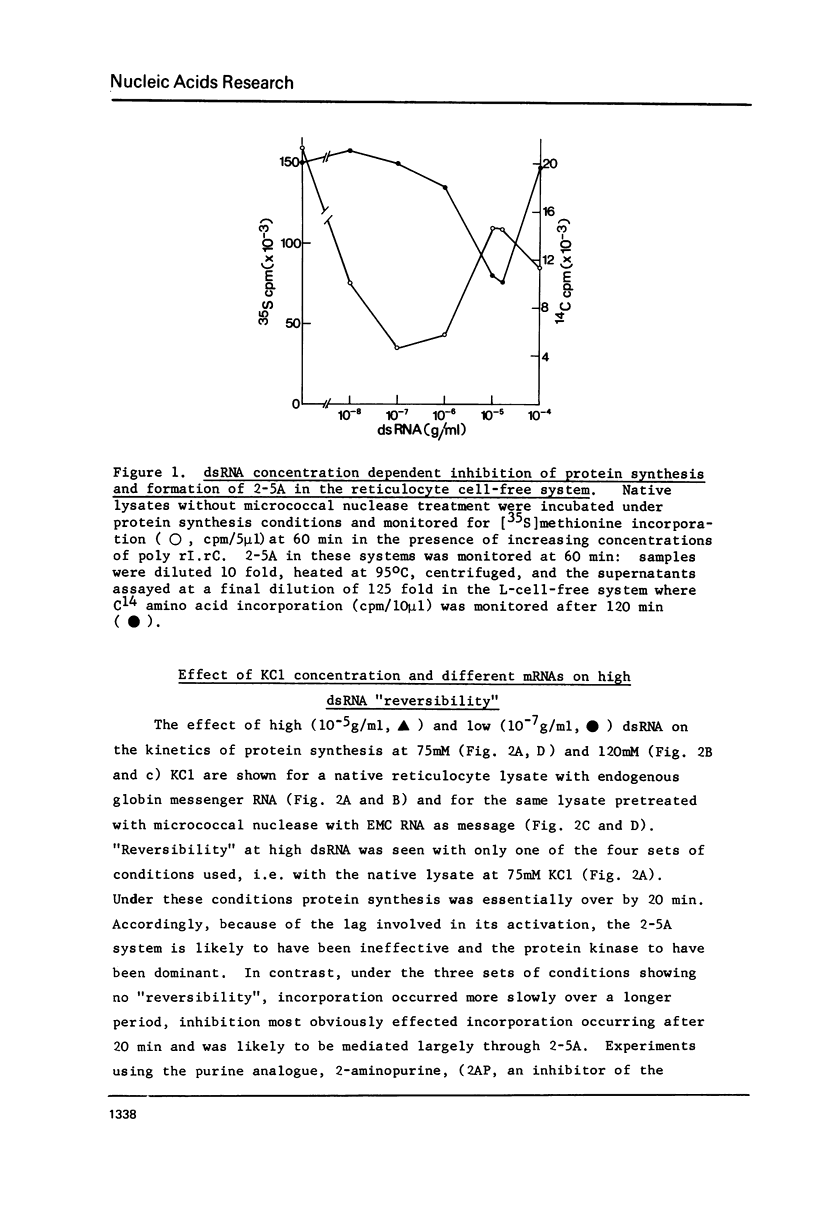
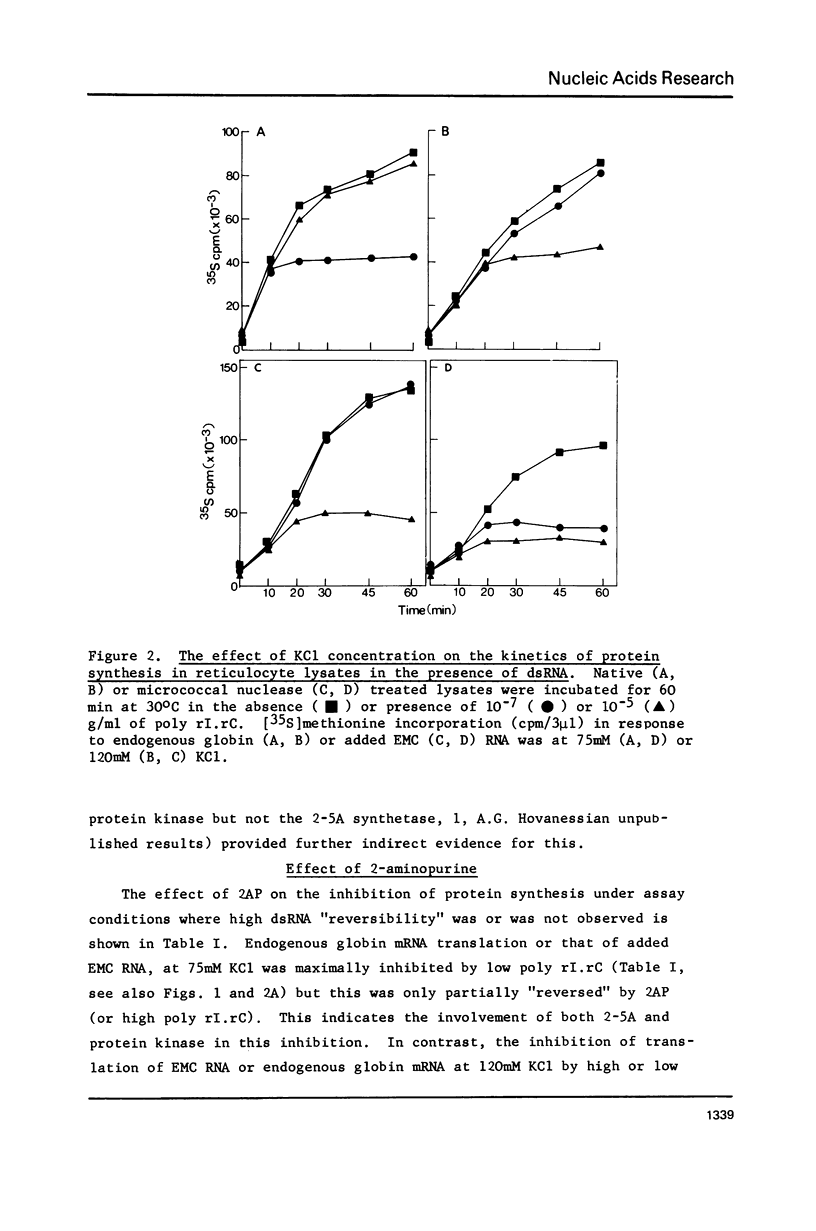
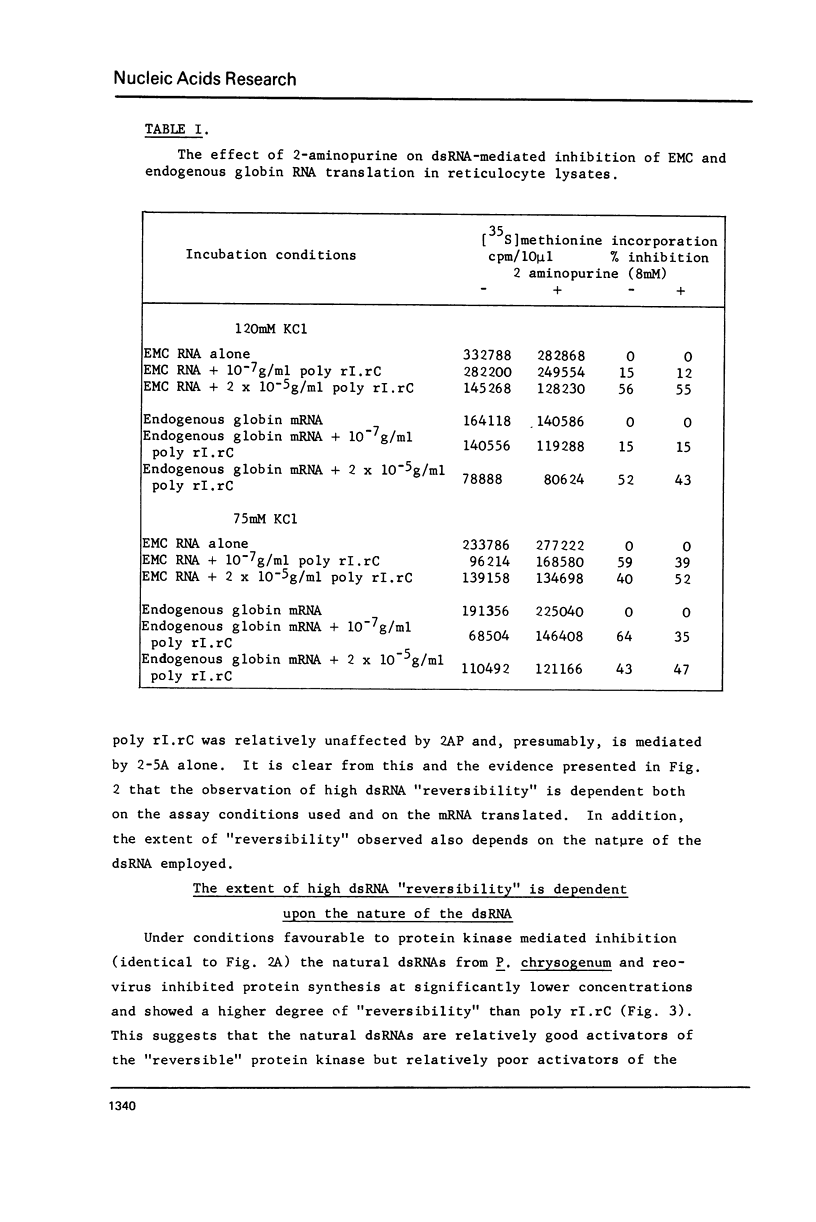
Abstract
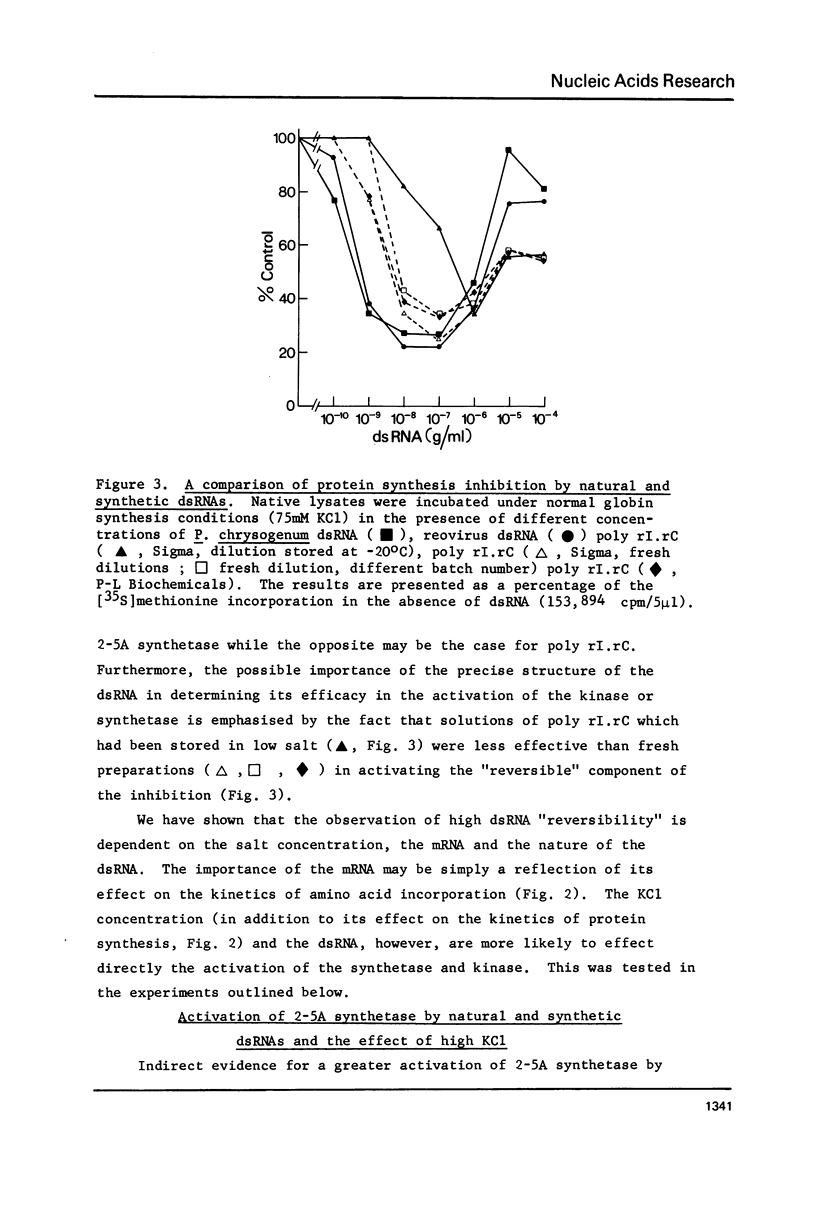
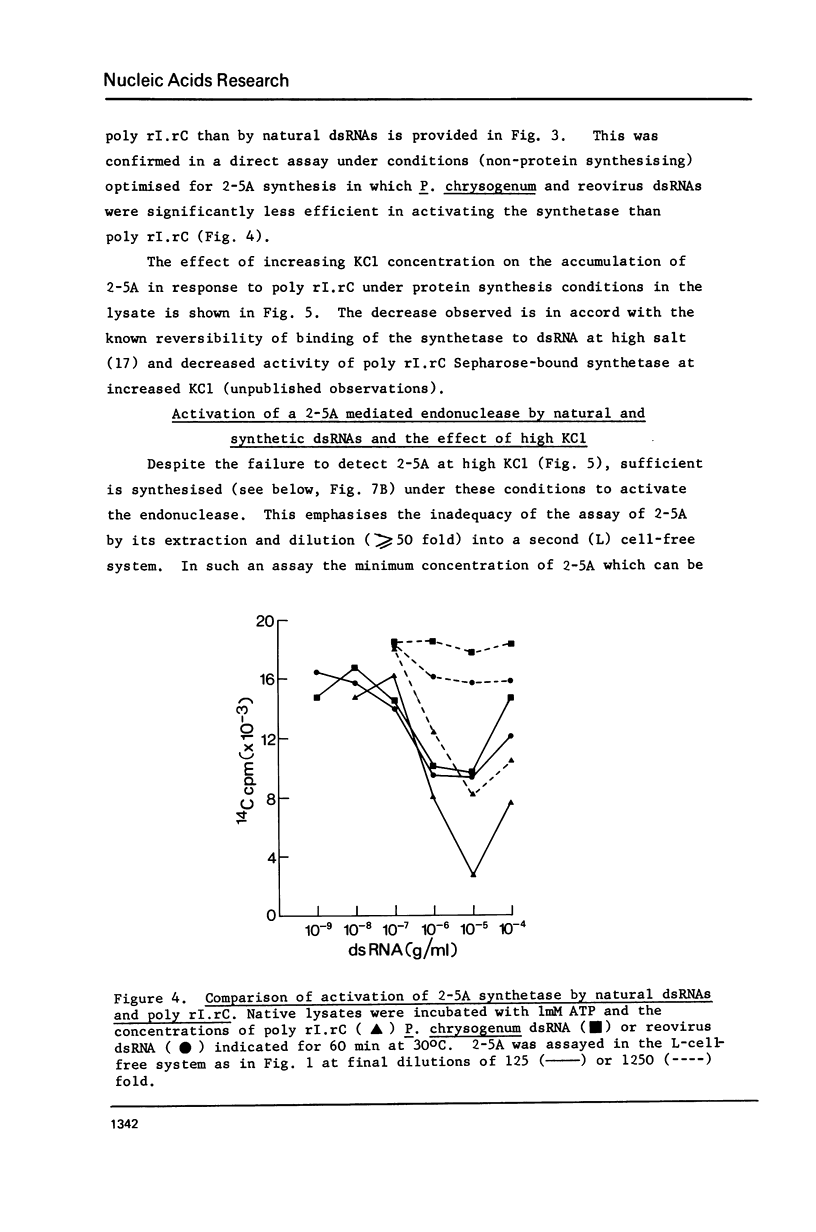
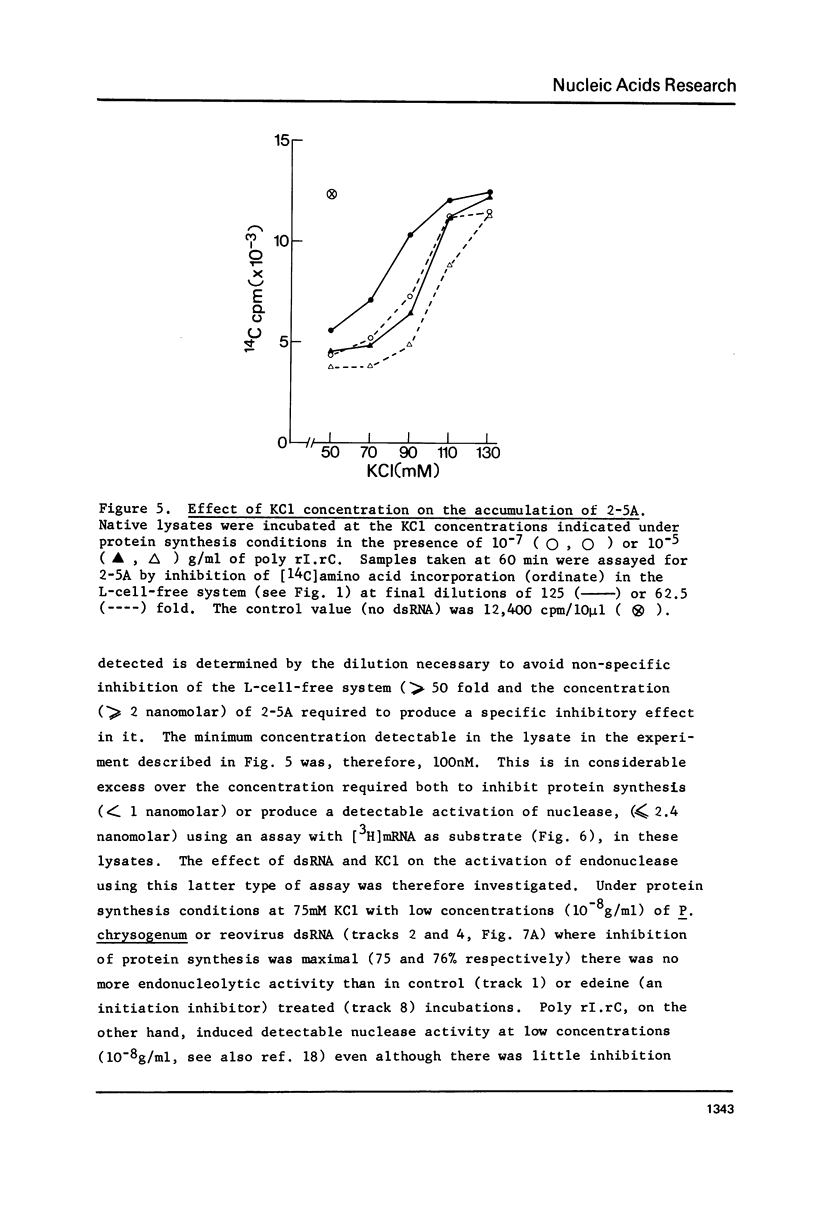
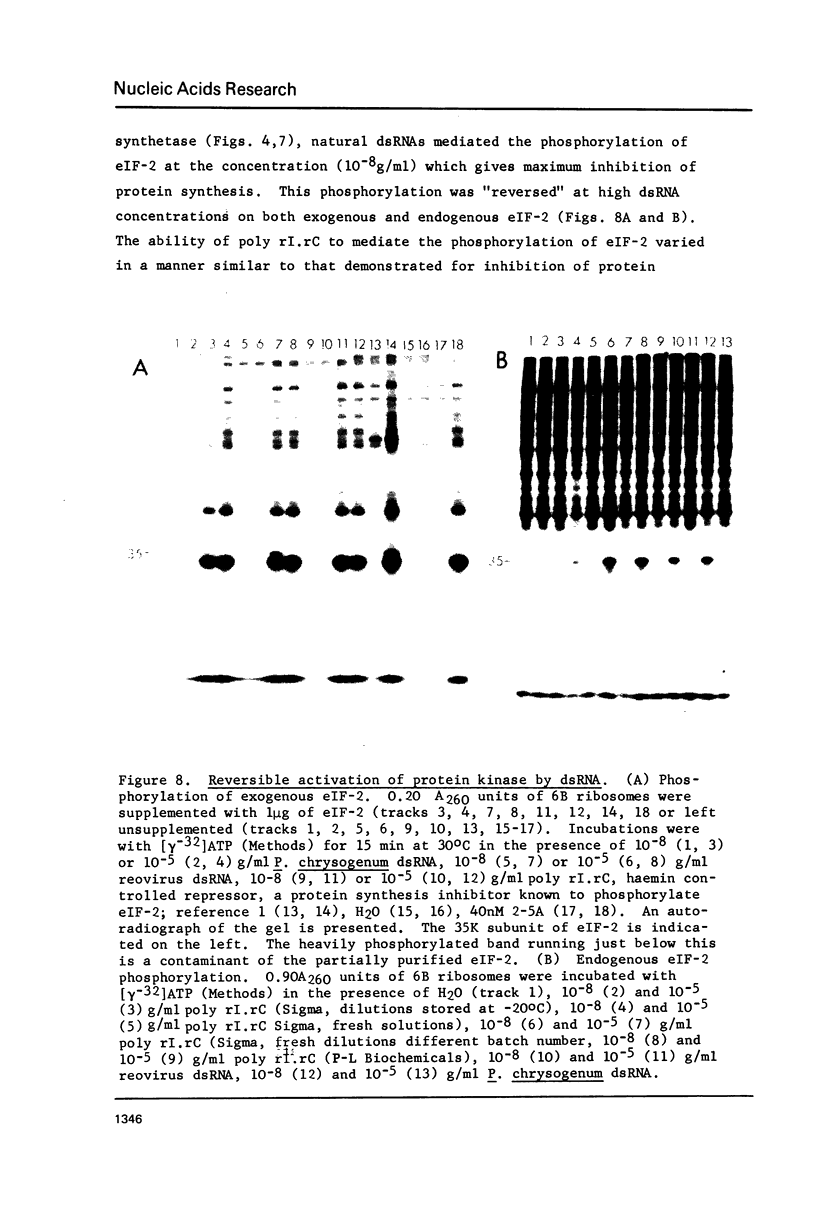
Double-stranded RNA (dsRNA) inhibits protein synthesis in rabbit reticulocyte lysates by activating the synthesis of the endonuclease effector pppA2' p5' A2' p5' A(2-5A) and a protein kinase which phosphorylates the protein synthesis initiation factor eIF-2. Under certain assay conditions, high concentrations of dsRNA are without inhibitory effect in many lysates (high