Abstract
Ankylosing spondylitis (AS) is a common, inflammatory rheumatic disease that primarily affects the axial skeleton and is associated with sacroiliitis, uveitis, and enthesitis. Unlike other autoimmune rheumatic diseases, such as rheumatoid arthritis or systemic lupus erythematosus, autoantibodies have not yet been reported to be a feature of AS. We therefore wished to determine whether plasma from patients with AS contained autoantibodies and, if so, characterize and quantify this response in comparison to patients with rheumatoid arthritis (RA) and healthy controls. Two high density nucleic acid programmable protein arrays expressing a total of 3498 proteins were screened with plasma from 25 patients with AS, 17 with RA, and 25 healthy controls. Autoantigens identified were subjected to Ingenuity Pathway Analysis to determine the patterns of signaling cascades or tissue origin. 44% of patients with ankylosing spondylitis demonstrated a broad autoantibody response, as compared with 33% of patients with RA and only 8% of healthy controls. Individuals with AS demonstrated autoantibody responses to shared autoantigens, and 60% of autoantigens identified in the AS cohort were restricted to that group. The autoantibody responses in the AS patients were targeted toward connective, skeletal, and muscular tissue, unlike those of RA patients or healthy controls. Thus, patients with AS show evidence of systemic humoral autoimmunity and multispecific autoantibody production. Nucleic acid programmable protein arrays constitute a powerful tool to study autoimmune diseases.
Ankylosing spondylitis (AS)1 is a chronic, debilitating, rheumatic disease with a predilection for the axial skeleton and large joints. It affects in excess of 0.1% of the population and can be associated with uveitis, apical pulmonary fibrosis, and cardiac disease (1, 2). AS is difficult to diagnose, and patients can suffer symptoms for years before receiving appropriate treatment (2). The etiology is unknown but is thought to be immune-mediated. The extremely strong association with the Class I human leukocyte antigen allotype HLA-B27 has led to hypotheses involving CD8 T cell-mediated immunity (3). More recently additional genetic associations, including IL23R, have suggested a role for Th17 T cells (4).
Autoantibodies are a common characteristic of many rheumatic autoimmune diseases (5). Rheumatoid factor, an autoantibody against the Fc portion of IgG, occurs in more than 85% of patients with rheumatoid arthritis (RA). Although not specific to RA, rheumatoid factor is used routinely as a diagnostic test for RA and other autoimmune disorders (6). More recently, autoantibodies to cyclic citrullinated peptides have proven more specific than rheumatoid factor in diagnosing RA and have been shown to have prognostic value (7).
Autoantibodies are not commonly considered to be a feature of AS. However, anti-leukocyte (8), anti-neutrophil (9), and autoantibodies to some collagen proteins have been reported (10). Increased levels of circulating plasma cells have also been reported in AS patients (11), as well as evidence of hypergammaglobulinemia (12). Aside from these findings, no comprehensive investigation into the presence of autoantibodies in patients with AS has been performed to date.
Protein microarrays are commonly used as tools for detecting protein-protein interactions, such as the binding of autoantibodies to their cognate antigens. However, technical issues involving the cloning and purification of thousands of proteins, protein folding and stability, and the shelf life of protein arrays have, until now, made this a challenging task. A novel technology, referred to as the nucleic acid programmable protein array (NAPPA) has recently surmounted these issues (13). NAPPA involves the in situ transcription-translation of thousands of glycosylated proteins in close spatial proximity. Biotinylated cDNAs containing GST-tagged query proteins are immobilized onto glass slides. Anti-GST antibodies spotted adjacent to the cDNA are used as capture molecules. To synthesize the proteins, slides are covered in a continuous layer of reticulocyte lysate. The C-terminal GST tag ensures that only full-length proteins are captured. Therefore, this novel protein microarray technology provides an ideal platform for the characterization of autoantibody responses in autoimmune diseases.
In this discovery stage study, we wished to determine whether patients with ankylosing spondylitis demonstrated autoantibody responses using two different NAPPA arrays expressing a total of 3498 proteins. We show that AS patients demonstrate multispecific autoantibody responses to several autoantigens, predominantly targeted toward connective tissue and skeletal proteins.
EXPERIMENTAL PROCEDURES
Patient Information
This study included 25 patients with ankylosing spondylitis attending a dedicated AS clinic run by Dr. Paul Bowness at the Nuffield Orthopaedic Centre (Oxford, UK) who met the modified New York criteria (14), and 17 individuals with rheumatoid arthritis attending Dr. Bowness' rheumatology outpatients clinic who met the American College of Rheumatology revised criteria (15). Ethical permission was obtained (Oxfordshire REC 06/Q1606/139), and all subjects gave informed consent. The AS patients were HLA-B*2705-positive by DNA typing. The mean age of the AS patients was 42, and the range was from 28 to 69. 16 were male, 14 received nonsteroidal anti-inflammatory drugs, eight received disease-modifying anti-rheumatic drugs, and none received anti-TNF agents. The mean and standard deviation scores of the AS patients on the Bath Ankylosing Spondylitis Disease Activity Index, Bath Ankylosing Spondylitis Functional Index, and Bath Ankylosing Spondylitis Metrology Index scales were 5.8 ± 2.6, 5.4 ± 2.8, and 5.2 ± 1.8, respectively. The mean age of the RA patients was 48, and the range was from 18 to 68. Eleven were female, six received nonsteroidal anti-inflammatory drugs, eleven received disease-modifying anti-rheumatic drugs, and none received anti-TNF agents. The mean disease activity score of the RA patients was 5.2 ± 2.3. Twenty-five healthy controls were studied (four HLA-B27+ individuals, and fourteen male) with a mean age of 40 and a range of 28 to 64.
