Abstract
Cell migration is critical for proper development of the embryo and is also used by many cell types to perform their physiological function. For instance, cell migration is essential for immune cells to monitor the body and for epithelial cells to heal a wound whereas, in cancer cells, acquisition of migratory capabilities is a critical step toward malignancy. Migratory cells are often categorized into two groups: (1) mesenchymal cells, produced by an epithelium-to-mesenchyme transition, that undergo solitary migration and (2) epithelial-like cells which migrate collectively. However, on some occasions, mesenchymal cells may travel in large, dense groups and exhibit key features of collectively migrating cells such as coordination and cooperation. Here, using data published on neural crest cells, a highly invasive mesenchymal cell population that extensively migrate throughout the embryo, we explore the idea that mesenchymal cells, including cancer cells, might be able to undergo collective cell migration under certain conditions and discuss how they could do so.
Key words: collective cell migration, epithelium-to-mesenchyme transition, neural crest cells, contact-inhibition of locomotion, cancer, metastasis
Introduction: How to Define Collective Cell Migration?
Collective cell migration can be simply presented as the migration of groups of cells as opposed to the migration of isolated cells. However, several definitions have been proposed which may include or exclude some types of cell migration. While some argue for a broad definition such as the “migration in loosely or closely associated groups”;1 others insist that cells should remain “physically and functionally connected such that the integrity of cell-cell junctions is preserved during movement.”2 According to the second definition loose groups of cells, where cell-cell junctions are transient and constantly remodeled, are not migrating collectively. Therefore, collective cell migration would apply solely to cells with an epithelial or epithelial-like phenotype. However, cells from loose groups may travel together in a directional fashion for long periods of time and display a high level of coordination and cooperation suggesting that the type of cell-cell adhesion may not be a relevant criterion to assess collectiveness. Here, we review studies published on neural crest (NC) cells, a mesenchymal and highly migratory cell population3–6 and discuss the implications of the findings of these works in the context of defining collective cell migration and its relevance to mesenchymal cell migration.
The Neural Crest at a Glance
The neural crest (NC) is a multipotent cell population specified at the interface between the neural and non-neural ectoderms by a combination of signals from the BMP, Wnt, FGF and Notch families.7,8 After induction, NC cells separate from their surrounding tissues during a delamination phase which involves an epithelium-to-mesenchyme transition (EMT).5,9,10 As part of the EMT process, NC cells reduce their cell-cell adhesion properties to become mesenchymal cells with extensive migratory capabilities.4,5 As a result, they colonize nearly all tissues and organs of the embryo (Fig. 1) where they give rise to a wide range of derivatives such as neurons, glia, bone, cartilage, endocrine cells, connective tissues and smooth muscle.3,5,6 Interestingly, the NC cells migrate as several independent subpopulations exhibiting a variety of migratory strategies and behaviors, which we review hereafter.
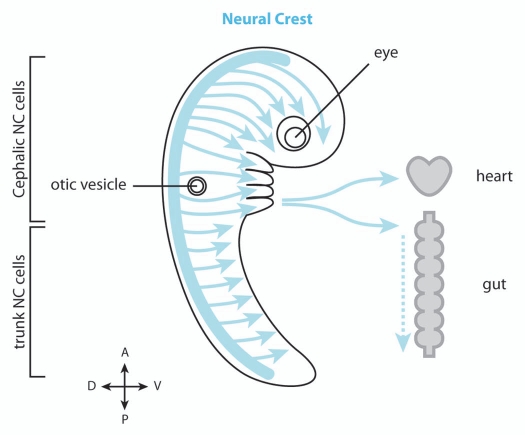
Figure 1.
Neural crest cell migration. Neural crest (NC) cells (blue) emerge from the dorsal neuroepithelium and migrate extensively throughout the embryo. The cephalic NC cells mainly migrate under the skin and toward the ventral portions of the face with some subpopulations migrating further ventrally toward the heart and along gut. The trunk NC cells mostly invade the ventral regions of the trunk and colonize the skin.
Collective and Solitary Behaviors during NC Cell Migration
Xenopus cephalic neural crest cells.
