Abstract
Our previous data indicate that M3 muscarinic receptors mediate carbachol induced bladder contractions. The data presented here were obtained by selective alkylation of M3 receptors with 4-DAMP mustard and suggest that the M2 receptor subtype may be involved in inhibition of β-adrenergic receptor induced relaxation, therefore, allowing recontraction. Alkylation resulted in 85% of M3 receptors and 65% of M2 receptors unable to bind radioligand as demonstrated by subtype selective immunoprecipitation. Rat bladder strips subjected to our alkylation procedure contracted submaximally, and direct carbachol contractions were inhibited by antagonists with affinities consistent with M3 receptor mediated contraction. In contrast, the affinities of antagonists for inhibition of carbachol induced recontractions following isoproterenol stimulated relaxation in the presence of 90 mM KCl, indicated a contractile function for the M2 receptor that was not observed in control strips. In conclusion, these studies demonstrate a possible role for the M2 subtype in bladder smooth muscle contraction.
INTRODUCTION
Radioligand binding data suggests that M2 muscarinic receptors comprise the major population of receptors in the rat urinary bladder (1). However, affinity values (PA2) derived from Schild plot analysis of a series of muscarinic antagonists for the inhibition of carbachol induced contractions of the rat urinary bladder are consistent with M2 receptors mediating this response (2, 3, 4). We have previously shown by subtype selective immunoprecipitation that the majority of the muscarinic receptors in the rat urinary bladder are of the M2 subtype, while the remaining receptors identifiable by this means are of the M3 subtype (4). The presence of prejunctional M1 facilitory and M2 inhibitory receptors on parasympathetic nerves innervating the rat bladder has been demonstrated both by acetylcholine release (5,6,7) and muscle contraction studies (2,3). Other data obtained by selective alkylation of M3 receptors with 4-DAMP mustard suggests that M2 receptors may be involved in blocking the increase in cAMP, and hence relaxation, induced by isoproterenol activation of β-adrenergic receptors (8,9) in the guinea pig ileum (10), trachea (11) and the rat esophageal muscularis mucosae (12). However in the guinea pig esophagus, rat stomach fundus (11) and guinea pig esophageal muscularis mucosae (13) no significant contractile function for the M2 receptor was observed using the same paradigm. Rat bladder smooth muscle contracts after depolarization with 90 mM KCl, and this can be reversed by isoproterenol through stimulation of β-adrenergic receptors. β-adrenergic receptors couple through Gs to stimulate adenylyl cyclase and raise the level of intracellular cAMP. Smooth muscle can be induced to contract in response to carbachol subsequent to isoproterenol induced relaxation. We will refer to these contractions after exposure to KCl and isoproterenol treatment as “recontractions” as used previously (12). Using a methodology similar to that previously used by other investigators (8,12,13), we alkylated bladder muscarinic receptors with 4-DAMP mustard in the presence of methoctramine in order to protect M2 subtypes and thus selectively inactivate M3 receptors. We protected bladder muscarinic receptors with 30 nM methoctramine (10 fold lower than used by other investigators) in order to maximize M3 receptor inactivation while protecting a substantial portion of the M2 receptor subtypes. In contrast to previous studies where no direct measure of receptor subtype inactivation was performed, this is the first report in which the success of the alkylation procedure was verified by subtype selective immunoprecipitation of [3H] QNB labeled receptors.
We compared the affinity of three competitive subtype selective muscarinic receptor antagonists to inhibit carbachol induced contractions of normal and alkylated bladder strips using two different contractile paradigms. The first consisted of measuring contractions due to carbachol, similar to the method we used previously to show that the M3 subtype mediates bladder contractility (4). In the second paradigm which we call recontractions, the muscle :strips were first contracted by depolarization with 90 mM KCl then relaxed by isoproterenol. Once a new stable baseline was achieved, the strips were recontracted using carbachol. Use of these two different contractile paradigms on both time control and alkylated rat bladder strips allowed us to separate and determine the contractile function of the M2 and M3 receptor subtypes in the rat urinary bladder.
