Abstract
Retinoic acid derivatives have shown their greatest benefit in acute promyelocytic leukemia, but have also demonstrated pre-clinical anti-cancer effects in some solid tumors. Histone deacetylase inhibitors, by upregulating gene expression, are able to limit cancer cell proliferation and induce apoptosis. The combination of all-trans retinoic acid (ATRA) and the histone deacetylase inhibitor valproic acid has been previously studied in hematologic malignancies. We conducted a Phase I two-step dose escalation trial of the liposomal ATRA analog ATRA-IV and divalproex sodium (Depakote®) in nine patients with advanced solid tumors refractory to prior therapy. Side effects attributed to therapy had a severity ≤grade 2 and included skin toxicity and thrombocytopenia. The best disease response seen was disease stabilization in one patient. Expression of cellular retinoic acid binding protein-2 in peripheral blood mononuclear cells was detected as a marker of drug effect. The maximum tolerated dose (MTD) of both drugs in combination could not be established due to early closure of the trial resulting from a halt in the commercial availability of ATRA-IV.
Key words: ATRA, valproic acid, Phase I trial, histone deacetylase inhibitors, cancer
Introduction
Retinoic acid is the most potent natural form of vitamin A and influences cellular growth and differentiation by transcriptionally regulating gene expression by binding to retinoic acid receptors (RARs) and retinoid X receptors (RXRs).1 The six subtypes of receptors, RARα, RARβ, RARγ, RXRα, RXRβ and RXRγ, are members of the steroid/thyroid hormone receptor superfamily. The natural metabolites all-trans-RA (ATRA) and 9-cis-RA strongly bind RARs, while only 9-cis-RA binds RXRs. These receptor complexes then bind the promoter areas of target genes, which contain DNA sequences known as retinoic acid response elements (RAREs) or retinoid X response elements (RXREs).
Dysregulated RAR expression has been linked to a number of malignancies. The t(15;17) translocation of the RARα gene on chromosome 17 to the promyelocytic leukemia (PML) gene on chromosome 15 has been implicated in the pathogenesis of acute promyelocytic leukemia. Additionally, loss of RARβ expression has been found in a number of tumor types, including oral cavity,2 esophageal,3 prostate,4 breast5 and lung.6 ATRA-containing regimens have become standard in the management of acute promyelocytic leukemia (APL).7,8 Additionally, a number of solid tumor cell lines, including carcinomas of the head and neck, breast and kidney, show growth inhibition in the presence of ATRA.9–11 Clinical activity of retinoids has been demonstrated in many non-hematological premalignant and malignant conditions, including squamous cell carcinomas of the skin, cutaneous T-cell lymphoma and leukoplakia.12 Lippman and colleagues observed a 40–50% response rate in advanced non-melanoma skin cancer in patients treated with the retinoid isotretinoin.13
ATRA-IV is a liposomal ATRA analog designed so that the liposomal delivery system alters the drug's pharmacokinetics to improve tissue distribution. Following intravenous injection, this lipid formulation is able to bypass the hepatic clearance mechanism that metabolizes the oral formulation of ATRA. The lipid encapsulation likely also decreases toxicities associated with oral tretinoin doses. Additionally, in vitro studies have shown that liposomal ATRA has a greater anti-proliferative effect on neoplastic cells than free ATRA and can inhibit the proliferation of lymphoma cells in a dose-dependent manner by inducing apoptosis.14–16 Previous Phase I studies have shown that ATRA-IV is well tolerated with a side effect profile, mainly headache and rash, similar to that of oral ATRA.
Histone deacetylase (HDAC) inhibitors represent another class of targeted agents that have shown promise in cancer therapy. Histone deacetylases work in balance with histone acetylases (HATs) in the regulation of histone acetylation, which modulates chromatin relaxation and gene transcription. HDAC inhibitors cause histone hyperacetylation, thereby decondensing the DNA-histone chromatin complex and allowing gene transcription to proceed. In contrast, silent genes are associated with low levels of histone acetylation.17 HDAC inhibitors have been shown to induce cell cycle arrest, cell differentiation, inhibition of angiogenesis and apoptosis.18 The various classes of these drugs include short-chain fatty acids, hydroxamic acids, cyclic peptides and benzamides. Several HDAC inhibitors have been investigated in clinical trials, and vorinostat has been approved for use in cutaneous T-cell lymphoma.19
Valproic acid (VPA) is a short-chain fatty acid that has been shown to induce degradation of HDAC2 and impede angiogenesis in vitro and in vivo.20–22 Animal experiments showed that VPA induced differentiation of carcinoma cells, transformed hematopoietic progenitor cells and leukemic blasts from acute myeloid leukemia patients, and significantly reduced tumor growth and metastasis formation.23 Single-agent VPA has been studied in children with pontine gliomas,24 as well as in adults with advanced, heavily treated cancers.25
A number of pre-clinical and clinical studies have investigated the combination of HDAC inhibitors and ATRA. Studies have shown that the combination of valproic acid and ATRA was capable of inducing differentiation of myeloid blasts,26,27 and clinical responses have been observed in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) receiving this combination.28–30 We previously demonstrated that the combination of ATRA and the histone deacetylase inhibitor trichostatin A (TSA) more strongly inhibited the proliferation of renal cell carcinoma cell lines as compared to either drug alone.31
We performed a Phase I study of the combination of ATRA-IV and Depakote® (Abbott Laboratories), comprised of sodium valproate and valproic acid in a 1:1 molar relationship, to determine the MTD of the combination of Depakote and ATRA-IV in patients with advanced solid tumor malignancies.