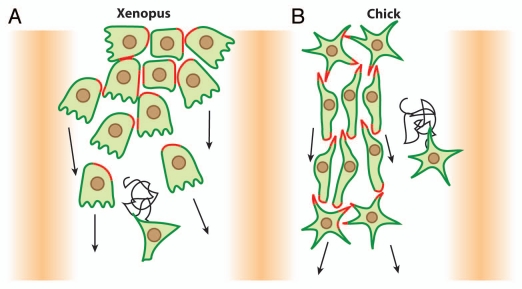
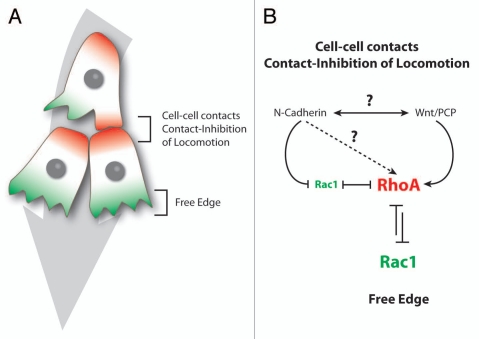
In Xenopus, the cephalic NC cells start their migration as a relatively tight pseudoepithelial cell sheet (Fig. 2A).11,12 At early stages of Xenopus NC cell migration, cells have relatively stable cell-cell junctions and motile cells can pull forward non-motile neighbors such as cells undergoing cell division or cells having recently collapsed cell protrusions.13 NC cells progressively turn mesenchymal. They exhibit highly dynamic and transient contacts and migrate in a cell streaming fashion (Fig. 2A, lower part).6,11 Throughout migration, in sheets or streams, cell-cell interactions promote Contact-Inhibition of Locomotion (CIL),14,15 the process by which cells collapse protrusions and repolarize upon contact with another cell.16,17 Since CIL promotes the collapse of the protrusions, it restricts the protrusive activity toward the cell-free space giving each cell a clear front-back polarity matching the free space-cell contact axis (Fig. 3A).13,15,18 CIL is mediated by the Wnt-PCP signaling pathway15,19–21 and requires the formation of N-Cadherin-based adherens junctions upon contact.13 Wnt-PCP activates RhoA at the contacts15 while N-Cadherin is required for the local inhibition of Rac1 (Fig. 3B).13 The link between N-Cadherin and PCP is unknown but one possibility is that N-Cadherin promotes the formation of a proper contact bringing the cell membranes in close proximity, which in turns helps trigger PCP signaling. Importantly, cells in groups show higher persistence than single cells15 suggesting that cell-cell interactions promote a certain degree of coordination within the group while single cells are free to wander without being restricted by direct neighbors. Besides cell-cell interactions polarizing the cells by maintaining a high-RhoA/low-Rac1 activity at the cell contacts, additional factors modulate polarity through small GTPases. For instance, Syndecan-4 is expressed by NC cells and inhibits Rac1 when bound to Fibronectin.19 Moreover, the chemokine Stromal cell-derived factor 1 (Sdf1) increases Rac1 activity through its receptor Cxcr4.13 Interestingly, cells in groups are able to chemotax toward Sdf1 with great efficiency whereas isolated NC cells fail to migrate directionally when placed in a gradient of Sdf1.13 The chemotactic abilities of NC cell groups are abolished if cell-cell junctions are impaired while single cells can be made responsive if cultured at high cell density.13 These data indicate that NC cells in sheets and mesenchymal NC cells cultured at high cell density acquire emergent properties that are not born by isolated cells.
Figure 2.
Migration of cephalic NC cells in Xenopus and chick embryos. (A) In Xenopus, NC cell migration start as a loose cell sheet (top part) and progressively turns into a cell streaming composed of mesenchymal cells (bottom part). (B) In chick, NC cells migrate as mesenchymal cells and form chains. In both models cells are polarized by interactions with other NC cells (red) and maintained as a dense group by the presence of inhibitors defining the borders of the NC routes (shades of orange). High cell density leads to directional movement while isolated cells exhibit poor directionality (sinuous path).
Figure 3.
Xenopus cephalic NC cells are polarized by contact-inhibition of locomotion. (A) Contact-Inhibition of Locomotion is triggered by cell interactions. Cells are polarized according to their cell-cell contacts; free edge is in green, cell contacts are in red. (B) Cell-cell interactions are mediated by N-Cadherin and Wnt/PCP signaling. N-Cadherin is required for a local inhibition of Rac1 at the cell junctions and Wnt/PCP induces an increase of RhoA activity at the contact. Both N-Cadherin and Wnt/PCP maintain low Rac1 and high RhoA activities at the cell-cell contact which restrict Rac1 activity at the free edge.
Migration of Xenopus cephalic NC cells toward the ventral region of the face does not lead to a complete spreading of the cell population along the dorso-ventral axis. NC cells remain in close proximity and migrate ventrally leaving a gap between the rear of the NC population and the neuroepithelium from which they emerge. This typical pattern of migration has been extensively described in references 4, 6, 11 and 22 and can be easily observed by in situ hybridization or time-lapse microscopy. Cells within a group locally exchange position with their direct neighbors but no dramatic movements happen such that the global organization of the NC cell population is relatively steady with cells at the front and at the back of the group keeping their relative position for long periods of time.13,15
Altogether, these data indicate that Xenopus cephalic NC cells migrate as an organized group of mostly mesenchymal cells with high cell cooperation and a relatively steady spatial organization.
Chick cephalic neural crest cells.
Chick cephalic NC cells turn mesenchymal at the onset of their migration and never engage in sheet-like migration.23–25 However, the description of chick cephalic NC cell migration highlights a global behavior that is very similar to that of the Xenopus cephalic NC cells. Chick NC cells migrate in a coordinated fashion toward the ventral region of the face leaving cell-free spaces behind.5 Importantly, when the total number of migratory cells is reduced experimentally the overall behavior is not affected and the cells still manage to reach the ventral most regions of the face.26,27 Cephalic chick NC cells mostly migrate in chains with leader cells and followers keeping their relative positions for long periods of time,23–25,27 suggesting that despite being mesenchymal some degree of spatial organization is achieved and maintained over time. Migrating chick NC cells have repeated cell-cell contacts with their migratory neighbors similar to the close contact observed in Xenopus neural crest (Fig. 2A and B), but in addition, chick neural crest exhibit long filopodia that allow cell-cell communication over long distances.25 Despite being transient, these contacts lead to an exchange of cell material between the cells28 and induce a collapse of cell protrusions25 similar to the contact-inhibition behavior described in Xenopus.15 The molecular effectors responsible for this phenomenon are unknown. Interestingly, cells within a group show higher directionality than cells wandering on their own25 indicating that cell-cell interactions lead to a more efficient migration. If such cell-cell contacts confer the ability to respond to external cues on NC cells as in Xenopus remains to be demonstrated. Finally, chick cephalic NC cells also exhibit a cell polarity based on a free space-cell contact axis. Cells exposed to cell-free spaces, located at the front or back of the chains, have more protrusive activity than cells within the chains (Fig. 2B).25
Altogether these data strongly support a model similar to Xenopus NC cells where chick cephalic NC cells migrate more efficiently than isolated cells and where cell-cell interactions are responsible for cell polarity and coordination.
Enteric NC cells.