METHODS
Materials
Frozen normal rat bladders were purchased from Pel Freeze Biologicals (Rogers, AR). The following drugs or chemicals were obtained from the sources indicated: carbachol, sodium cholate, protease inhibitors, atropine (Sigma Chemical Company, St. Louis, Mo), methoctramine, 4-DAMP, 4-DAMP mustard, and p-F- HHSiD (Research Biochemicals International, Nantick, MA), [3H]QNB (43 Ci/mM Dupont-New England Nuclear Research Products, Wilmington, DE), pansorbin (Calbiochem Inc., La Jolla. CA), digitonin (Gallard-Schlesinger Industries Inc., Carle Place, N Y ).
Muscle Strips
Urinary bladders were removed from rats euthanized by decapitation. The urinary bladder body (tissue above the ureteral orifices) was dissected free of the serosa and surrounding fat. The bladder was divided in the mid-sagittal plane, then cut into longitudinal smooth muscle strips (approximately 4 mm × 10 mm). The muscle strips were then suspended with 1 g of isometric tension in tissue baths containing 15 ml of modified Tyrodes solution (125 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 1.8 mM CaCl2, 0.5 mM MgCl2, 23.8 mM NaHCO3, and 5.6 mM glucose) and equilibrated with 95/5% O2/CO2, at 37 °C. The strips were tested for their ability to contract in response to electric field stimulation of 8 volts, 30 Hz, 1 ms duration.
Carbachol Concentration-Effect
Following equilibration to the bath solution for 30 minutes, bladder strips were incubated for 30 minutes in the presence or absence of antagonist. For the recontraction paradigm, after equilibration with antagonist, KCl was added to a final concentration of 90 mM followed by the addition of isoproterenol to a final concentration of 30 μM. After a stable baseline was achieved, contractions were induced by the cumulative addition of carbachol. Concentration-effect curves were derived from the peak tension developed following cumulative addition of carbachol (10 nM to 300 μM final bath concentration).
4-DAMP Mustard Studies
Based on the reported affinity of methoctramine for the M2 and M3 receptor subtypes (7.8–8.3 for M2, 6.3–6.9 for M3, 15) and on the decrease in [3H]-NMS binding seen in tissue homogenates of submaxillary gland (predominately M3 receptors) and heart (predominately M2 receptors) reported by Thomas et. al. (14), we chose to protect muscarinic receptors with 30 nM methoctramine. This concentration of methoctramine is lower than that used by other investigators performing this type of study (8–11) and was chosen to result in maximal M3 receptor inactivation. The reported affinity of 4-DAMP for the M2 and M3 subtypes is 8.0–8.4 and 8.9–9.3 respectively (15). For these experiments, bladder strips were incubated for 20 min at 37 °C in the presence of 30 nM methoctramine. In the continued presence of 30 nM methoctramine, 40 nM 4-DAMP mustard (dissolved in distilled water at 4 μM) was added and incubated for an additional 60 min at 37 °C. Following alkylation, the strips were washed 6 times for 10 min each with modified Tyrodes containing 30 nM methoctramine, then 9 times for 10 minutes each with modified Tyrodes solution. Concentration response curves were derived from the peak tension developed following cumulative addition of carbachol (10 nM to 300 μM final bath concentration). Three concentrations of each antagonist was used for each Schild plot and each concentration was tested on 5–6 strips. For comparison, time control experiments were performed without exposure to 4-DAMP mustard. Dose ratios were determined based on the average (of 5–6 antagonist free strips. An EC50 value for each strip was determined from an nonlinear least squares sigmoidal curve fit of the data (Origin, MicroCal Software, Inc., Northampton, Mass). The EC50 values determined in the presence of antagonist were used to generate Schild plots in order to calculate pA2 values for each antagonist. If the slope of the Schild plot was not significantly different from unity, the slope of the Schild plot was constrained to unity in order to calculate the pKb value.