Results
A total of nine patients were entered onto this trial. Table 1 illustrates the baseline characteristics and diagnoses of enrolled patients. The median age was 67, with a range of 25–77. All patients were males. Tumor types represented were kidney, bladder, prostate, esophageal, mesothelioma and sarcoma. The majority of patients, eight of nine, had received prior chemotherapy. All patients had metastatic disease at the time of study entry.
Table 1.
Baseline patient characteristics
| Characteristic | Number of patients (%) |
| Age (y) | 67 |
| Range | 25–77 |
| Karnofsky performance status | |
| 70 | 1 (11) |
| 80 | 8 (89) |
| Type of cancer | |
| Renal cell carcinoma | 2 (22) |
| Bladder urothelial carcinoma | 2 (22) |
| Prostate adenocarcinoma | 2 (22) |
| Gastroesophageal adenocarcinoma | 1 (11) |
| Mesothelioma | 1 (11) |
| Sarcoma | 1 (11) |
| Prior therapy | |
| Chemotherapy | 8 (89) |
| Radiotherapy | 3 (33) |
| Immunotherapy | 1 (11) |
| Hormonal | 2 (22) |
| Surgical | 4 (44) |
| Other | 7 (78) |
Toxicity.
All nine patients were evaluable for toxicity (Table 2). Among the three patients who reached a serum valproic acid level of 50–80 µg/ml, one patient developed airway obstruction related to progression of his tumor, resulting in respiratory failure and death. One patient at this dose level developed grade 1 thrombocytopenia. Other side effects seen at this dose level were cough and mood alterations in two patients and nocturia, dysphagia, edema, chest pain and arthralgias, each in one patient. None of these were more severe than grade 2. One patient started treatment with a target serum valproic acid level of 50–80 µg/ml but developed an allergic skin rash one day after initiating the medication and was taken off study.
Table 2.
Adverse events graded by national cancer institute common toxicity criteria
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
| Fatigue | 2 | 1 | |||
| Skin | 3 | 2 | |||
| Constipation | 1 | ||||
| Cough | 1 | ||||
| Pleural effusion | 1 | ||||
| Dyspnea | 3 | ||||
| Airway obstruction | 1a | ||||
| Epistaxis | 1 | ||||
| Anorexia | 1 | ||||
| Neurosensory | 2 | ||||
| Lower extremity paralysis | 1b | ||||
| Headache | 1 | ||||
| Diarrhea | 3 | ||||
| Nocturia | 1 | 1 | |||
| Nausea/vomiting | 1 | 1 | |||
| Altered mood | 1 | 2 | |||
| Dysphagia | 1 | ||||
| Thrombocytopenia | 1 | ||||
| Edema | 1 | ||||
| Hot flashes | 1 | ||||
| Abdominal pain | 1 | ||||
| Chest pain | 1 | 1 | |||
| Bone pain | 1 | ||||
| Arthralgias | 1 | 1 | |||
| Other pain | 3 |
Airway obstruction related to progression of disease rather than study drug.
Lower extremity weakness related to epidural disease rather than study drug.
Among the two patients who achieved a serum valproic acid level of 80–100 µg/ml, adverse events included fatigue, pleural effusion, epistaxis, anorexia, peripheral neuropathy, nocturia, nausea/vomiting, hot flashes, chest pain and arthralgias, and bone pain, each in one patient, and skin changes, dyspnea and diarrhea, each in two patients. Additionally, three patients started valproic acid therapy with a target serum level of 80–100 µg/ml but stopped therapy before reaching this serum level—one patient due to a grade 2 allergic skin rash, one due to a headache which started after he took one dose of medication, and one due to grade 4 right lower extremity weakness attributed to epidural disease.
In total, three patients were treated at a serum valproic acid level of 50–80 µg/ml and two at a serum valproic acid level of 80–100 µg/ml, all with ATRA-IV at a dose of 60 mg/m2. The MTD of Depakote in combination with ATRA-IV was not reached because patient accrual at higher doses than those achieved was not possible due to the commercial discontinuation of ATRA-IV, which necessitated study closure.