The enteric subpopulation of NC cells arises from the caudal hindbrain and the sacral region of the trunk.5,29–33 These cells move toward the gut and colonize its entire length where they form the chains of enteric ganglia.5 Enteric NC cells first reach the anterior region of the gut and progress caudally without leaving a NC-free space behind. The migration is mostly achieved by a vanguard of actively migrating and proliferating cells.29,34,35 Behind these leading cells is a rearguard of NC cells that migrate and proliferate less.35 Proliferation of the enteric NC cells has been shown as critical for the complete innervation of the gut35 suggesting that the caudalward extension of the population was due to population pressure progressively shifting the leading edge of the migratory population further caudally. However, proliferation is mainly observed at the front of the population indicating that the front is not pushed forward by cells from the rearguard. Experiments where two groups of enteric cells were grafted, in anterior and posterior regions of the gut, show that both populations progress toward the middle of the gut.35 They both exhibit a migratory behavior similar to that observed in a control situation despite one of the group is migrating backward. However, when the two populations meet, both proliferation and migration are reduced.35 This supports the idea that the progression of the vanguard is mainly driven by the availability of a NC-free space at the front, such that the progression rate of the population is directly linked to the time needed to fully populate a given region of the gut. When one given segment is occupied by a critical number of NC cells the migration proceeds further to occupy the next segment. Analysis of cell movement indicates that even if NC cells are progressively moving in a rostro-caudal manner the movement of cells within the population cannot be predicted.34 More precisely, the average movement of the cells does not match the average progression of the group indicating that there is no global coordination. Also in contrast with cephalic NC cells, enteric NC cells keep only poor spatial relationships over long periods of time. Cells in close proximity at the beginning of the migration may end far apart as a constant supply of new cells by proliferation passes cells from the vanguard to the rearguard where they soon stop migrating and differentiate.29,34–36 However, some enteric NC cells do engage in chains and isolated cells migrate slower than cells at high cell density.37 This indicates that cell-cell interactions take place and influence cell behavior but these interactions are not sufficient to promote collective behavior when we consider the population of enteric NC cells as a whole. It is possible however that some degree of collectiveness may be seen transiently when considering subpopulations of the enteric NC cells such as cells within the vanguard. More analyses are needed to address these points.
Altogether, these observations indicate that enteric NC cell migration is mostly driven by migratory and proliferative activities at the vanguard, leading to a progressive movement of the cells toward NC-free regions of the gut. These data also indicate that enteric NC cells show little coordination and do not keep spatial relationships during their extensive rostro-caudal migration.
Trunk NC cells.
Trunk NC cells migrate following two main routes: a ventromedial pathway toward the anlagen of the dorsal root (DRG) and sympathetic (SG) ganglia3,5 and a dorsolateral pathway underneath the ectoderm only used by pigment cells.5,38,39 Trunk NC cells start their migration as mesenchymal cells.9,39 Cells using the ventral path keep a relatively stable spatial organization throughout migration with early delaminating cells colonizing regions located farther away than cells emigrating later on.5,40 In chick some of these cells were even observed migrating as part of chains,41 similarly to their cephalic counterpart, confirming that trunk NC cells engage into migratory units involving cell-cell interactions. It is only after cells have reached the anlagen of the DRG and SG that some cell mixing can be observed and that the spatial distribution is blurred.41 Interestingly, in vitro cultures of trunk NC cells indicate that cells at high cell density exhibit a higher directionality than dissociated cells.42 In these experiments, NC cells migrate in a nearly confluent fashion and often slightly overlap with one another. The authors suggest that the higher directionality is achieved because each cell is restricted by its direct neighbors. They also describe how cells collapse their filopodia upon contact with other NC cells. Moreover, cell-cell interactions were shown to directly promote motility as single cells have poorer activity than cells in dense cultures.43 More precisely, isolated trunk NC cells were observed to alternate between short periods of migration and tumbling with no net movement whereas cells interacting with one another exhibited a more active behavior with longer periods of cell motility and greater speed. Cells were seen constantly colliding and moving away from each other in a manner reminiscent of the cell behavior induced by Contact-Inhibition of Locomotion.14 In vivo, NC cells migrating through the ventromedial pathway are restricted to a narrow path surrounded by inhibitory cues4,5,39 that maintain a high cell density. Based on the direct correlation between cell density and directionality observed in vitro it is tempting to suggest that a similar effect is achieved in vivo when cells encounter local inhibitors. CIL has not been directly assessed in trunk NC cells. However, the fact that these cells are influenced by their contact with other cells and the fact that Wnt/PCP is required for zebrafish and chick trunk NC migration19,20,44 in vivo strongly suggest a critical role for CIL in the directional migration of trunk NC cells.
NC cells using the dorsolateral pathway give rise to pigment cells.38 These cells migrate in a non-segmented manner. They migrate extensively to cover the entire surface of the skin. In contrast with cephalic NC cells, but similarly to enteric NC cells, when the size of the population is reduced the most distal regions fail to be colonized and white patches devoid of pigment cells are generated.38 This observation strongly suggests that the initial distribution of pigments cell precursors is primarily driven by repulsion among NC cells. The cells move away from each other until they reach a region where they are no longer surrounded by other NC cells to maximize the coverage of the skin. Such mechanism would make contact-inhibition the primary driving force of pigment cell distribution as proposed by some authors.45 Whether or not this is the case remains to be evaluated.
Altogether these data indicate that trunk NC cells migrating along the ventromedial pathway keep a relative spatial organization throughout migration and engage in highly directional migration when maintained at high cell density, which indicate collective cell migration behavior. On the contrary, migration of pigment cells suggests a contact-inhibition driven mechanism with no apparent coordination.
Can Mesenchymal Cells Undergo Collective Cell Migration?