dsRNA "reversible" lysates). In these lysates natural dsRNA at low concentrations stimulated protein kinase activity to a greater extent than did the synthetic dsRNA poly rI.rC. Synthesis of 2--5A was greater when poly rI.rC was used. However, a number of factors, including the salt concentration and messenger RNA used, combine to determine the overall effect of dsRNA on protein synthesis under any given set of experimental conditions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Lenz J. R., Maroney P. A. The effect of salt concentration on the inhibition of protein synthesis by double-stranded RNA. Eur J Biochem. 1978 Dec 1;92(1):155–163. doi: 10.1111/j.1432-1033.1978.tb12733.x. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Coupled transcription and translation in mammalian and avian cell-free systems. Virology. 1978 Feb;84(2):479–495. doi: 10.1016/0042-6822(78)90264-7. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Effect of interferon pretreatment on coupled transcription and translation in cell-free extracts of primary chick embryo cells. Virology. 1978 Feb;84(2):496–508. doi: 10.1016/0042-6822(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Henshaw E. C., Rahamimoff H., London I. M. Met-tRNAfMet binding to 40S ribosomal subunits: a site for the regulation of initiation of protein synthesis by hemin. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2946–2950. doi: 10.1073/pnas.71.8.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Content J., Lebleu B., De Clercq E. Differential effects of various double-stranded RNAs on protein synthesis in rabbit reticulocyte lysates. Biochemistry. 1978 Jan 10;17(1):88–94. doi: 10.1021/bi00594a012. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Sen G. C., Dubois M. F., Ratner L., Slattery E., Lengyel P. Interferon action: two distinct pathways for inhibition of protein synthesis by double-stranded RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5893–5897. doi: 10.1073/pnas.75.12.