Blood Samples and Plasma Isolation
Venesection was performed using a 21-gauge needle and a 20-ml syringe. Heparinized blood samples were transferred into 50-ml falcon tubes. To isolate the plasma, the samples were centrifuged at 500 × g for 10 min at room temperature. Isolated plasma was aliquoted in 1-ml fractions in 1.5-ml microcentrifuge tubes and stored at −80 °C for 1–2 years. All of the samples underwent one freeze-thaw cycle prior to analysis. All of the samples were collected contemporaneously.
Preparation of NAPPA Slides and DNA Preparation
Glass slides were treated with 2% aminosilane in acetone, washed with acetone and then water, and dried using forced air. The slides were then stored in a dry container with silica packs until ready for printing. Bacteria harboring the expression plasmids were cultured in 1.5 ml of Terrific Broth containing 10% potassium phosphate and 100 μg/ml ampicillin in 96-well plates for 24 h and then pelleted by centrifugation at 3000 × g for 30 min. DNA was prepared according to published protocols (16). DNA concentrations were measured by spectrophotometry at 260 nm, and plates were deemed acceptable if 90% of the wells had a total of 15 μg or more.
Preparation of DNA Samples and Array Printing
DNA samples were precipitated by the addition of 0.8× volume of isopropanol and centrifugation at 4000 × g for 30 min. They were then washed with 80% ethanol and allowed to air dry. Each well was resuspended in 20 μl of spotting buffer (50 μg/ml capture antibody, 3.6 mg/ml BSA, 2 mm bis-sulfosuccinimidyl suberate and mixed for 30 min. Four 96-well plates were then transferred to one 384-well plate, which was used for printing. The arrays were printed on aminosilane-treated glass slides using a Genetix Q Array2 printer. Standard conditions of 60% humidity were applied.
NAPPA Protein Expression
Printed slides were blocked for 1 h at room temperature on a rocking platform in SuperBlock (Thermo Scientific Pierce) using 30 ml for four slides to wash away any unbound NAPPA reagents (plasmid, BSA, or capture antibody). The slides were then rinsed with water and dried with filtered compressed air. 100 μl of rabbit reticulocyte lysate in vitro transcription-translation mix (Promega, Madison, WI) was prepared per slide (4 μl of TNTTM buffer, 2 μl of T7 polymerase, 1 μl of -Met, 1 μl of -Leu, 2 μl of RNaseOUT, and 90 μl of DEPC water) and Hybriwell gaskets (Grace Biolabs, Bend, OR) were applied. In vitro transcription-translation mix was pipetted onto the array through the hole in the gasket. Port seals were applied to both ports to avoid evaporation. The arrays were then incubated for 1.5 h at 30 °C for protein expression, followed by 30 min at 15 °C to allow the query protein to bind the immobilized capture antibody. The HybriWells were removed, and the arrays were washed with milk three times for 3 min on a rocking platform. The protein arrays were then blocked with milk at room temperature for 1 h.
Antibody Capture and Visualization
Plasma samples were diluted between 1/170 and 1/2000 in 2 ml of 5% milk in PBS to achieve an equal amount of nonspecific background binding. The samples were pipetted onto the arrays, assembled into gaskets (Corning), and incubated overnight while rotating at 4 °C. The arrays were then disassembled and washed three times in milk for 5 min on a rocking platform. Secondary antibody (horseradish peroxidase-conjugated anti-human IgG) was applied to the slide under a coverslip and incubated for 1 h at room temperature. The slides were washed in PBS three times for 5 min, once with water, and dried. Tyramide signal amplification solution covering the arrays was applied under a coverslip and incubated for 10 min at room temperature. Arrays were rinsed with water and dried with filtered compressed air. Arrays were then scanned in a microarray scanner, using settings for Cy3.
NAPPA Data Analysis
For each protein, spot signals were averaged over the cohort, and a statistical distribution of the intensities of those signals among the controls was calculated. From this, the mean and standard deviation of the signal intensity was calculated. Z scores were calculated for every query protein in every individual. Z score = (signal − mean)/(standard deviation). Proteins were deemed hits if their Z score exceeded 3. Thus, for every target protein screened, the response of the control population formed a benchmark.
NAPPA Validation
50 μl of pooled plasma from four IL-6 autoantibody-positive AS patients and four IL-6 autoantibody negative AS patients or eight healthy donors was diluted in 1 ml of PBS. 20 μl of protein A-Sepharose beads (Invitrogen) was added, and the samples were mixed at room temperature. After 1 h the beads were washed with 1 ml of each PBS, TBS-T, TBS-T + 0.5 m NaCl, TBS-T + 0.5% Triton X-100, and PBS. The beads were then blocked in 1 ml of 5% milk in TBS-T for 1 h at room temperature. 1, 10, or 100 ng of recombinant IL-6 (Miltenyi Biotec) was added to the samples. Controls were BSA-coated (Sigma) and empty beads. The samples were mixed and incubated overnight at 4 °C. After washing three times in 1 ml of TBS-T, the bound proteins were eluted with Laemmli buffer. 10% of the eluate was separated by a 16% Tris-glycine SDS-PAGE followed by immunoblotting. IL-6 was detected using a polyclonal antibody (R & D Systems). Densitometric analysis of the IL-6 signal was performed using ImageJ.