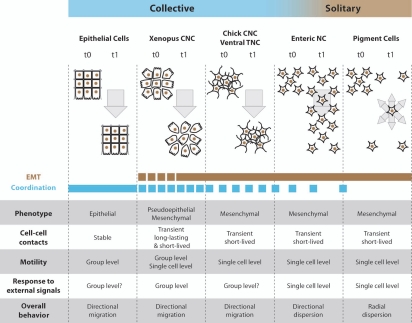
The broad definition of collective cell migration stated as the “migration in loosely or closely associated groups”1 would include nearly all migratory NC cell subpopulations apart from pigment cell precursors. On the contrary, the more restrictive definition based on stable physical contacts would exclude all NC cell migratory behaviors but the very early steps of Xenopus cephalic NC cell migration. The Xenopus cephalic NC cell migration starts as an epithelial-like cell sheet migration and quickly turns into a cell streaming with more transient cell-cell contacts (Fig. 4). However the effect of cell-cell interactions on cell polarity and competence to respond to external guidance cues is, in both cases, similar and involves the same molecular effectors (N-Cadherin/PCP signaling).13,15,19–21 Despite turning into cell streaming, Xenopus cephalic NC cells still move forward in a coordinated manner. Cells at the back do not undergo a reverse migration toward the free space created between the rear of the NC population and the neuroepithelium. These observations indicate that the transition toward a more mesenchymal phenotype does not reduce the ability of these cells to migrate together. In addition, it strongly supports the idea that the stability of the cell-cell contacts cannot be used to assess collectiveness. Importantly, in Xenopus and mouse cephalic crests cell-cell interactions are essential for the response to external signals such as Sdf1 or semaphorins.4,6,13,46 This indicates that the guidance is controlled at the group level (Fig. 4). If such collective guidance occurs in other cell collectives such as epithelial cell sheet or other NC cells remains to be determined. Nevertheless, in chick, cephalic NC cells and trunk NC cells using the ventral route, which exhibit typical mesenchymal phenotypes from the early steps of migration, also migrate in directional coordinated fashion with a relatively stable spatial organization, including chains, but no stable cell-cell contacts. We believe these NC cell subpopulations should be considered as collectively migrating mesenchymal cell populations (Fig. 4).
Figure 4.
Neural crest cells exhibit solitary and collective behaviors. Comparison between epithelial cells, cephalic, enteric and trunk NC cells. For each cell population their epithelial or mesenchymal phenotype and the type of cell-cell contacts are indicated. Motility at the group level means that non-motile cells can be pulled by adjacent motile cells while motility at the single cell level indicates that each cell is properly motile. Response to external signals at the group level means that the ability to respond to these signals depends on the fact that cells are part of a collective as opposed to a situation where each cell responds to external cues independently. Such collective guidance has been shown for Xenopus and mouse cephalic NC cells13,46 and is therefore likely to apply to other cephalic crests in other species. CNC, cephalic NC cells; TNC, trunk NC cells.
A relatively similar situation of a loosely connected group of cells, fitting the broad definition of a cell collective mentioned above, is observed in the vanguard of enteric NC cells.29,34–36 However, these cells show no apparent coordination. Cells across the population keep very poor spatial relationships and the general displacement of the population does not match the individual cell movements.29,34,36,37 This suggests that having a high density of loosely connected cells is not sufficient to promote collective migration since enteric NC cells do not seem to travel together but rather spread over a large surface (Fig. 4).
Based on the aforementioned examples, we propose that cell collectives should be classified as such if they meet the two following criteria: (1) cells are migrating together in a coordinated directional fashion such that the movement of cells within the group correlates in some way to the average movement of the group as a whole; (2) cell-cell interactions affect the migratory behavior of cells within the group. To test if a particular kind of cells meets these criteria several parameters related to cell migration (velocity, persistence, polarity, tracks, etc.) should be analyzed. In addition, the behavior of an isolated cell, a cell within a group and the average behavior of the group should be compared. It should be stressed that none of these criteria would by themselves suffice to define collectiveness. For instance, if coordination is circumstantial then cells in clustered and isolated cells would perform equally in similar conditions suggesting that the group itself is not required for the migratory behavior observed. Furthermore, a tissue with a very high proliferative activity could show directional expansion and keep spatial organization overtime but there would be no actual migration at the population level and cells at different regions of the group would exhibit dramatically different behavior. The front would progress but the rear would exhibit a mainly static behavior with cells oscillating around their original positions. Based on these criteria all the cephalic and most of the trunk NC cells would be considered as collectively migrating whereas pigment cells precursors and enteric NC cells would not (Fig. 4). The case of the enteric cells is of particular relevance. The size of enteric NC cell population has a direct effect on how far the population can migrate; the enteric NC cells clearly migrate toward NC-free spaces and cell interactions influence cell motility.29,35–37,47 These three observations support the fact that enteric NC cells interact with each other and that the general expansion of the population is driven by cell-cell interactions. This indicates that being part of a large group of cells matters and has an influence of the behavior of the overall population. However, there is no coordination among cells and there is a general spreading of the cells toward less populous areas but no collective effort to shift the group from one location to another.
How do Neural Crest Cells Undergo Collective Cell Migration without Stable Cell-Cell Adhesion?