Inmunoprecipitations
A two step solubilization procedure for imrnunoprecipitation (16) and antibodies (4) were used as previously described. Muscarinic receptor density is reported as fmoles/mg protein in the solubilized receptor preparations. Control precipitations were performed 3 times in triplicate on pooled normal bladders (8–10 bladders per experiment). For time control and mustard treated tissue precipitations, 24 muscle strips were pooled and used in one experiment performed in quadruplicate. There was no difference between time control strips and normal bladders.
Statistical and Data Analysis
Results are reported as means ± SEM. The contractility data curves were generated by a curve fitting program (Origin, MicroCal Software, Inc., Northampton, Mass) based on a sigmoidal fit of the data for the concentration-response curves and a linear fit for the Schild plots. Statistical analysis of multiple group comparison was performed by analysis of variance (ANOVA) with a post hoc Scheffé test (GB-STAT, Dynamic Microsystems, Silver Spring, MD) or Student’s t test where appropriate. Statistically significant differences in the affinity values and departure from unity in the slopes derived from the Schild plots were determined using 95% confidence intervals.
RESULTS
Antagonist Affinities
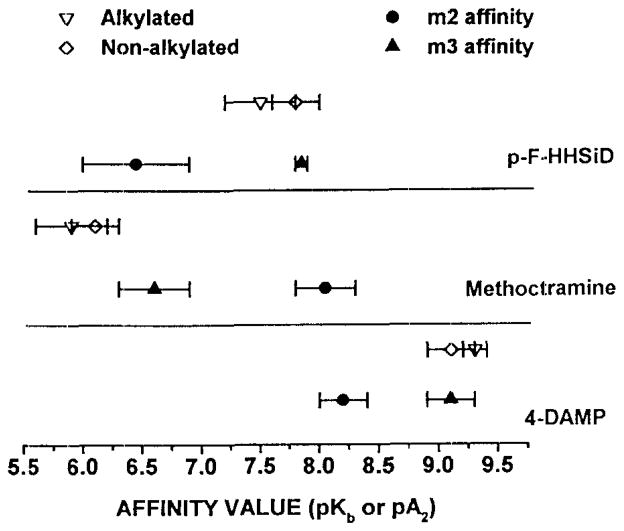
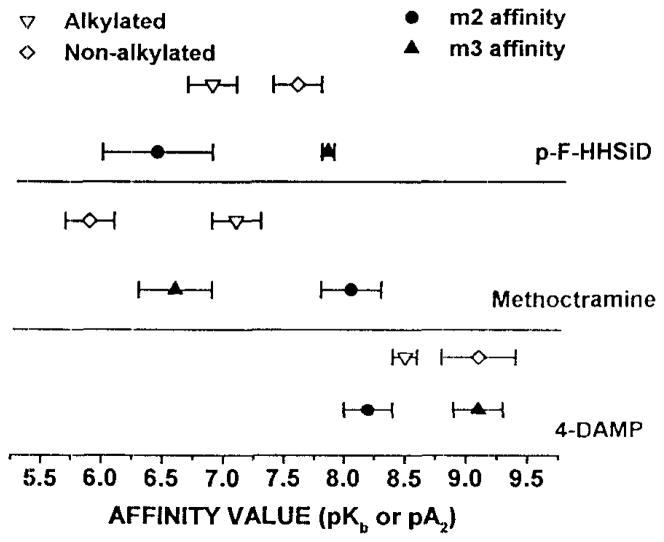
As summarized in table 1, we measured the affinity of 4-DAMP, p-F-HHSiD and rnethoctramine for inhibition of (a) carbachol induced recontractions of bladder strips not exposed to 4-DAMP mustard, (b) carbachol induced contractions on alkylated bladder strips, and (c) carbachol induced recontractions on alkylated bladder strips. The carbachol EC50 for both contractions and recontractions increased 2.7-fold following alkylation (Table 1). Analysis of antagonist affinities is consistent with M3 receptor mediated direct contractions for both alkylated and nonalkylated muscle strips (Figure 1). Similarly, recontractions performed on unalkylated tissue were also mediated by M3 receptors. However antagonist affinities derived using the recontraction paradigm following alkylation are consistent with an M2 receptor participation (Figures 2, 3).