RESULTS
Screening of AS and Control Plasma Samples Using Two Dedicated NAPPA Arrays
Prior to the NAPPA screening, plasma samples were coded, blinded, and randomized, and each sample was screened on a mini-array, consisting of 100 proteins, to measure background signal. All of the plasma samples were diluted to at least 1/170 to normalize for background intensity. Fig. 1 shows the workflow of this study. After the proteins were synthesized in situ using a transcription-translation coupled rabbit reticulocyte lysate system, the slides were washed and blocked to minimize nonspecific interactions between plasma proteins and those present on the array. The slides were incubated with plasma overnight at 4 °C to permit binding of autoantibodies to their target antigens. They were then incubated with a secondary horseradish peroxidase-conjugated anti-human IgG antibody. Visualization was performed using a Tyramide-Cyanine 3-conjugated amplification system and scanned using a slide fluorescence scanner. Signals for each spot were averaged over the cohort and a statistical distribution of the intensities of those signals among the controls was calculated. From these distributions, the means and standard deviations were calculated for each spot and individual. On each array, positive controls included the Epstein-Barr virus nuclear antigen protein and human IgG protein (to which the horseradish peroxidase anti-human secondary antibody would bind). Negative controls included the parental expression vector containing the GST tag but lacked a cDNA, as well as spots that carry the spotting mix but lacked any DNA.
Fig. 1.
Schematic view of the workflow for NAPPA analysis used in this study. See text for details. Ab, antibody.
Fig. 2 shows the plasma screening result from one AS patient (AS4) and one healthy control (HC8) on NAPPA array GST#1. Seven autoantigens were detected in patient AS4, and none were detected in control HC8. This array contained 1749 target proteins and was chosen because it expressed 279 proteins associated with immunological disease, 297 proteins associated with connective tissue disorders, 273 proteins associated with inflammatory disease, and 204 proteins involved in skeletal and muscular disorders. NAPPA array GST#2 array also expressed 1749 target proteins, 220 involved in inflammatory disease, and 196 involved in immunological disease but expressed fewer proteins involved in connective tissue and skeletal and muscular disorders: 109 and 143, respectively.
Fig. 2.
Representative screenings on NAPPA array GST#1 of one AS patient plasma sample (AS4) (A and B) compared with one healthy control plasma sample (HC8) (C and D). A, screening of one AS plasma sample with seven autoantigens (ringed spots) annotated. MAPK13, mitogen-activated protein kinase 13; APLP2, amyloid β precursor-like protein 2; DSCR2, proteasome assembly chaperone 1; TRIM27, tripartite motif-containing 27; FCGRT, Fc fragment of IgG receptor transporter; HSPA2, heat shock 70-kDa protein 2; SULT2A1, alcohol sulfotransferase. B, magnified section of A boxed in red. C, screening of one HC plasma sample with no autoantigens detected. The ringed spots are for comparative purposes only. D, magnified section of B boxed in green. The color code for array intensity ranges from least intense to most intense is: blue < green < yellow < orange < red. CTRL, control.
44% of AS Patients Demonstrate Multi-specific Autoantibody Responses
Each one of the 67 plasma samples (25 AS, 17 RA inflammatory controls, and 25 healthy controls) was screened against two NAPPA arrays comprising a total of 3498 human proteins. These proteins are listed as supplementary data (supplemental Table S1). The numbers of autoantigens detected in each individual plasma sample from the screening of NAPPA arrays GST#1 and GST#2 are listed in Tables I and II, respectively. The plasma samples of AS patients reacted consistently with more autoantigens than the RA or healthy controls. Thus, 28% (7 of 25) of AS patients, compared with 18% (3 of 17) of RA patients and 4% (1 of 25) of healthy controls, bound at least 50 proteins from the screening of array GST#1. Similarly, from the screening of the array GST#2, 20% (5/25) of AS patients, 19% (3/16) of RA patients, and 4% (1/25) of healthy controls demonstrated a multi-specific autoantibody response of 50 proteins. Combining arrays, 44% (11/25) of AS patients, 36% (6/17) of RA patients, and 8% (2/25) of healthy controls demonstrated a response to more than 50 autoantibodies. From the screening of NAPPA array GST#1, 92% of healthy control individuals expressed fewer than five autoantibodies, compared with 48% of AS patients and 53% of RA patients. From the screening of NAPPA array GST#2, 70% of healthy control individuals expressed fewer than five autoantibodies, compared with 36% of AS patients and 19% of RA patients.
Table I. Number of putative autoantigens detected per individual in the screening of NAPPA array GST#1.