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. The (2'-5') oligoadenylate (pppA2'-5'A2'-5'A) synthetase and protein kinase(s) from interferon-treated cells. Eur J Biochem. 1979 Feb 1;93(3):515–526. doi: 10.1111/j.1432-1033.1979.tb12850.x. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Ball L. A. Increased sensitivity of cell-free protein synthesis to double-stranded RNA after interferon treatment. Nature. 1974 Jul 5;250(461):57–59. doi: 10.1038/250057a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Zilberstein A., Shulman L., Federman P., Berissi H., Revel M. Interferon action: isolation of nuclease F, a translation inhibitor activated by interferon-induced (2'-5') oligo-isoadenylate. FEBS Lett. 1978 Nov 15;95(2):257–264. doi: 10.1016/0014-5793(78)81006-0. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Kerr I. M., Gilbert C. S., White C. N., Ball L. A. Synthesis and breakdown of pppA2'p5'A2'p5'A and transient inhibiton of protein synthesis in extracts from interferon-treated and control cells. Eur J Biochem. 1978 Dec;92(2):455–462. doi: 10.1111/j.1432-1033.1978.tb12767.x. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Kerr I. M. Inhibition of protein synthesis by 2'-5' linked adenine oligonucleotides in intact cells. Nature. 1978 Nov 2;276(5683):88–90. doi: 10.1038/276088a0. [DOI] [PubMed] [Google Scholar]