TABLE 1.
Antagonist Affinity, Carbachol EC50, and Subtype Implicated for Mediating Contractions and Recontractions of Time Control and 4-DAMP Mustard Treated Rat Bladder Strips in-vitro.
| Control Contractions | Control Recontractions | Alkylated Contractions | Alkylated Recontractions | |
|---|---|---|---|---|
| methoctramine | 6.1±0.2a | 5.9±0.2 | 5.9±0.3 | 7.1±0.2d |
| Schild Slope | 0.86±0.1a | 0.98±.1 | 0.82±0.1 | 0.6±0.1b |
| p-F-HHSid | 7.5±0.2 | 7.3±0.2 | 7.5±0.3 | 6.9±0.2 |
| Schild Slope | 1.07±0.1 | 0.92±0.1 | 0.93±0.2 | 0.75±0.3 |
| 4-DAMP | 9.1±0.2 | 9.1±0.3 | 9.3±0.1 | 8.5±0.1 |
| Schild Slope | 0.93±0.1 | 1.03±0.2 | 1.23±0.2 | 0.99±0.2 |
| Carb EC50 (μM) | 1.3±0.3 | 1.9±0.3 | 3.2±0.1c | 5.1±0.3d |
|
| ||||
| Subtype Implicated | M3 | M3 | M3 | M2, M3 |
from (17),
slope significantly different from unity.
Denotes significant difference (p<0.05) from control contraction (c) or control recontraction (d).
Figure 1. Affinity of Anticholinergics for Inhibition of Contraction of Control and Alkylated Rat Bladder Strips in-vitro.
The closed symbols with error bars represent reported affinity ranges for the individual subtypes (15). The open symbols (±S.E.M.) represent the affinities derived from control and alkylated rat bladder strips.
Figure 2. Affinity of Anticholinergics for Inhibition of Contraction of Control and Alkylated Rat Bladder Strips in-vitro in the presence of 90 mM KCl and 30 μM Isoproterenol.
The closed symbols with error bars represent reported affinity ranges for the individual subtypes (15). The open symbols (±S.E M.) represent the affinities derived from control and alkylated rat bladder strips
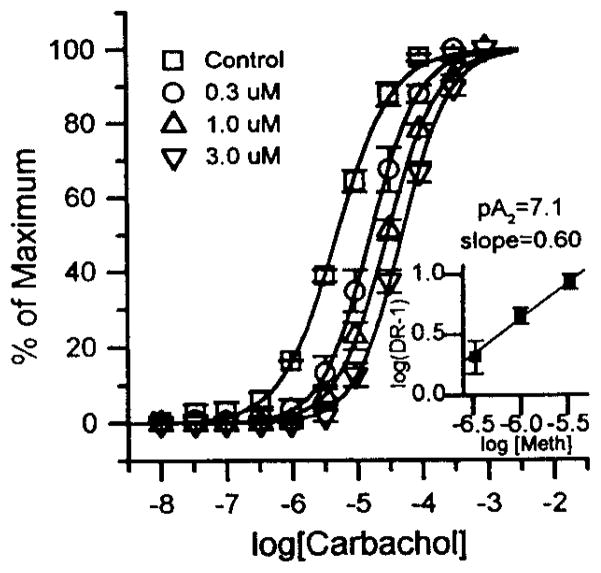
Figure 3. Carbachol concentration-response displacement curves and Schild plot (inserts) for methoctramine’s effect on recontractions of rat bladder strips in which muscarinic receptors were alkylated by 40 nM 4-DAMP mustard in the presence of 30 nM methoctramine in-vitro.