For each target protein, the mean and standard deviation of the control cohort was calculated. Z scores ((signal − mean)/(standard deviation)) were calculated for every query protein in every individual in the three groups. Proteins with Z scores greater than 3 are assigned as positive hits.
| Healthy controls |
AS patients |
RA patients |
|||
|---|---|---|---|---|---|
| Sample name | No. of putative autoantigens detected | Sample name | No. of putative autoantigens detected | Sample name | No. of putative autoantigens detected |
| HC1 | 0 | AS1 | 10 | RA1 | 1 |
| HC2 | 0 | AS2 | 3 | RA2 | 231 |
| HC3 | 0 | AS3 | 3 | RA3 | 5 |
| HC4 | 0 | AS4 | 7 | RA4 | 12 |
| HC5 | 0 | AS5 | 136 | RA5 | 120 |
| HC6 | 1 | AS6 | 0 | RA6 | 39 |
| HC7 | 1 | AS7 | 244 | RA7 | 3 |
| HC8 | 0 | AS8 | 2 | RA8 | 1 |
| HC9 | 7 | AS9 | 11 | RA9 | 0 |
| HC10 | 0 | AS10 | 219 | RA10 | 0 |
| HC11 | 0 | AS11 | 152 | RA11 | 11 |
| HC12 | 2 | AS12 | 2 | RA12 | 3 |
| HC13 | 1 | AS13 | 218 | RA13 | 3 |
| HC14 | 0 | AS14 | 7 | RA14 | 130 |
| HC15 | 0 | AS15 | 3 | RA15 | 0 |
| HC16 | 1 | AS16 | 0 | RA16 | 10 |
| HC17 | 1 | AS17 | 1 | RA17 | 2 |
| HC18 | 0 | AS18 | 158 | ||
| HC19 | 1 | AS19 | 26 | ||
| HC20 | 0 | AS20 | 2 | ||
| HC21 | 0 | AS21 | 6 | ||
| HC22 | 0 | AS22 | 94 | ||
| HC23 | 182 | AS23 | 2 | ||
| HC24 | 0 | AS24 | 0 | ||
| HC25 | 1 | AS25 | 3 | ||
Table II. Number of putative autoantigens detected per individual in the screening of NAPPA array GST #2.
For each target protein, the mean and standard deviation of the control cohort was calculated. Z scores ((signal − mean)/(standard deviation)) were calculated for every query protein in every individual in the three groups. Proteins with Z scores greater than 3 are assigned as positive hits. ND denotes sample not done, because of experimental error.
| Healthy controls |
AS patients |
RA patients |
|||
|---|---|---|---|---|---|
| Sample name | No. of putative autoantigens detected | Sample name | No. of putative autoantigens detected | Sample name | No. of putative autoantigens detected |
| HC1 | 1 | AS1 | 12 | RA1 | 202 |
| HC2 | 0 | AS2 | 3 | RA2 | 32 |
| HC3 | 0 | AS3 | 2 | RA3 | 22 |
| HC4 | 1 | AS4 | 3 | RA4 | 1 |
| HC5 | 0 | AS5 | 19 | RA5 | 38 |
| HC6 | 11 | AS6 | 75 | RA6 | 44 |
| HC7 | 0 | AS7 | 11 | RA7 | 2 |
| HC8 | 22 | AS8 | 19 | RA8 | 97 |
| HC9 | 3 | AS9 | 59 | RA9 | 19 |
| HC10 | 2 | AS10 | 32 | RA10 | 28 |
| HC11 | 0 | AS11 | 11 | RA11 | 7 |
| HC12 | 23 | AS12 | 4 | RA12 | 7 |
| HC13 | 8 | AS13 | 34 | RA13 | 1 |
| HC14 | 2 | AS14 | 28 | RA14 | ND |
| HC15 | ND | AS15 | 2 | RA15 | 69 |
| HC16 | 0 | AS16 | 370 | RA16 | 10 |
| HC17 | 9 | AS17 | 35 | RA17 | 32 |
| HC18 | 0 | AS18 | 144 | ||
| HC19 | 3 | AS19 | 1 | ||
| HC20 | 0 | AS20 | 2 | ||
| HC21 | 39 | AS21 | 15 | ||
| HC22 | 101 | AS22 | 10 | ||
| HC23 | 0 | AS23 | 3 | ||
| HC24 | 1 | AS24 | 1 | ||
| HC25 | 2 | AS25 | 142 | ||
NAPPA Method Validation
Although the NAPPA method is a highly reproducible and robust system for detecting protein-protein interactions (16), we wished to validate one of the NAPPA results via an independent experimental method. Eight AS patient plasma samples were pooled, half in which IL-6 had been identified as a putative autoantigen, as were eight plasma samples from healthy control individuals. From these two pools, IgG molecules were immunoprecipitated using protein A beads. These IgG immunoprecipitates were then tested in a dose-dependent manner for recovery efficiency of recombinant IL-6. After blotting the immunoprecipitated material for the presence of IL-6, densitometry analysis showed that IgG from the AS plasma pool recovered more than 60% of recombinant IL-6, compared with just over 5% recovery from the healthy control plasma pool (Fig. 3).
Fig. 3.
AS patient plasma can immunoprecipitate IL-6. 1, 10, and 100 ng of recombinant IL-6 was used as bait and immunoprecipitated with IgG molecules from 50 μl of pooled AS patient or control plasma samples. Immunoprecipitated (IP) material was separated by 16% SDS-PAGE and detected by anti-IL-6 immunoblotting.
Multiple AS patients show autoantibody responses to shared autoantigens. We next asked whether any of the autoantibodies were present in multiple plasma samples within the AS patient group. From the screening of NAPPA array GST#1, 193 autoantigens were shared by three AS patients, 82 autoantigens were shared by four AS patients, 30 autoantigens were shared by five AS patients, and three autoantigens were shared by six AS patients (Fig. 4A). The data from array GST#2 was similarly analyzed. Fig. 4B shows that 130 autoantigens were detected in at least two AS patients, 18 autoantigens were detected in three AS patients, and two autoantigens were detected in four AS patients. Fewer autoantibodies were present in multiple plasma samples in the RA patients, and no autoantibodies were found in multiple healthy control individuals from the screening of either the GST#1 or GST#2 arrays.