Some important questions are raised by the collective behavior of the NC cells. If these cells do not have proper long-lasting cell junctions how is the group maintained over time? Additional mechanisms are required to explain why cells located at the rear of the population move in one direction while they face a cell-free space in the opposite direction. Several alternatives can be proposed: (1) cells are maintaining the group by attracting each other; (2) migratory cells at the front of the population modify the environment so that the migration of the following cells is biased toward the front of migration; (3) contacts between the cells mediate local forces and tensions across the cell body which would align cells at the rear of the group to the overall direction of the population. The first idea, mutual attraction between cells, is reminiscent of the situation observed in starving Dictyostelium cells48 and has also been observed among bacteria.49 When confronted to restricted resources Dicty cells secrete cyclic-AMP which acts as a chemoattractant for other Dicty cells, where cells progressively migrate toward each other to form a multicellular structure called a slug. Interestingly, it has recently been found that such system is at work among cephalic Xenopus NC cells.50 These cells express a chemoattractant (Complement factor C3a) and its receptor (C3aR).50–52 Cells leaving the group are attracted back to the group by repolarization mediated by the C3a/C3aR signaling. C3a promotes Rac1 activation and protrusion formation (Fig. 5). Impairing C3a or C3aR expression leads to cell dispersion and a loss of collectiveness both in vivo and in vitro.50 Furthermore, expression of C3a/C3aR into individually migrating cells, like myeloid cells,53 transforms their solitary behavior into collective cell migration.50 These results indicate that, in absence of stable contacts, mutual attraction among mesenchymal cells can maintain cells as a group. One can imagine that other mesenchymal cells could prevent dispersion by a similar mechanism where each cell would sense a signal released locally by the other cells. Interestingly, some cancer cells, like glioma cells, have extensive autocrine activity involving multiple growth factors and chemokines such as FGFs, PDGFs, GDNFs, HGF, LPA, Sdf1 and their cognate receptors.54–56 Such co-expressions of ligands and receptors are thought to mainly promote tumor growth. Since some of these factors have chemotactic abilities, it is therefore possible that cancer cells may also actively attract each other to maintain critical mass for survival or to promote collectiveness. However, information on collective behavior in cancer cells is so far restricted to epithelial tumors. Thus, the ideas of mesenchymal collectives and mutual attraction during cancer invasion, despite being valid hypotheses, still remain to be explored. The second idea, modification of the environment by leader cells, is in line with studies showing that specific organization of the extracellular matrix (ECM) can favor directional migration.57–60 In addition, local remodeling of the ECM by pioneering cells via metalloproteinases is thought to promote migration of the following cells.61–63 Interestingly, NC cells express several molecules with matrix remodeling abilities64–73 suggesting that the leading NC cells may modify the surrounding tissues in a way that would promote a more efficient migration of the following cells into the same region. Moreover, the modification of the local environment could also include the modulation of the availability of external guidance cues. In fact, NC cells express proteases capable of cleaving their attractant, stromal cell-derived factor 1.74,75 Therefore, one can imagine that leading cells would locally digest the attractant, progressively shaping a gradient that cells at the rear of the population would follow. In that case, leading cells would be driven by the presence of a NC-free space at the front while followers would be guided by local patterns of external cues. Alternatively, if all migratory NC cells can digest Sdf1, the attractant would only be available at the border of the NC population, therefore promoting outward migration of the leading cells. A similar situation is observed in the zebrafish lateral line where the back of the population expressed Cxcr7, a decoy receptor that traps Sdf1, while the front cells express the functional Sdf1 receptor, Cxcr4.76–80 It has been proposed that the trapping of Sdf1, by Cxcr7, on one side of the lateral line population might generate a local gradient promoting directional movement. This suggests that local changes in the attractant availability, directly controlled by the cells, could drive directional movement. That this change occurs by local trapping or degradation may not matter. Interestingly, traveling pulses of bacteria exhibit a collective behavior based on two of the mechanisms discussed above: (1) a gradient of nutrients generated by leading cells and (2) a co-attraction system that maintains bacteria close to each other.81 The external gradient is generated while the bacteria eat the nutrients, an equivalent to local degradation or trapping. Finally, the third idea, alignment between cells by forces generated at cell-cell contacts, is supported by works showing that, within epithelia, cells can align to patterns of tensions and forces occurring as a consequence of cell-cell interactions.82–85 In mesenchymal cells such forces and tensions would be transmitted by transient but repeated contacts that, over time, might carry similar positional/directional information.
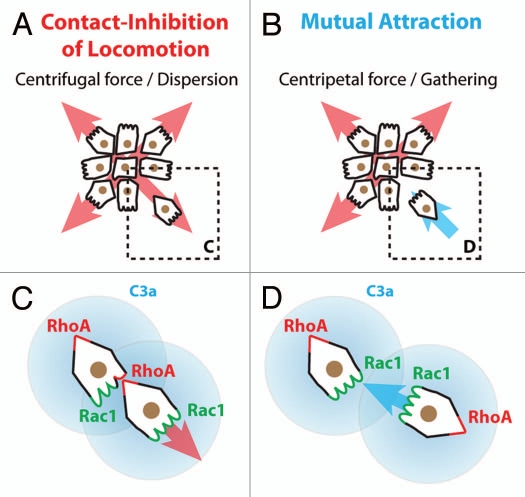
Figure 5.
Collectiveness of Xenopus cephalic neural crest cells is maintained by mutual attraction based on autocrine C3a/C3aR signaling. (A) Contact-Inhibition of Locomotion (CIL) polarizes cells toward the cell-free space and therefore acts as a centrifugal force leading to dispersion. (B) Mutual attraction driven by chemoattractant C3a acts as centripetal force promoting gathering of cells. (C) Cell-cell interactions through CIL polarize the distribution and activity of small GTPases within the cells. (D) C3a/C3aR signaling promotes Rac1 activity in cells that have recently left the group. This leads to repolarization and gathering.
Altogether, these observations in other systems suggest that cell cooperation and coordination leading to collective cell migration can emerge from positional information between cells, mediated either by chemical or physical means and combined with external guidance cues.