The concentration of methoctramine used is shown in the legend. Each curve represents the average responses of 5–6 muscle strip preparations expressed as the percent of each individual strip’s maximal carbachol response. These maximal responses (average ± S.E.M.) in grams of tension were 2.1 ± 0.18 for control (square); 2.1 ± 0.36, 2.1 ± 0.36 and 2.2 ± 0.38 for 0.3 (circle), 1 (triangle), and 3 μM (upside down triangle) methoctramine respectively. The carbachol EC50 for the antagonist-free strips was 5.1 ± 0.34 μM. The slope of the Schild plot was significantly less than unity.
Immunoprecipitation
To confirm the efficiency of alkylation, both the 4-DAMP mustard treated and the time control bladder strips were collected and the densities of total muscarinic receptors and of the M2 and M3 subtypes were determined by subtype selective immunoprecipitation (Figure 4). Total receptor and M2 receptor density in the soluble membrane fraction in both normal and time control strips were approximately 3-fold higher than in the 4-DAMP mustard treated bladder strips. The density of M3 receptors in control strips was approximately 6-fold higher than in the 4-DAMP mustard treated strips. The actual density of M3 receptors in the 4-DAMP mustard treated strips was 12.8 fmoles/mg protein, near the limit of detectability. This concentration of receptor corresponds to 107 ± 6 cpm in the immunoprecipitation assay, while background obtained using normal rabbit serum instead of a subtype specific antibody in the precipitations was 44 ± 4 cpm. As a result of the selective alkylation, the M2:M3 ratio was higher in the mustard treated bladder strips (11.0) than in control strips (5.8). The sum of M2 and M3 receptors precipitated accounted for 86% and 83% of the total receptors solubilized from control and mustard treated strips, respectively.
Figure 4. Precipitation of M2 and M3 Muscarinic Receptor Subtypes from Normal Control, Time Control, and 4-DAMP Mustard Treated Rat Bladder Strips.
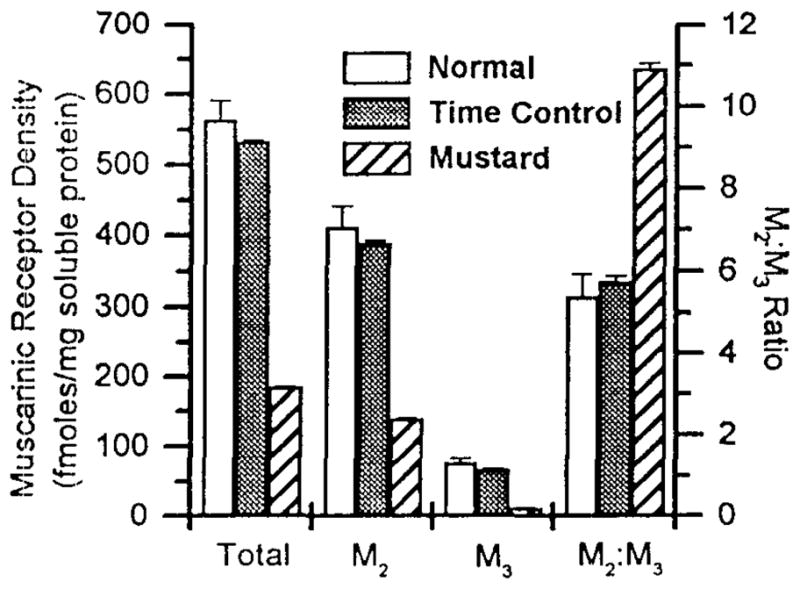
Receptors were labeled with [3H] QNB and solubilized as described in the text. Data shown are average fmoles of receptor/mg solubilized protein ±S.E.M. or the ratio of M2:M3 receptors from pooled normal control (clear), time control (shaded) and 4-DAMP mustard treated (striped) bladders Approximately 1 g of tissue per assay was used, n= 3 performed in triplicate for normal control, n= 1 performed in quadruplicate for time control and n= 1 performed in quadruplicate for 4-DAMP mustard treated bladders.