Antitumor activity.
The best response among all patients was disease stabilization of disease in patient 6, who had prostate cancer and was treated for four cycles at a serum valproic acid level of 80–100 µg/ml and ATRA-IV at 60 mg/m2 (Table 3). Stabilization lasted 16 w, at which time he progressed by serum PSA measurements. All other patients either progressed or were taken off study before the required serum valproic acid level was achieved, and thus, were not evaluable for tumor response. Patients 1 and 2 achieved the required serum valproic acid level of 50–80 µg/ml and started ATRA-IV at a dose of 60 mg/m2 but were then found to have progression of their soft tissue metastases. Patient 4, who had gastroesophageal adenocarcinoma, achieved the required serum valproic acid level of 50–80 µg/ml and completed one cycle of therapy, but then died from respiratory failure attributed to airway compression by progressive tumor. Patient 7 was treated for 1.5 cycles at a serum valproic acid level of 80–100 µg/ml and ATRA-IV at 60 mg/m2, but was taken off study after he had progression of his bone metastases.
Table 3.
Summary of patient responses
| Patient | Gender | Cancer type | Established valproic acid serum level (µg/ml) | Best response | Time to progression (weeks) | Site of progression | ATRA-IV dose (mg/m2) | Number of cycles completed |
| 1 | M | Renal cell carcinoma | 50–80 | POD | 8 | Brain/bone/lung | 60 | 2 |
| 2 | M | Bladder urothelial carcinoma | 50–80 | POD | 7 | Lung | 60 | 1.75 |
| 3 | M | Renal cell carcinoma | -a | - | - | - | - | 0b |
| 4 | M | Gastroesophageal adenocarcinoma | 50–80 | Deathc | 4 | - | 60 | 1 |
| 5 | M | Mesothelioma | -a | - | - | - | - | 0d |
| 6 | M | Prostate adenocarcinoma | 80–100 | SD | 16 | PSA progression | 60 | 4 |
| 7 | M | Sarcoma | 80–100 | POD | 6 | Bone | 60 | 1.5 |
| 8 | M | Bladder urothelial carcinoma | -a | - | - | - | - | 0e |
| 9 | M | Prostate adenocarcinoma | -a | - | - | - | - | 0f |
One cycle of therapy lasts 4 w.
Patient did not establish a serum concentration of valproic acid within the expected range. Patient not evaluable for response.
Patient developed grade 2 allergic skin rash one day following initiation of Depakote.
Patient expired after 5 w on study due to airway obstruction related to progression of disease.
Patient developed grade 2 allergic skin rash two days following initiation of Depakote.
Patient was taken off study after 2 w due to recurrent intermittent grade 2 headache.
Patient developed grade 4 right lower extremity paralysis possibly related to epidural disease. POD, progression of disease; SD, stable disease.
Pharmacokinetics and retinoid gene expression.
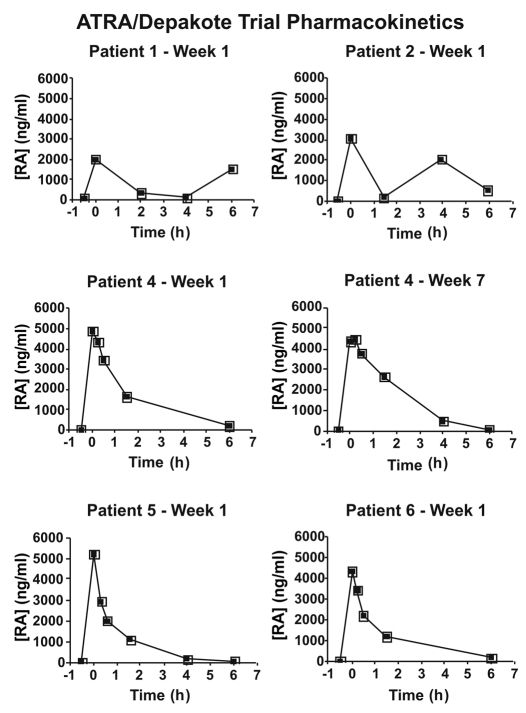
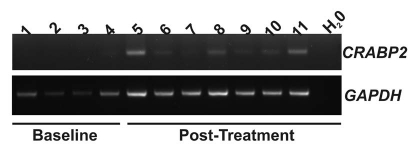
Pharmaco kinetic data for ATRA are presented in Figure 1 and Table 4. Expression of the human retinoid regulated gene, CRABP2 (NM_001878), was not detected in peripheral blood mononuclear cells (PBMCs) of untreated patients (N = 4). Expression of CRABP2 was detectable and persisted following treatment with depakote and ATRA-IV (N = 3) (Fig. 2). Consistent with our previous report,33 expression of the RARβ2 was not detected in PBMCs following ATRA-IV and Depakote treatment (data not shown).