Conclusion
Work on epithelium-to-mesenchyme transitions has emphasized the fact that cell-cell junctions are abolished when epithelial cells adopt a mesenchymal phenotype.10 However, epithelial cells are not static and cell-cell junctions are also dynamic and remodeled.86–88 This suggests that the notion of stable or transient cell junctions is somehow relative and may simply represent different degree of turnover of the components of the junctions between epithelial and mesenchymal cells. In addition, during EMT, cells lose their adhesion with their original tissue and exhibit a loose phenotype but they do not stop expressing cell adhesion molecules. Instead, they rather switch to a different repertoire or start expressing additional cadherins at the onset of migration.4,6,9,89,90 For instance, chick trunk NC cells switch from N-Cadherin/Cadherin-6B expressions to Cadherin 7 and 11.91–93 Xenopus cephalic NC cells maintain N-Cadherin expression but additionally express Cadherin-11 while migrating.11,94 Finally, most cancer cells exhibit dramatic changes of Cadherin expression upon EMT mostly involving E, N and P-Cadherins.10,89,90,95 This indicates that, even after EMT, mesenchymal cells, including mesenchymal cancer cells, are still expressing cell surface molecules capable of mediating homotypic interactions among them. Moreover, we have shown in NC cells that N-Cadherin is playing a similar role in cell polarity and competence to respond to external cues in pseudoepithelial and mesenchymal NC cells.13 These observations suggest that mesenchymal cells do form cell-cell junctions, even tough transient ones, and that, in some cases, these junctions are required for the emergence of specific properties at the group level. Therefore, we think that collective cell migration is likely to happen in mesenchymal cells under certain conditions. And if it does, there is an exciting field of investigation ahead of us to understand how coordination and cooperation can emerge in cell collectives with no stable cell-cell interactions.
Acknowledgments
We are grateful to Helen Matthews and Mae Woods for comments and corrections on the manuscript. This investigation was supported by grants from MRC, BBSRC and Wellcome Trust to R.M.
References
- 1.Rørth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–429. doi: 10.1146/annurev.cellbio.042308.113231. [DOI] [PubMed] [Google Scholar]
- 2.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 3.Hall B. The neural crest and neural crest cells in vertebrate development and evolution. New York: Springer; 2008. [Google Scholar]
- 4.Kuriyama S, Mayor R. Molecular analysis of neural crest migration. Philos Trans R Soc Lond B Biol Sci. 2008;363:1349–1362. doi: 10.1098/rstb.2007.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Douarin N, Kalcheim C. The neural crest. Cambridge UK; New York NY, USA: Cambridge University Press; 1999. [Google Scholar]
- 6.Theveneau E, Mayor R. Collective cell migration of the cephalic neural crest: The art of integrating information. Genesis. 2011;49:164–176. doi: 10.1002/dvg.20700. [DOI] [PubMed] [Google Scholar]
- 7.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 8.Steventon B, Carmona-Fontaine C, Mayor R. Genetic network during neural crest induction: from cell specification to cell survival. Semin Cell Dev Biol. 2005;16:647–654. doi: 10.1016/j.semcdb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Duband JL. Diversity in the molecular and cellular strategies of epithelium-to-mesenchyme transitions: Insights from the neural crest. Cell Adh Migr. 2010;4:458–482. doi: 10.4161/cam.4.3.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Alfandari D, Cousin H, Marsden M. Mechanism of xenopus cranial neural crest cell migration. Cell Adh Migr. 2010;4:553–560. doi: 10.4161/cam.4.4.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadaghiani B, Thiebaud CH. Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Dev Biol. 1987;124:91–110. doi: 10.1016/0012-1606(87)90463-5. [DOI] [PubMed] [Google Scholar]
- 13.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, et al. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayor R, Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010;20:319–328. doi: 10.1016/j.tcb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, et al. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abercrombie M, Dunn GA. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp Cell Res. 1975;92:57–62. doi: 10.1016/0014-4827(75)90636-9. [DOI] [PubMed] [Google Scholar]
- 17.Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953;5:111–131. doi: 10.1016/0014-4827(53)90098-6. [DOI] [PubMed] [Google Scholar]
- 18.Theveneau E, Mayor R. Integrating chemotaxis and contact-inhibition during collective cell migration: small GTPases at work. Small GTPases. 2010;1:113–117. doi: 10.4161/sgtp.1.2.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews HK, Marchant L, Carmona-Fontaine C, Kuriyama S, Larrain J, Holt MR, et al. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–1780. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 20.Carmona-Fontaine C, Matthews H, Mayor R. Directional cell migration in vivo: Wnt at the crest. Cell Adh Migr. 2008;2:240–242. doi: 10.4161/cam.2.4.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- 22.Hörstadius SO. The neural crest; its properties and derivatives in the light of experimental research. London, New York: Oxford University Press; 1950. [Google Scholar]
- 23.Kulesa PM, Fraser SE. Neural crest cell dynamics revealed by time-lapse video microscopy of whole embryo chick explant cultures. Dev Biol. 1998;204:327–344. doi: 10.1006/dbio.1998.9082. [DOI] [PubMed] [Google Scholar]
- 24.Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development. 2000;127:1161–1172. doi: 10.1242/dev.127.6.1161. [DOI] [PubMed] [Google Scholar]