DISCUSSION
The density of M2 receptors is approximately 6 times higher than the density of M3 receptors in the rat urinary bladder. Nevertheless, pharmacologic evidence, based on the affinity of a panel of subtype selective muscarinic antagonists is most consistent with the M3 muscarinic receptor subtype mediating smooth muscle contraction (3, 4, 9). An M3 mediated contractile response was seen for direct contractions both before (4) and after alkylation. Similarly, recontractions prior to selective alkylation were also mediated by the M3 receptor subtype (Table 1).
A contractile function for the M2 receptor was only seen using the recontraction paradigm following selective alkylation of M3 receptors by 4-DAMP mustard treatment (Table 1, Figure 2). The affinity of methoctramine for inhibition of recontractions increased greater than 10-fold after alkylation while the affinity of both 4-DAMP and p-F-HHSiD decreased (Table 1). The affinity of methoctramine for inhibition of recontractions following alkylation (PA2 of 7.1, Figure 3) is intermediate between the reported affinity of methoctramine for the M2 and M3 receptor subtypes (15), while the affinity of both 4-DAMP and p-F-HHSiD is consistent with M2 mediated recontractions following selective alkylation. As discussed below, the recontraction following alkylation, therefore, could be the net result of the remaining M3 receptors inducing a direct contraction combined with M2 receptors counteracting the relaxant effect of isoproterenol. Consistent with this interpretation is the finding that the slope of the methoctramine Schild plot for recontractions following alkylation is significantly less than unity. The reason that the Schild plot for methoctramine has a slope of less than one while the slopes of the Schild plots for 4-DAMP and p-F-HHSiD are not less than one is not clear. These results are in general agreement with those of Hegde et al. (9) in which 300 nM methoctramine was used to protect against alkylation by 4-DAMP mustard, a concentration which would protect a larger percentage of M3 receptors than the 30 nM methoctramine used in this study. Consistent with inactivation of more M3 receptors, we found that the affinity of methoctramine for inhibition of recontractions following 4-DAMP mustard treatment was higher: pA2=7.1 vs. 6.8 reported by Hegde et al. (9).
Unlike other reports (8,9, 10), we determined the proportion of the M2 and M3 receptor subtypes unable to bind ligand and therefore, likely to be alkylated and consequently inactivated. The alkylation procedure resulted in inactivation of 85% of M3 and 65% of M2 receptors as demonstrated by receptor subtype selective immunoprecipitation (Figure 4). Because the population of M2 receptors is much higher than that of M3 receptors in the bladder, this still leaves 140 fmoles/mg solubilized protein of M2 receptors. Using our alkylating procedure, a role for the remaining M2 receptors can thus be established as contributing to bladder recontractions. However, even after alkylation, in the absence of increased intracellular cAMP, an M2 receptor contribution to contraction was not detected. Based on these results, it appears that a combination of M2 and M3 receptors can act to mediate recontraction in the face of increased intracellular cAMP following M3 receptor inactivation. After the 4-DAMP mustard treatment, which inactivated 85% of M3 receptors, the magnitude of contractions was only reduced by 25%. This result, combined with the antagonist affinity data that M3 receptors, mediate contractions following alkylation, indicate that M3 receptors have an overriding ability to mediate contraction. These findings are consistent with a functional reserve of M3 receptors for contraction and this conclusion is only possible based on our ability to directly measure the percentage of M3 receptors inactivated by the 4-DAMP mustard treatment in contrast to previous investigations (9–11,13,14).
The above findings are consistent with the hypothesis that M2 receptors function in smooth muscle to oppose the relaxing effect of β-adrenergic receptor stimulation (10, 12). However, it remains to be shown whether the above paradigm is physiologically relevant. A recent study by Hegde et. al. (9) supports the physiological relevance of this function for M2 receptors. In urethane-anaesthetized rats the rank order of potency of i.v. injected muscarinic antagonists for inhibiting volume-induced bladder contractions correlated most favorably with M2 receptor involvement. However, the location of the receptors effected by antagonists administered in-vivo is not clear. It is possible that these antagonists influence volume-induced bladder contractions at the neuronal level and not by acting directly on the smooth muscle of the bladder. One key question that remains to be answered in order to provide more convincing evidence in support of the proposed model is whether β-receptor and M2 receptors are located on the same smooth muscle cells. If they are on the same cells. they could function to modulate, in opposing ways, the intracellular level of cAMP and hence muscle tone. The localization of these receptors by single cell techniques awaits further study.