Fig. 4.
AS patients have autoantibodies to a number of common antigens. The numbers of autoantibodies detected in two to five AS patients from the screening of GST#1 (A) and two to four AS patients from the screening of GST#2 (B) are shown.
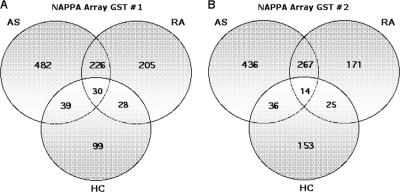
60% of Autoantibodies Detected Are Specific to the AS Cohort
We then asked whether the detected autoantibodies were largely common between AS and RA patients or specific to the AS group. From the screening of array GST#1, 482 (62%) were specific to the AS patients (Fig. 5A). The AS and RA cohort shared 256 common autoantigens. Results from the screening of array GST#2 (Fig. 5B) were comparable, with 436 (58%) of these autoantibodies restricted to the AS cohort and 281 shared by the AS and RA groups.
Fig. 5.
Most AS autoantibodies are not found in RA or healthy controls. A, Venn diagram showing distribution of all autoantigens detected in the screening of NAPPA array GST#1 shows 482 (62%) of autoantigens detected were specific to AS patients. B, screening of NAPPA array GST#2 shows 436 (58%) of autoantigens were restricted to the AS cohort.
Autoantibodies from AS Patients Show a Bias toward Antigens Involved in Skeletal and Connective Tissue Disorders
We next asked whether autoantigens detected in the AS cohort showed a bias toward any particular biological pathway. For this purpose, we used Ingenuity Pathway Analysis® (IPA), which is based on a comprehensive collection of literature data on protein networks. Fig. 6A shows that, when compared with the pathways assigned to the 1749 target proteins expressed by the array itself, the autoantigens identified in the AS patients demonstrated a distinct bias toward the pathway involving connective tissue development and function. The GST#1 array expressed 95 proteins involved in connective tissue development and function. Of these, 65 proteins (68%) were detected as autoantigens in the AS cohort. By contrast, only 0–37% of pathway proteins were recognized in the other 26 pathways assigned to the array. This was expressed by IPA as a more significant p value for connective tissue development and function in the AS autoantigen analysis (p value = 4.09 × 10−11) compared with the analysis of the entire GST#1 array (p value = 1.54 × 10−10) after correction for multiple comparisons. The same analysis, conducted on results from the independent screening of NAPPA array GST#2, was consistent with the analysis from NAPPA array GST#1 and showed that the autoantigens detected in the AS cohort were specifically biased toward connective tissue disorder and skeletal and muscular disorder pathways (Fig. 6B). We next explored whether autoantigens from these significant pathways occurred in multiple AS patients and were restricted to the AS cohort. 83 such proteins were identified. Table III lists a subset of these autoantigens, specifically those involved in extracellular matrix and bone remodeling.
Fig. 6.
IPA comparison of proteins expressed by the NAPPA arrays and autoantigens detected in the AS cohort demonstrates a bias toward antigens involved in connective tissue and skeletal antigens. A, IPA compared the p values calculated to significant pathways assigned to all proteins expressed on the NAPPA array GST#1 with the p values calculated to significant pathways assigned to all autoantigens detected in the AS cohort only. The p value for the analysis of AS autoantigens in the connective tissue development and function pathway (p value = 4.09 × 10−11) was found to be more significant than it was in the analysis of all proteins expressed on NAPPA array GST#1 (1.54 × 10−10). B, IPA compared the p values calculated for significant pathways assigned to all proteins expressed on the NAPPA array GST#2 with the p values calculated to the significant pathways assigned to all autoantigens detected in the AS cohort only. The p values obtained from the analysis of AS autoantigens in the connective tissue disorders (p = 5.7 × 10−4) and skeletal and muscular disorders (p = 2.55 × 10−5) pathways from the NAPPA GST#2 screen were lower than they were in the analysis of all proteins expressed on NAPPA array GST#2 for those pathways, with the array p value for connective tissue disorders of 6.82 × 10−2 and skeletal and muscular disorders 1.22 × 10−2. All of the p values were calculated using a right tailed Fisher's exact test and corrected for multiple comparison with the Benjamini-Hochberg method. *, pathway significance threshold where p = 0.05.
Table III. Autoantigens involved in skeletal and connective tissue remodeling restricted to the AS cohort.
ECM, extracellular matrix.