- 25.Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- 26.McLennan R, Kulesa PM. In vivo analysis reveals a critical role for neuropilin-1 in cranial neural crest cell migration in chick. Dev Biol. 2007;301:227–239. doi: 10.1016/j.ydbio.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Kulesa PM, Bailey CM, Kasemeier-Kulesa JC, McLennan R. Cranial neural crest migration: new rules for an old road. Dev Biol. 2010;344:543–554. doi: 10.1016/j.ydbio.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinney MC, Stark DA, Teddy J, Kulesa PM. Neural crest cell communication involves an exchange of cytoplasmic material through cellular bridges revealed by photoconversion of KikGR. Dev Dyn. 2011;240:1391–1401. doi: 10.1002/dvdy.22612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns AJ, Pachnis V. Development of the enteric nervous system: bringing together cells, signals and genes. Neurogastroenterol Motil. 2009;21:100–102. doi: 10.1111/j.1365-2982.2008.01255.x. [DOI] [PubMed] [Google Scholar]
- 30.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- 31.Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–541. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- 32.Epstein ML, Mikawa T, Brown AM, McFarlin DR. Mapping the origin of the avian enteric nervous system with a retroviral marker. Dev Dyn. 1994;201:236–244. doi: 10.1002/aja.1002010307. [DOI] [PubMed] [Google Scholar]
- 33.Burns AJ, Le Douarin NM. Enteric nervous system development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat Rec. 2001;262:16–28. doi: 10.1002/1097-0185(20010101)262:1<16::AID-AR1007>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Landman KA, Fernando AE, Zhang D, Newgreen DF. Building stable chains with motile agents: Insights into the morphology of enteric neural crest cell migration. J Theor Biol. 2011;276:250–268. doi: 10.1016/j.jtbi.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 35.Simpson MJ, Zhang DC, Mariani M, Landman KA, Newgreen DF. Cell proliferation drives neural crest cell invasion of the intestine. Dev Biol. 2007;302:553–568. doi: 10.1016/j.ydbio.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Landman KA, Simpson MJ, Newgreen DF. Mathematical and experimental insights into the development of the enteric nervous system and Hirschsprung's disease. Dev Growth Differ. 2007;49:277–286. doi: 10.1111/j.1440-169X.2007.00929.x. [DOI] [PubMed] [Google Scholar]
- 37.Young HM, Bergner AJ, Anderson RB, Enomoto H, Milbrandt J, Newgreen DF, et al. Dynamics of neural crest-derived cell migration in the embryonic mouse gut. Dev Biol. 2004;270:455–473. doi: 10.1016/j.ydbio.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Kelsh RN, Harris ML, Colanesi S, Erickson CA. Stripes and belly-spots—a review of pigment cell morphogenesis in vertebrates. Semin Cell Dev Biol. 2009;20:90–104. doi: 10.1016/j.semcdb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo BR, Erickson CA. Regional differences in neural crest morphogenesis. Cell Adh Migr. 2010;4:567–585. doi: 10.4161/cam.4.4.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krispin S, Nitzan E, Kassem Y, Kalcheim C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development. 2010;137:585–595. doi: 10.1242/dev.041509. [DOI] [PubMed] [Google Scholar]
- 41.Kasemeier-Kulesa JC, Kulesa PM, Lefcort F. Imaging neural crest cell dynamics during formation of dorsal root ganglia and sympathetic ganglia. Development. 2005;132:235–245. doi: 10.1242/dev.01553. [DOI] [PubMed] [Google Scholar]
- 42.Rovasio RA, Delouvee A, Yamada KM, Timpl R, Thiery JP. Neural crest cell migration: requirements for exogenous fibronectin and high cell density. J Cell Biol. 1983;96:462–473. doi: 10.1083/jcb.96.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas LA, Yamada KM. Contact stimulation of cell migration. J Cell Sci. 1992;103:1211–1214. doi: 10.1242/jcs.103.4.1211. [DOI] [PubMed] [Google Scholar]
- 44.Rios AC, Serralbo O, Salgado D, Marcelle C. Neural crest regulates myogenesis through the transient activation of NOTCH. Nature. 2011;473:532–535. doi: 10.1038/nature09970. [DOI] [PubMed] [Google Scholar]
- 45.Erickson CA. Control of neural crest cell dispersion in the trunk of the avian embryo. Dev Biol. 1985;111:138–157. doi: 10.1016/0012-1606(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 46.Xu X, Francis R, Wei CJ, Linask KL, Lo CW. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006;133:3629–3639. doi: 10.1242/dev.02543. [DOI] [PubMed] [Google Scholar]
- 47.Anderson RB, Newgreen DF, Young HM. Neural crest and the development of the enteric nervous system. Adv Exp Med Biol. 2006;589:181–196. doi: 10.1007/978-0-387-46954-6_11. [DOI] [PubMed] [Google Scholar]
- 48.King JS, Insall RH. Chemotaxis: finding the way forward with Dictyostelium. Trends Cell Biol. 2009;19:523–530. doi: 10.1016/j.tcb.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Zusman DR, Scott AE, Yang Z, Kirby JR. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat Rev Microbiol. 2007;5:862–872. doi: 10.1038/nrmicro1770. [DOI] [PubMed] [Google Scholar]
- 50.Carmona-Fontaine C, Theveneau E, Tzekou A, Woods M, Page K, Tada M, et al. Complement fragment C3a controls mutual cell attraction during collective cell migration. Dev Cell. 2011 doi: 10.1016/j.devcel.2011.10.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollet N, Muncke N, Verbeek B, Li Y, Fenger U, Delius H, et al. An atlas of differential gene expression during early Xenopus embryogenesis. Mech Dev. 2005;122:365–439. doi: 10.1016/j.mod.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costa RM, Soto X, Chen Y, Zorn AM, Amaya E. spib is required for primitive myeloid development in Xenopus. Blood. 2008;112:2287–2296. doi: 10.1182/blood-2008-04-150268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011;437:199–213. doi: 10.1042/BJ20101603. [DOI] [PubMed] [Google Scholar]
- 55.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, et al. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- 56.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 57.Nagel M, Winklbauer R. Establishment of substratum polarity in the blastocoel roof of the Xenopus embryo. Development. 1999;126:1975–1984. doi: 10.1242/dev.126.9.1975. [DOI] [PubMed] [Google Scholar]