M2 receptors appear to participate directly in mediating smooth muscle contraction in denervated rat bladders (17). The situation here, with acute inactivation of M3 receptors is apparently different. Even though the M2:M3 ratio is increased by selective alkylation as is the case following denervation, carbachol induced contractions continue to be inhibited by muscarinic antagonists with an affinity consistent with M3 receptor mediated contraction. One possible interpretation is that the absolute density of M2 receptors, which was decreased by the alkylation procedure but was increased following denervation, and not the M2:M3 ratio is the key factor in determining whether direct contractions are mediated by the M2 or the M3 receptor. However, other differences resulting from adaptation or remodeling of the bladder induced by either hypertrophy or the increased mechanical stretch imposed on the denervated bladders may not allow for straight forward comparison of the results from these two paradigms.
The mechanism by which the M2 receptor participates in contraction appears to be different in the denervated versus alkylated bladder muscle. In the denervated bladder the M2 receptor can be shown to mediate direct carbachol induced contractions in the absence of exogenous β-adrenergic stimulation (17), while in the normal bladder this subtype can only be shown to participate in contraction when the majority of the M3 receptor population is inactivated and following β-adrenergic induced relaxation. These differences suggest that the M2 receptor can induce contraction in smooth muscle by two different mechanisms. In the normal bladder in vivo, the M2 receptor may contribute to initiation of a micturition contraction by inhibiting the increased cAMP (by coupling to Gi) associated with the dominant β-adrenergic tone during bladder filling. However in the denervated bladder, the M2 receptor may mediate contraction by a different mechanism, possibly by inducing phosphoinositide (PI) hydrolysis through activation of phospholipase C by coupling to a member of the Gq family of guanine nucleotide binding regulatory proteins (4).
Other investigators (11) have used the shift in agonist concentration-effect curves as a basis for estimating the percent of receptors alkylated. This is likely to lead to an underestimation of the actual percentage of receptors inactivated in a tissue such as the rat urinary bladder with a functional reserve of M3 receptors mediating contraction, as suggested by our results. This method would also underestimate the percentage of receptors inactivated if receptor inactivation decreases the maximum response or if the response is the due to activation of more than one receptor subtype. Our treatment protocol resulted in alkylation of approximately 65% of M2 and 85% of M3 receptors (Figure 4) as opposed to the predicted values of 50% M2 and 95% of M3 receptors. Even though our predicted values were similar to the actual percentage of M2 and M3 receptors inactivated, the precipitation results confirm the need to directly measure the fraction of receptor subtypes alkylated in order to draw firm conclusions based on pharmacological analysis of tissue treated in this manner.
In conclusion, we demonstrate that our selective alkylation procedure resulted in an 85% reduction of M3 receptor and a 65% reduction in M2 receptors. Rat bladder strips subjected to this procedure, contracted submaximally, and were inhibited by subtype selective antagonists with affinities consistent with M3 receptor mediated contraction. In contrast, in an environment of increased cAMP, a contractile function for the M2 receptor was evident.
Acknowledgments
This study was supported by PHS grant # RO1 DK43333 from the National Institute of Diabetes, Digestive and Kidney Diseases of the National Institutes of Health.