| Autoantigen | Number of patients detected | Subcellular location | Function | Reference |
|---|---|---|---|---|
| P2RX7 | 4 | Plasma membrane | Ion channel, increases bone mineralization and ossification | 31, 32 |
| Chondromodulin 1 | 4 | Cartilage matrix | Bone resorption and remodeling | 30 |
| Osteoglycin | 3 | Extracellular matrix | Induces ectopic bone formation | 34 |
| Melanocortin 4 receptor | 3 | Plasma membrane | Increases bone resorption | 33 |
| Osteonectin | 2 | Cell surface, extracellular | Bone formation and remodeling | 35 |
| Connective tissue growth factor | 2 | Extracellular matrix | Ossification, cartilage condensation | 25 |
| Glypican 3 | 2 | Extracellular Matrix | Mediates osteogenesis | 26 |
| Glypican 4 | 2 | Extracellular matrix | ECM structural protein | 27 |
| Matrix Gla protein | 2 | Extracellular matrix | Regulation of ECM calcification | 28 |
| SMOC1 | 2 | Extracellular matrix | Involved in ECM assembly | 29 |
DISCUSSION
We have used a novel type of protein array screening tool to characterize the autoantibody response in patients with ankylosing spondylitis. In total, 44% of AS patients demonstrated a broad autoantibody response, with over 750 reactivities seen at plasma dilutions of greater than 1/170, which is considered clinically significant for autoimmune disease (17). AS patients demonstrated autoantibody responses to several shared autoantigens, and 60% of the autoantibodies in AS patients appeared specific to that group in that they were not found in RA or healthy controls. Further evidence of biological relevance is provided by the fact that only autoantibodies from AS patients showed a bias toward autoantigens involved in skeletal and connective tissue. Our studies indicate that NAPPA is a powerful new technique to screen for autoantibodies in human autoimmune diseases. The use of NAPPA arrays has the advantage that large numbers of proteins can be screened and that these proteins are translated and transcribed in a eukaryotic cell extract, promoting proper folding and glycosylation. However, a disadvantage is that antibodies to proteins with post-translational modifications would not be detected. Similarly, epitopes whose conformation may be membrane-dependent might not be detected.
Autoantibodies are associated with many systemic autoimmune diseases and can be highly specific (e.g. myasthenia gravis) (18) or broadly recognize multiple specificities (e.g. systemic lupus erythematosus) (19). Our data show for the first time that plasma from AS patients contains multiple autoantibodies recognizing a variety of antigens, with a bias toward proteins expressed in connective tissue.
We propose two possible interrelated mechanisms for this. First, production of IL-17 by T cells in AS (20) may directly stimulate B cell maturation and Ig production, as shown for systemic lupus erythematosus by Doreau et al. (21). Second, the presence of professional antigen-presenting cells together with T and B cells within inflamed areas of connective tissue, as demonstrated histopathologically in AS (22), may lead to autoimmunity in the presence of appropriate cytokine stimulation. Interestingly, we identified and validated interleukin-6, a cytokine implicated in both AS and the Th17 response, as a target autoantigen. Accumulating evidence from immune-mediated diseases supports the hypothesis that the tissue damage caused by immune responses can result in “epitope spreading” following priming of self-reactive lymphocytes. Here presentation of pathogenic epitopes in draining lymph nodes leads to the migration of activated lymphocytes to the site of inflammation, recruiting more phagocytes, and contributing to further tissue destruction. The debris from this destruction can result in extracellular matrix and inflammatory self-proteins being proteolytically digested and their resultant peptides (inappropriately) presented by professional antigen-presenting cells. This may lead to the activation of autoreactive T and B lymphocytes, perpetuating the cycle of inflammation and tissue destruction (23).
Autoantibodies to extracellular matrix components such as collagen I, II, III, IV, and V have previously been reported in AS patients (24, 25), and elevated levels of IgA antibodies to keratin proteins have been detected in patients with spondyloarthropathy (26). Also, higher levels of IgA antibodies have been detected in immune complexes precipitated from AS patient plasma (27). We were not able to look for autoantibodies to collagen proteins I–IV in the screening of the NAPPA GST#1 and GST#2 arrays, because they were not expressed (supplemental Table S1). However, we did identify several extracellular matrix proteins as autoantigens in multiple AS patients. These included connective tissue growth factor (28), glypican 3 (29), glypican 4 (30), matrix Gla protein (31), and SMOC1, a protein involved in extracellular matrix assembly (32). These proteins were all specific to the AS cohort (see Table III).
Furthermore, in addition to proteins involved in extracellular matrix remodeling, proteins involved in ossification and bone remodeling were also identified as autoantigens in multiple AS patients and were restricted to the AS cohort. For example, chondromodulin is a bone remodeling factor (33) that is thought to function by allowing cartilaginous tissue to be vascularized and replace by bone. The purinergic receptor P2RX7 is involved in ossification (34) and has been shown to regulate bone formation (35). Similarly, the melanocortin 4 receptor has been shown to increase bone resorption (36). Finally, the cartilage matrix protein osteoglycin induces ectopic bone formation (37), and the extracellular matrix glycoprotein osteonectin is involved in osteocyte differentiation and bone formation (38). New bone formation is a characteristic feature of ankylosing spondylitis. It would be interesting to investigate whether a correlation between the presence of these autoantibodies and the ankylosis characteristic of late stage AS existed.
It remains to be determined whether the autoantibodies observed in this study occur in early or late stage disease. Unfortunately, the cohort studied in this discovery stage experiment was too small to subclassify the AS patients according to disease duration, disease activity, flares and remission, age, sex, or treatment and produce statistically significant results. If, as with diabetes, patients produced autoantibodies prior to the presentation of clinical symptoms, a smaller microarray specifically expressing hundreds of proteins involved in connective and skeletal tissue might prove to be a valuable diagnostic tool. Alternatively, if these autoantibodies occurred later as a result of disease activity, they may be of prognostic use for tailored treatment. For example, such AS patients may benefit from B cell ablative therapy, which has recently been shown to improve clinical symptoms in patients with rheumatoid arthritis and dermatological autoimmune conditions (39). Indeed, although we have not demonstrated a direct pathogenic role for the autoantibodies identified in AS, our study suggests a scientific rational for a therapeutic trial of B cell depletion in this disease.