Figure 1.
Pharmacokinetic plot of high performance liquid chromatography analysis of serum ATRA/Depakote levels measured before and at various times prior to (−0.5 h) and following (1–6 h) ATRA-IV infusion (n = 5). In the case of patient 4, serum ATRA levels were measured at weeks 1 and 7. All patients received 60 mg/m2 ATRA-IV. Infusion occurred at time 0.0 on the x-axis. ATRA was eliminated from plasma by about 6–10 h after ATRA-IV infusion. Y-axis, serum concentration (ng/ml) of ATRA.
Table 4.
ATRA pharmacokinetics data
| Total peak area (ng* h/ml) | Maximum concentration (ng/ml) | |
| Patient 1—week 1 | 5,098 | 2,009 |
| Patient 2—week 1 | 8,612 | 3,071 |
| Patient 4—week 1 | 10,111 | 4,873 |
| Patient 4—week 7 | 11,020 | 4,486 |
| Patient 5—week 1 | 6,718 | 5,214 |
| Patient 6—week 1 | 7,535 | 4,353 |
Figure 2.
mRNA expression of the retinoid regulated CRABP2 gene was assessed in peripheral blood mononuclear cells (PBMC) derived cDNA using reverse transcriptase PCR. Expression of CRABP2 was not detected in four patients prior to initiation of treatment (baseline, samples 1–4). Expression of CRABP2 was detectable following four treatments courses, immediately prior to the fifth course (samples 5 and 8: N = 2) and sixth treatment courses (sample 10). Expression of CRABP2 persists following the fifth (samples 6 and 8: 2 h post infusion; sample 7 and 9: 24 h post infusion) and sixth (sample 11: 2 h post infusion) drug infusions. Expression of GAPDH was used to confirm cDNA integrity.
Discussion
This Phase I study of ATRA-IV and Depakote in patients with advanced solid tumors is among the first investigating the combination of a retinoid and a HDAC inhibitor in solid malignancies. Three patients were treated at a serum valproic acid level of 50–80 µg/ml and two were treated at a level of 80–100 µg/ml, all at an ATRA-IV dose of 60 mg/m2. The MTD of both drugs in combination could not be established due to early closure of the trial resulting from a halt in the commercial availability of ATRA-IV.
Side effects seen in this trial were similar to those seen in the trial by Kuendgen et al. investigating the combination of valproic acid and ATRA in patients with AML, including skin and gastrointestinal toxicity, thrombocytopenia and pleural effusion.30 These side effects were not more severe than grade 2. The grade 4 lower extremity paralysis in patient 9 occurred after he had received only a few doses of Depakote, and imaging studies revealed epidural disease. One death occurred and was attributed to disease progression, not treatment.
This is the first study evaluating the combination of ATRA and valproic acid in patients with solid tumor malignancies. The best response seen was stabilization of disease in one patient. Improved responses possibly could have been achieved with higher doses of both drugs. This drug combination has been studied to the greatest extent in AML and MDS, with pre-clinical support for this regimen based on data such as those of Guel et al.34 who found that VPA and ATRA given together drastically stimulated self-renewal of normal hematopoietic progenitor cells, while each drug individually had a less pronounced effect. In a study enrolling 23 patients with either MDS or AML secondary to MDS, five patients were treated with a combination of oral ATRA and valproic acid from therapy initiation, with the remainder receiving only valproic acid initially and eligible for combination therapy if no response was seen.29 In those patients treated with combination therapy from the outset, no responses were seen. Since the response to single-agent valproic acid was better than that with combination therapy, the authors hypothesized that “pre-treatment” with valproic acid may be necessary for drug synergy, as HDAC inhibition could be necessary to relieve repression of retinoic acid signaling pathways.35 Consistent with this, expression of a prototypical retinoid target gene, CRABP2, that was not detectable in patient peripheral blood mononuclear cells prior to treatment, was found to be increased and persistent following treatment with depakote and ATRA (Fig. 2). This suggests that epigenetic barriers to transcription can be overcome by prolonged exposure to HDAC inhibitors. These data may also indicate the utility of CRABP2 expression as a surrogate and peripheral marker of the effects of ATRA and Depakote treatment on gene expression in solid tumors. In another small study of 11 elderly (>age 70) patients with non-M3 AML who were not candidates for standard chemotherapy, the combination of ATRA and valproic acid was able to induce a complete marrow response in three patients with one complete remission.36 The patient with the complete remission had a response duration of 22 mo with an overall survival of 28 mo. In another study of patients with de novo/secondary AML, ATRA (45 mg/m2) and valproic acid at higher doses than those used here resulted in hematological improvement in 27% of patients.37 Finally, the combination of 5-azacytidine, valproic acid and ATRA in acute myeloid leukemia patients resulted in a response rate of 42%.38 These data and the patient data reported here together suggest that a demethylating drug such as 5-aza-2′-deoxycytidine may be a useful addition.