References
- 1.Monferini E, Firaldo E, Ladinski J. Characterization of muscarinic receptor subtypes in the rat urinary bladder. Eur J Pharmacol. 1988;147:453. doi: 10.1016/0014-2999(88)90180-x. [DOI] [PubMed] [Google Scholar]
- 2.Braverman AS, Kohn IJ, Luthin GR, Ruggieri MR. Prejunctional M1 facilitory and M2 inhibitory muscarinic receptors mediate rat bladder contractility. Am J Physiol. 1998;274:R51. doi: 10.1152/ajpregu.1998.274.2.r517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohn IJ, Braverman AS, Ruggieri MR, Hanno PM. Pharmacological, molecular, and functional classification of muscarinic receptor subtypes in the rat urinary bladder. Surgical Forum. 1995;46:762. [Google Scholar]
- 4.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995;273:959. [PMC free article] [PubMed] [Google Scholar]
- 5.D’Agostine G, Kilbinger H, Chiari MC, Garna E. Presynaptic inhibitory muscarinic receptors modulating [3H] acetylcholine release in the rat urinary bladder. J Pharmacol Exp Ther. 1986;239:522. [PubMed] [Google Scholar]
- 6.Somogyi GT, de Groat WC. Evidence for inhibitory nicotinic and facilitatory muscarinic receptors in cholinergic nerve terminals of the rat urinary bladder. J Aut Nerv Sys. 1992;37:89. doi: 10.1016/0165-1838(92)90237-b. [DOI] [PubMed] [Google Scholar]
- 7.Somogyi GT, Tanowitz M, de Groat WC. M1 muscarinic receptor- mediated facilitation of acetylcholine release in the rat urinary bladder. J Physiol. 1994;480:81. doi: 10.1113/jphysiol.1994.sp020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eglen RM, Peelle B, Pulido-Rios MT, Leung E. Functional interactions between muscarinic M2 receptors and 5-hydroxytryptamine (5-HT)4 receptors and beta 3-adrenoceptors in isolated oesophageal muscularis mucosae of the rat. Brit J Pharm. 1996;119:595. doi: 10.1111/j.1476-5381.1996.tb15714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Brit J Pharm. 1997;120:1409. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlert FJ, Thomas EA. Functional role of M2 muscarinic receptors in the guinea pig ileum. Life Sciences. 1995;56:965. doi: 10.1016/0024-3205(94)00035-q. [DOI] [PubMed] [Google Scholar]
- 11.Thomas EA, Ehlert FJ. Involvement of the M2 muscarinic receptor in contractions of the guinea pig trachea, guinea pig esophagus, and rat fundus. Biochem Pharmacol. 1996;51:779. doi: 10.1016/0006-2952(95)02396-8. [DOI] [PubMed] [Google Scholar]
- 12.Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharm Rev. 1996;48:531. [PubMed] [Google Scholar]
- 13.Watson N, Reddy H, Eglen RM. Characterization of muscarinic receptor and beta-adrenoceptor interactions in guinea-pig oesophageal muscularis mucosae. Eur J Pharmacol. 1995;294:779. doi: 10.1016/0014-2999(95)00656-7. [DOI] [PubMed] [Google Scholar]
- 14.Thomas EA, Hsu HH, Griffin MT, Hunter AL, Luong T, Ehlert FJ. Conversion of N-(2-chloroethyl)-4-piperidinyl diphenylacetate (4-DAMP mustard) to an aziridinium ion and its interaction with muscarinic receptors in various tissues. Mol Pharm. 1992;41:718. [PubMed] [Google Scholar]
- 15.Caulfield MP. Muscarinic receptors-characterization, coupling and function. Pharmac Ther. 1993;58:319. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 16.Luthin GR, Harkness J, Artymyshyn RP, Wolfe BB. Antibodies to a synthetic peptide can be used to distinguish between muscarinic acetylcholine receptor binding sites in brain and heart. Mol Pharm. 1988;34:327. [PubMed] [Google Scholar]
- 17.Braverman AS, Luthin GR, Ruggieri MR. M2 muscarinic receptor contributes to contraction of the denervated rat urinary bladder. Am J Physiol. 1998;275:R1654. doi: 10.1152/ajpregu.1998.275.5.R1654. [DOI] [PMC free article] [PubMed] [Google Scholar]