In summary, the screening of two independent nucleic acid programmable protein arrays with plasma from AS patients has shown consistent evidence of the presence of multiple autoantibodies compared with healthy controls and patients with RA. Both arrays demonstrated that AS patients produced autoantibodies to shared autoantigens and that a number of these antigens were restricted to the AS cohort. Similarly, when subjected to Ingenuity Pathway Analysis, AS autoantigens from both arrays demonstrated a bias toward proteins expressed in connective and skeletal tissue. This novel finding has implications for our understanding of the pathogenic processes underlying AS and may in future aid diagnosis and treatment.
Acknowledgments
We thank the members of the Bowness, LaBaer, and Kessler group for insightful discussions.
Footnotes
* This work was supported by funding provided by the National Institutes of Health Research Biomedical Research Centre Oxford, the Medical Research Council, Action Medical Research (UK), National Science and Engineering Research Council of Canada, and the European Molecular Biology Organization. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- AS
- ankylosing spondylitis
- RA
- rheumatoid arthritis
- NAPPA
- nucleic acid programmable protein arrays
- IPA
- Ingenuity Pathway Analysis.
REFERENCES
- 1. Sukenik S., Pras A., Buskila D., Katz A., Snir Y., Horowitz J. (1987) Cardiovascular manifestations of ankylosing spondylitis. Clin. Rheumatol. 6, 588–592 [DOI] [PubMed] [Google Scholar]
- 2. Braun J., Sieper J. (2006) Early diagnosis of spondyloarthritis. Nat. Clin. Pract. Rheumatol. 2, 536–545 [DOI] [PubMed] [Google Scholar]
- 3. Benjamin R., Parham P. (1990) Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol. Today 11, 137–142 [DOI] [PubMed] [Google Scholar]
- 4. Burton P. R., et al. (2007) Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Mühlen C. A., Tan E. M. (1995) Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin. Arthritis Rheum. 24, 323–358 [DOI] [PubMed] [Google Scholar]
- 6. Dörner T., Egerer K., Feist E., Burmester G. R. (2004) Rheumatoid factor revisited. Curr. Opin. Rheumatol. 16, 246–253 [DOI] [PubMed] [Google Scholar]
- 7. Vallbracht I., Rieber J., Oppermann M., Förger F., Siebert U., Helmke K. (2004) Diagnostic and clinical value of anti-cyclic citrullinated peptide antibodies compared with rheumatoid factor isotypes in rheumatoid arthritis. Ann. Rheum. Dis. 63, 1079–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenberg J. N., Johnson G. D., Holborow E. J. (1979) Antinuclear antibodies in ankylosing spondylitis, psoriatic arthritis, and psoriasis. Ann. Rheum. Dis. 38, 526–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Locht H., Skogh T., Kihlström E. (1999) Anti-lactoferrin antibodies and other types of anti-neutrophil cytoplasmic antibodies (ANCA) in reactive arthritis and ankylosing spondylitis. Clin. Exp. Immunol. 117, 568–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tani Y., Sato H., Hukuda S. (1997) Autoantibodies to collagens in Japanese patients with ankylosing spondylitis. Clin. Exp. Rheumatol 15, 295–297 [PubMed] [Google Scholar]
- 11. Eghtedari A. A., Davis P., Bacon P. A. (1976) Immunological reactivity in ankylosing spondylitis: Circulating immunoblasts, autoantibodies, and immunoglobulins. Ann. Rheum. Dis. 35, 155–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burgos-Vargas R., Naranjo A., Castillo J., Katona G. (1989) Ankylosing spondylitis in the Mexican mestizo: Patterns of disease according to age at onset. J. Rheumatol. 16, 186–191 [PubMed] [Google Scholar]
- 13. LaBaer J., Ramachandran N. (2005) Protein microarrays as tools for functional proteomics. Curr. Opin. Chem. Biol. 9, 14–19 [DOI] [PubMed] [Google Scholar]
- 14. van der Linden S., Valkenburg H. A., Cats A. (1984) Evaluation of diagnostic criteria for ankylosing spondylitis: A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 [DOI] [PubMed] [Google Scholar]
- 15. Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31, 315–324 [DOI] [PubMed] [Google Scholar]
- 16. Ramachandran N., Raphael J. V., Hainsworth E., Demirkan G., Fuentes M. G., Rolfs A., Hu Y., LaBaer J. (2008) Next-generation high-density self-assembling functional protein arrays. Nat. Methods 5, 535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang R., Hellmark T., Zhao J., Cui Z., Segelmark M., Zhao M. H., Wang H. Y. (2009) Levels of epitope-specific autoantibodies correlate with renal damage in anti-GBM disease. Nephrol. Dial. Transplant. 24, 1838–1844 [DOI] [PubMed] [Google Scholar]
- 18. Vincent A., Newsom Davis J. (1980) Anti-acetylcholine receptor antibodies. J. Neurol. Neurosurg. Psychiatry 43, 590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes G. R. (1984) Autoantibodies in lupus and its variants: Experience in 1000 patients. Br. Med. J. 289, 339–342, doi:10.1136/bmj.289.6441.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shen H., Goodall J. C., Hill Gaston J. S. (2009) Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 60, 1647–1656 [DOI] [PubMed] [Google Scholar]