Patients and Methods
Between June 2004–December 2005, nine patients with metastatic solid tumor malignancies who had failed at least one standard therapy, if available, were entered into this trial following approval by the institutional review board of Weill Cornell Medical College. All patients, who were age 21 or older, gave informed consent and were required to have a Karnofsky performance status (KPS) greater than or equal to 60 and a life expectancy of at least three months. Necessary laboratory parameters were as follows: WBC ≥3,000 cells/mm3, hemoglobin ≥9.0 gm/dl, platelet count ≥100,000, bilirubin <1.5 mg/dl, creatinine <2 mg/dl or creatinine clearance >50 cc/minute, and both AST/SGOT and ALT/SGPT each ≤twice the upper limit of normal. Exclusion criteria included the presence of active brain metastases or epidural tumor, concomitant steroid administration, radiation therapy or chemotherapy within 14 d of the start of protocol, presence of another active malignancy except non-melanoma skin carcinoma and in-situ cervical carcinoma, pancreatitis, coagulation disorders, psychiatric disorders, epilepsy, uncontrollable thyroid abnormalities or other severe clinically significant medical conditions.
Pretreatment evaluation included complete history and physical examination, complete blood count, complete metabolic panel, amylase, lipase, lipid panel and a coagulation profile. Urinalysis, chest X-ray and electrocardiogram (EKG) were also performed. Prostate cancer patients had baseline PSA and testosterone measured; breast cancer patients had baseline carcinoembryonic antigen (CEA), CA-125 and CA 15-3; colon cancer patients had baseline CEA. All patients underwent appropriate radiographic imaging of measurable disease and bony disease. Women in whom pregnancy was possible were required to have a negative pregnancy test, and they received counseling regarding the teratogenicity of ATRA-IV and Depakote and the necessity of using two forms of birth control during the study and for one month after completing therapy.
Treatment plan.
This trial was designed as a two-step dose escalation study. Depakote dose escalation was performed first in order to determine the MTD of Depakote with a baseline ATRA-IV dose of 60 mg/m2 weekly. Once an individual patient started Depakote at a designated dose level, it was not escalated for that patient. Three patients at each dose level had to complete 4 w of therapy before dose escalation in future patients could proceed. If no patients experienced a dose-limiting toxicity (DLT), then three new patients could be treated at the next higher dose level. If one instance of DLT was observed among the three patients treated at an individual dose level, an additional three patients had to be treated at that same dose level. If fewer than two DLT events subsequently occurred, then dose level escalation could proceed. If two or more DLT events were seen at any dose level, that level was defined as the dose limiting level and the preceding dose level was defined as the MTD.
DLT was defined as the occurrence of one or more of the following toxicities (as graded by NCI CTC version 2.0,32) considered by the investigator to be possibly related to study drug: grade 3 or 4 neutropenia or thrombocytopenia, any non-hematological toxicity ≥grade 3; or any non-hematological toxicity resulting in a dose-delay of greater than 3 w.
Depakote dose levels were defined by serum trough levels measured beginning on day 8 of cycle 1, and patients were divided into three cohorts based on dose level. Dose level I was defined as 50–80 µg/ml; level 2 as 80–100 µg/ml; level 3 as 100–120 µg/ml. Patients started Depakote at a dose of 10 mg/kg/day. Following the initial serum trough measurement on day 8, the Depakote dose could be increased by 5–10 mg/kg/weekly with weekly trough serum concentration measurements until the appropriate Depakote trough serum concentration had been achieved. After patients had been on Depakote for at least one week and they had shown the appropriate Depakote serum concentration, they started ATRA-IV at a fixed dose of 60 mg/m2 weekly until the MTD of Depakote had been reached or until a Depakote serum concentration of 100–120 µg/ml had been reached. Trough Depakote serum concentrations were measured weekly for the first 3 w following ATRA-IV administration and then monthly thereafter.
Once the MTD of Depakote was reached or a serum concentration of 100–120 µg/ml was achieved without toxicity, the ATRA-IV dose could be escalated by patient cohort to 90, 120 and then a maximum of 140 mg/m2, which was defined as the MTD in a Phase I study of ATRA-IV alone. Three-six patients could be treated at each ATRA-IV dose level.