- 58.Winklbauer R, Nagel M, Selchow A, Wacker S. Mesoderm migration in the Xenopus gastrula. Int J Dev Biol. 1996;40:305–311. [PubMed] [Google Scholar]
- 59.Gunzer M, Friedl P, Niggemann B, Brocker EB, Kampgen E, Zanker KS. Migration of dendritic cells within 3-D collagen lattices is dependent on tissue origin, state of maturation and matrix structure and is maintained by proinflammatory cytokines. J Leukoc Biol. 2000;67:622–629. doi: 10.1002/jlb.67.5.622. [DOI] [PubMed] [Google Scholar]
- 60.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 62.Ilina O, Bakker GJ, Vasaturo A, Hofmann RM, Friedl P. Two-photon laser-generated microtracks in 3D collagen lattices: principles of MMP-dependent and -independent collective cancer cell invasion. Phys Biol. 2011;8:15010. doi: 10.1088/1478-3975/8/1/015010. [DOI] [PubMed] [Google Scholar]
- 63.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 64.Cai DH, Vollberg TM, Sr, Hahn-Dantona E, Quigley JP, Brauer PR. MMP-2 expression during early avian cardiac and neural crest morphogenesis. Anat Rec. 2000;259:168–179. doi: 10.1002/(SICI)1097-0185(20000601)259:2<168::AID-AR7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 65.Giambernardi TA, Sakaguchi AY, Gluhak J, Pavlin D, Troyer DA, Das G, et al. Neutrophil collagenase (MMP-8) is expressed during early development in neural crest cells as well as in adult melanoma cells. Matrix Biol. 2001;20:577–587. doi: 10.1016/S0945-053X(01)00166-4. [DOI] [PubMed] [Google Scholar]
- 66.Cantemir V, Cai DH, Reedy MV, Brauer PR. Tissue inhibitor of metalloproteinase-2 (TIMP-2) expression during cardiac neural crest cell migration and its role in proMMP-2 activation. Dev Dyn. 2004;231:709–719. doi: 10.1002/dvdy.20171. [DOI] [PubMed] [Google Scholar]
- 67.Duong TD, Erickson CA. MMP-2 plays an essential role in producing epithelial-mesenchymal transformations in the avian embryo. Dev Dyn. 2004;229:42–53. doi: 10.1002/dvdy.10465. [DOI] [PubMed] [Google Scholar]
- 68.Anderson RB. Matrix metalloproteinase-2 is involved in the migration and network formation of enteric neural crest-derived cells. Int J Dev Biol. 2010;54:63–69. doi: 10.1387/ijdb.082667ra. [DOI] [PubMed] [Google Scholar]
- 69.Alfandari D, Cousin H, Gaultier A, Smith K, White JM, Darribere T, et al. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr Biol. 2001;11:918–930. doi: 10.1016/S0960-9822(01)00263-9. [DOI] [PubMed] [Google Scholar]
- 70.Alfandari D, Wolfsberg TG, White JM, DeSimone DW. ADAM 13: a novel ADAM expressed in somitic mesoderm and neural crest cells during Xenopus laevis development. Dev Biol. 1997;182:314–330. doi: 10.1006/dbio.1996.8458. [DOI] [PubMed] [Google Scholar]
- 71.Hall RJ, Erickson CA. ADAM 10: an active metalloprotease expressed during avian epithelial morphogenesis. Dev Biol. 2003;256:147–160. doi: 10.1016/S0012-1606(02)00133-1. [DOI] [PubMed] [Google Scholar]
- 72.Neuner R, Cousin H, McCusker C, Coyne M, Alfandari D. Xenopus ADAM19 is involved in neural, neural crest and muscle development. Mech Dev. 2009;126:240–255. doi: 10.1016/j.mod.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silver DL, Hou L, Somerville R, Young ME, Apte SS, Pavan WJ. The secreted metalloprotease ADAMTS20 is required for melanoblast survival. PLoS Genet. 2008;4:1000003. doi: 10.1371/journal.pgen.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 75.Rodríguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 76.Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell. 2006;10:673–680. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 77.Valentin G, Haas P, Gilmour D. The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr Biol. 2007;17:1026–1031. doi: 10.1016/j.cub.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 78.Dambly-Chaudière C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol. 2007;7:23. doi: 10.1186/1471-213X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, et al. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 80.Naumann U, Cameroni E, Pruenster M, Mahabaleshwar H, Raz E, Zerwes HG, et al. CXCR7 functions as a scavenger for CXCL12 and CXCL11. PLoS ONE. 2010;5:9175. doi: 10.1371/journal.pone.0009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saragosti J, Calvez V, Bournaveas N, Buguin A, Silberzan P, Perthame B. Mathematical description of bacterial traveling pulses. PLOS Comput Biol. 2010;6:1000890. doi: 10.1371/journal.pcbi.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petitjean L, Reffay M, Grasland-Mongrain E, Poujade M, Ladoux B, Buguin A, et al. Velocity fields in a collectively migrating epithelium. Biophys J. 2010;98:1790–1800. doi: 10.1016/j.bpj.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mege RM, et al. Traction forces exerted through N-cadherin contacts. Biol Cell. 2006;98:721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- 84.Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, et al. Physical forces during collective cell migration. Nat Phys. 2009;5:426–430. doi: 10.1038/nphys1269. [DOI] [Google Scholar]
- 86.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance and remodeling. J Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol. 2009;1:2998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–756. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- 89.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann NY Acad Sci. 2004;1014:155–163. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 90.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 91.Chalpe AJ, Prasad M, Henke AJ, Paulson AF. Regulation of cadherin expression in the chicken neural crest by the Wnt/beta-catenin signaling pathway. Cell Adh Migr. 2010;4:431–438. doi: 10.4161/cam.4.3.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- 93.Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- 94.Vallin J, Girault JM, Thiery JP, Broders F. Xenopus cadherin-11 is expressed in different populations of migrating neural crest cells. Mech Dev. 1998;75:171–174. doi: 10.1016/S0925-4773(98)00099-9. [DOI] [PubMed] [Google Scholar]
- 95.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]