- 21. Doreau A., Belot A., Bastid J., Riche B., Trescol-Biemont M. C., Ranchin B., Fabien N., Cochat P., Pouteil-Noble C., Trolliet P., Durieu I., Tebib J., Kassai B., Ansieau S., Puisieux A., Eliaou J. F., Bonnefoy-Bérard N. (2009) Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat. Immunol. 10, 778–785 [DOI] [PubMed] [Google Scholar]
- 22. Kuhne M., Erben U., Schulze-Tanzil G., Köhler D., Wu P., Richter F. J., John T., Radbruch A., Sieper J., Appel H. (2009) HLA-B27-restricted antigen presentation by human chondrocytes to CD8+ T cells: Potential contribution to local immunopathologic processes in ankylosing spondylitis. Arthritis Rheum. 60, 1635–1646 [DOI] [PubMed] [Google Scholar]
- 23. Vanderlugt C. L., Miller S. D. (2002) Epitope spreading in immune-mediated diseases: Implications for immunotherapy. Nat. Rev. Immunol. 2, 85–95 [DOI] [PubMed] [Google Scholar]
- 24. Mort J. S., Billington C. J. (2001) Articular cartilage and changes in arthritis: Matrix degradation. Arthritis Res. 3, 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maciewicz R. A., Wotton S. F., Etherington D. J., Duance V. C. (1990) Susceptibility of the cartilage collagens types II, IX and XI to degradation by the cysteine proteinases, cathepsins B and L. FEBS Lett. 269, 189–193 [DOI] [PubMed] [Google Scholar]
- 26. Borg A. A., Nixon N. B., Dawes P. T., Mattey D. L. (1994) Increased IgA antibodies to cytokeratins in the spondyloarthropathies. Ann. Rheum. Dis. 53, 391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. MacLean I. L., Archer J. R., Cawley M. I., Kidd B. L., O'Hara B. P., Pegley F. S., Thompson P. W. (1992) Immune complexes in ankylosing spondylitis. Ann. Rheum. Dis. 51, 83–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Twigg S. M., Joly A. H., Chen M. M., Tsubaki J., Kim H. S., Hwa V., Oh Y., Rosenfeld R. G. (2002) Connective tissue growth factor/IGF-binding protein-related protein-2 is a mediator in the induction of fibronectin by advanced glycosylation end-products in human dermal fibroblasts. Endocrinology 143, 1260–1269 [DOI] [PubMed] [Google Scholar]
- 29. Haupt L. M., Murali S., Mun F. K., Teplyuk N., Mei L. F., Stein G. S., van Wijnen A. J., Nurcombe V., Cool S. M. (2009) The heparan sulfate proteoglycan (HSPG) glypican-3 mediates commitment of MC3T3-E1 cells toward osteogenesis. J. Cell. Physiol. 220, 780–791 [DOI] [PubMed] [Google Scholar]
- 30. Kirn-Safran C., Farach-Carson M. C., Carson D. D. (2009) Multifunctionality of extracellular and cell surface heparan sulfate proteoglycans. Cell. Mol. Life Sci. 2009. November; 66(21): 3421–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murshed M., Schinke T., McKee M. D., Karsenty G. (2004) Extracellular matrix mineralization is regulated locally: Different roles of two gla-containing proteins. J. Cell Biol. 165, 625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manabe R., Tsutsui K., Yamada T., Kimura M., Nakano I., Shimono C., Sanzen N., Furutani Y., Fukuda T., Oguri Y., Shimamoto K., Kiyozumi D., Sato Y., Sado Y., Senoo H., Yamashina S., Fukuda S., Kawai J., Sugiura N., Kimata K., Hayashizaki Y., Sekiguchi K. (2008) Transcriptome-based systematic identification of extracellular matrix proteins. Proc. Natl. Acad. Sci. U.S.A. 105, 12849–12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamichi Y., Shukunami C., Yamada T., Aihara K., Kawano H., Sato T., Nishizaki Y., Yamamoto Y., Shindo M., Yoshimura K., Nakamura T., Takahashi N., Kawaguchi H., Hiraki Y., Kato S. (2003) Chondromodulin I is a bone remodeling factor. Mol. Cell. Biol. 23, 636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panupinthu N., Rogers J. T., Zhao L., Solano-Flores L. P., Possmayer F., Sims S. M., Dixon S. J. (2008) P2X7 receptors on osteoblasts couple to production of lysophosphatidic acid: A signaling axis promoting osteogenesis. J. Cell Biol. 181, 859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ke H. Z., Qi H., Weidema A. F., Zhang Q., Panupinthu N., Crawford D. T., Grasser W. A., Paralkar V. M., Li M., Audoly L. P., Gabel C. A., Jee W. S., Dixon S. J., Sims S. M., Thompson D. D. (2003) Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 17, 1356–1367 [DOI] [PubMed] [Google Scholar]
- 36. Ahn J. D., Dubern B., Lubrano-Berthelier C., Clement K., Karsenty G. (2006) Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology 147, 3196–3202 [DOI] [PubMed] [Google Scholar]
- 37. Madisen L., Neubauer M., Plowman G., Rosen D., Segarini P., Dasch J., Thompson A., Ziman J., Bentz H., Purchio A. F. (1990) Molecular cloning of a novel bone-forming compound: Osteoinductive factor. DNA Cell Biol. 9, 303–309 [DOI] [PubMed] [Google Scholar]
- 38. Delany A. M., Amling M., Priemel M., Howe C., Baron R., Canalis E. (2000) Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Invest. 105, 915–923, doi:10.1172/JCI7039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edwards J. C., Szczepanski L., Szechinski J., Filipowicz-Sosnowska A., Emery P., Close D. R., Stevens R. M., Shaw T. (2004) Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350, 2572–2581 [DOI] [PubMed] [Google Scholar]