Depakote dose reductions occurred for any grade 3 or 4 toxicity, except for anemia. In these cases, treatment was resumed following a decrease in toxicity to grade I or less with three weeks of withholding the drug. Treatment resumption occurred at the previous dose level or at a 25% dose reduction if toxicity was observed at the lowest dose level. If more than 3 w were required for a toxicity to decrease to grade I or less, then the patient was removed from study. If grade 3 or 4 toxicity recurred following the resumption of therapy, then the patient was removed from the study. Grade 3 or 4 anemia could be treated with packed red blood cell transfusion and/or erythropoietin as needed.
ATRA-IV treatment was interrupted in any patient experiencing a grade 3 or 4 toxicity, except for anemia or dermatologic toxicity. If the toxicity decreased to grade 1 or less within 3 w, therapy could be resumed at the previous dose level or at a 25% dose reduction if the patient was already at the lowest dose level. If more than three weeks were required for the toxicity to decrease to grade 1 or less, than the patient was removed from study. If grade 3 or 4 toxicity recurred on attenuated ATRA-IV doses, then treatment was discontinued. ATRA-IV treatment was interrupted for grade 4 skin toxicity and restarted at a 50% dose reduction when toxicity had improved to grade 2 or less. If grade 4 skin toxicity recurred following dose reduction, then the patient was removed from the study.
One cycle of therapy comprised four weeks of both ATRA-IV and Depakote. Patients underwent physical examination weekly for the first month of the study and then every 2 w thereafter. CBC and blood urea nitrogen (BUN), creatinine, amylase and lipase were obtained at 1, 2 and 4 w after the initiation of therapy and every 2 w thereafter. Reassessment of tumor status by the appropriate studies (bloodwork and/or radiologic imaging) was done every 8 w. Peripheral blood was drawn to extract lymphocytes following Depakote and ATRA-IV administration to test for retinoid-target gene expression, as well as to evaluate retinoic acid pharmacokinetics. Plasma pharmacokinetics of ATRA were evaluated using high performance liquid chromatography, and expression of the retinoid regulated, cellular retinoid binding protein-2 (CRABP2) gene expression was analyzed as previously described.11,33 PCR conditions were 95°C for 5 min; followed by 28 (GAPDH) or 35 (CRABP2) cycles of 95°C, primer annealing at 58°C for CRABP2 or 63°C for GAPDH for 30 s and extension at 72°C for 45 s. The identity of the CRAPB2 product was confirmed by automated DNA sequencing.
Patients were considered evaluable for toxicity and response if they completed 4 w of therapy. Overt progression was designated a treatment failure. Patients who achieved a complete response (CR), partial response (PR), minor response (MR) or disease stabilization were continued on therapy until progression. Patients who experienced a clinical PR and then underwent surgical resection of remaining disease were taken off therapy at the time of surgery. Patients who progressed with greater than 25% increase in tumor size were taken off protocol after 8 w on treatment. Those who showed rapid progression of disease with greater than 50% increase in tumor size were taken off study after 4 w on therapy.
Acknowledgements
We thank Martin Albert for technical assistance with the high performance liquid chromatography experiments.
Support: NCI CA92542 and CA85609; NIH General Clinical Research Center Program (NCRR grant M01RR00047); Antigenics, Inc., Abbott Laboratories, the Turobiner Kidney Cancer Research Fund and the Empire Clinical Research Investigator Program, Project 10 (K.A.D.).
Abbreviations
- ATRA
all-trans retinoic acid
- MTD
maximum tolerated dose
- RAR
retinoic acid receptor
- RAREs
retinoic acid response elements
- RXREs
retinoid X response elements
- PML
promyelocytic leukemia
- APL
acute promyelocytic leukemia
- HDAC
histone deacetylase
- HAT
histone acetylase
- VPA
valproic acid
- AML
acute myeloid leukemia
- MDS
myelodysplastic syndrome
- TSA
trichostatin A
- KPS
Karnofsky performance status
- WBC
white blood cell
- PBMC
peripheral blood mononuclear cell
- EKG
electrocardiogram
- PSA
prostate specific antigen
- CEA
carcinoembryonic antigen
- DLT
dose-limiting toxicity
- BUN
blood urea nitrogen
- CR
complete response
- PR
partial response
- MR
minor response
Footnotes
Previously published online: www.landesbioscience.com/journals/cbt/article/11436
References
- 1.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 2.Hu L, Gudas LJ. Cyclic AMP analogs and retinoic acid influence the expression of retinoic acid receptor alpha, beta and gamma mRNAs in F9 teratocarcinoma cells. Mol Cell Biol. 1990;10:391–396. doi: 10.1128/mcb.10.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu H, Zhang W, El-Naggar AK, Lippman SM, Lin P, Lotan R, et al. Loss of retinoic acid receptor-beta expression is an early event during esophageal carcinogenesis. Am J Pathol. 1999;155:1519–1523. doi: 10.1016/s0002-9440(10)65467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotan Y, Xu XC, Shalev M, Lotan R, Williams R, Wheeler TM, et al. Differential expression of nuclear retinoid receptors in normal and malignant prostates. J Clin Oncol. 2000;18:116–121. doi: 10.1200/JCO.2000.18.1.116. [DOI] [PubMed] [Google Scholar]
- 5.Xu XC, Sneige N, Liu X, Nandagiri R, Lee JJ, Lukmanji F, et al. Progressive decrease in nuclear retinoic acid receptor beta messenger RNA level during breast carcinogenesis. Cancer Res. 1997;57:4992–4996. [PubMed] [Google Scholar]
- 6.Xu XC, Sozzi G, Lee JS, Lee JJ, Pastorino U, Pilotti S, et al. Suppression of retinoic acid receptor beta in non-small-cell lung cancer in vivo: implications for lung cancer development. J Natl Cancer Inst. 1997;89:624–629. doi: 10.1093/jnci/89.9.624. [DOI] [PubMed] [Google Scholar]
- 7.Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 8.Sanz MA, Tallman MS, Lo-Coco F. Tricks of the trade for the appropriate management of newly diagnosed acute promyelocytic leukemia. Blood. 2005;105:3019–3025. doi: 10.1182/blood-2004-09-3475. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, Gudas LJ. Metabolism of all-trans-retinol in normal human cell strains and squamous cell carcinoma (SCC) lines from the oral cavity and skin: reduced esterification of retinol in SCC lines. Cancer Res. 1998;58:166–176. [PubMed] [Google Scholar]
- 10.Chen AC, Guo X, Derguini F, Gudas LJ. Human breast cancer cells and normal mammary epithelial cells: retinol metabolism and growth inhibition by the retinol metabolite 4-oxoretinol. Cancer Res. 1997;57:4642–4651. [PubMed] [Google Scholar]
- 11.Guo X, Nanus DM, Ruiz A, Rando RR, Bok D, Gudas LJ. Reduced levels of retinyl esters and vitamin A in human renal cancers. Cancer Res. 2001;61:2774–2781. [PubMed] [Google Scholar]
- 12.Smith MA, Parkinson DR, Cheson BD, Friedman MA. Retinoids in cancer therapy. J Clin Oncol. 1992;10:839–864. doi: 10.1200/JCO.1992.10.5.839. [DOI] [PubMed] [Google Scholar]
- 13.Lippman SM, Meyskens FL., Jr Results of the use of vitamin A and retinoids in cutaneous malignancies. Pharmacol Ther. 1989;40:107–122. doi: 10.1016/0163-7258(89)90078-8. [DOI] [PubMed] [Google Scholar]
- 14.Nastruzzi C, Walde P, Menegatti E, Gambari R. Liposome-associated retinoic acid. Increased in vitro antiproliferative effects on neoplastic cells. FEBS Lett. 1990;259:293–296. doi: 10.1016/0014-5793(90)80030-m. [DOI] [PubMed] [Google Scholar]
- 15.Parthasarathy R, Sacks PG, Harris D, Brock H, Mehta K. Interaction of liposome-associated all-transretinoic acid with squamous carcinoma cells. Cancer Chemother Pharmacol. 1994;34:527–534. doi: 10.1007/BF00685666. [DOI] [PubMed] [Google Scholar]
- 16.Sundaresan A, Claypool K, Mehta K, Lopez-Berestein G, Cabanillas F, Ford RJ., Jr Retinoid-mediated inhibition of cell growth with stimulation of apoptosis in aggressive B-cell lymphomas. Cell Growth Differ. 1997;8:1071–1082. [PubMed] [Google Scholar]
- 17.Acharya MR, Sparreboom A, Venitz J, Figg WD. Rational development of histone deacetylase inhibitors as anticancer agents: a review. Mol Pharmacol. 2005;68:917–932. doi: 10.1124/mol.105.014167. [DOI] [PubMed] [Google Scholar]
- 18.Pandolfi PP. Histone deacetylases and transcriptional therapy with their inhibitors. Cancer Chemother Pharmacol. 2001;48:17–19. doi: 10.1007/s002800100322. [DOI] [PubMed] [Google Scholar]
- 19.Mann BS, Johnson JR, He K, Sridhara R, Abraham S, Booth BP, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13:2318–2322. doi: 10.1158/1078-0432.CCR-06-2672. [DOI] [PubMed] [Google Scholar]
- 20.Kramer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA, et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 2003;22:3411–3420. doi: 10.1093/emboj/cdg315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaelis M, Michaelis UR, Fleming I, Suhan T, Cinatl J, Blaheta RA, et al. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol Pharmacol. 2004;65:520–527. doi: 10.1124/mol.65.3.520. [DOI] [PubMed] [Google Scholar]
- 22.Eyal S, Yagen B, Sobol E, Altschuler Y, Shmuel M, Bialer M. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia. 2004;45:737–744. doi: 10.1111/j.0013-9580.2004.00104.x. [DOI] [PubMed] [Google Scholar]
- 23.Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaheta RA, Michaelis M, Driever PH, Cinatl J., Jr Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med Res Rev. 2005;25:383–397. doi: 10.1002/med.20027. [DOI] [PubMed] [Google Scholar]
- 25.Atmaca A, Al-Batran SE, Maurer A, Neumann A, Heinzel T, Hentsch B, et al. Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Br J Cancer. 2007;97:177–182. doi: 10.1038/sj.bjc.6603851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cimino G, Lo-Coco F, Fenu S, Travaglini L, Finolezzi E, Mancini M, et al. Sequential valproic acid/alltrans retinoic acid treatment reprograms differentiation in refractory and high-risk acute myeloid leukemia. Cancer Res. 2006;66:8903–8911. doi: 10.1158/0008-5472.CAN-05-2726. [DOI] [PubMed] [Google Scholar]
- 27.Trus MR, Yang L, Suarez Saiz F, Bordeleau L, Jurisica I, Minden MD. The histone deacetylase inhibitor valproic acid alters sensitivity towards all trans retinoic acid in acute myeloblastic leukemia cells. Leukemia. 2005;19:1161–1168. doi: 10.1038/sj.leu.2403773. [DOI] [PubMed] [Google Scholar]
- 28.Kuendgen A, Knipp S, Fox F, Strupp C, Hildebrandt B, Steidl C, et al. Results of a phase 2 study of valproic acid alone or in combination with all-trans retinoic acid in 75 patients with myelodysplastic syndrome and relapsed or refractory acute myeloid leukemia. Ann Hematol. 2005;84:61–66. doi: 10.1007/s00277-005-0026-8. [DOI] [PubMed] [Google Scholar]
- 29.Kuendgen A, Strupp C, Aivado M, Bernhardt A, Hildebrandt B, Haas R, et al. Treatment of myelodysplastic syndromes with valproic acid alone or in combination with all-trans retinoic acid. Blood. 2004;104:1266–1269. doi: 10.1182/blood-2003-12-4333. [DOI] [PubMed] [Google Scholar]
- 30.Kuendgen A, Schmid M, Schlenk R, Knipp S, Hildebrandt B, Steidl C, et al. The histone deacetylase (HDAC) inhibitor valproic acid as monotherapy or in combination with all-trans retinoic acid in patients with acute myeloid leukemia. Cancer. 2006;106:112–119. doi: 10.1002/cncr.21552. [DOI] [PubMed] [Google Scholar]
- 31.Touma SE, Goldberg JS, Moench P, Guo X, Tickoo SK, Gudas LJ, et al. Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model. Clin Cancer Res. 2005;11:3558–3566. doi: 10.1158/1078-0432.CCR-04-1155. [DOI] [PubMed] [Google Scholar]
- 32.Common Toxicity Criteria, version 2.0. Cancer Therapy Evaluation Program. 1998. [Google Scholar]
- 33.Boorjian SA, Milowsky MI, Kaplan J, Albert M, Cobham MV, Coll DM, et al. Phase 1/2 clinical trial of interferon alpha2b and weekly liposome-encapsulated all-trans retinoic acid in patients with advanced renal cell carcinoma. J Immunother. 2007;30:655–662. doi: 10.1097/CJI.0b013e31805449a8. [DOI] [PubMed] [Google Scholar]
- 34.Guel H, Wassmann B, Romanski A. Effect of the his-tone deacetylase inhibitor valproic acid in combination with all-trans retinoic acid on normal and malignant hematopiesis. Blood. 2003;102:626. [Google Scholar]
- 35.Ferrara FF, Fazi F, Bianchini A, Padula F, Gelmetti V, Minucci S, et al. Histone deacetylase-targeted treatment restores retinoic acid signaling and differentiation in acute myeloid leukemia. Cancer Res. 2001;61:2–7. [PubMed] [Google Scholar]
- 36.Raffoux E, Chaibi P, Dombret H, Degos L. Valproic acid and all-trans retinoic acid for the treatment of elderly patients with acute myeloid leukemia. Haematologica. 2005;90:986–988. [PubMed] [Google Scholar]
- 37.Bellos F, Mahlknecht U. Valproic acid and all-trans retinoic acid: meta-analysis of a palliative treatment regimen in AML and MDS patients. Onkologie. 2008;31:629–633. doi: 10.1159/000160599. [DOI] [PubMed] [Google Scholar]
- 38.Soriano AO, Yang H, Faderl S, Estrov Z, Giles F, Ravandi F, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]