Abstract
Activation of muscarinic acetylcholine receptors is primarily responsible for urinary bladder emptying. Because multiple subtypes of muscarinic receptors exist, we wished to characterize those present in bladder and ultimately to attribute function to those that regulate bladder contractility, neurotransmitter release and perhaps other cholinergic functions in this tissue. Although the m2 and m3 subtypes could be immunoprecipitated after solubilization from human, rat, rabbit and guinea pig bladder membranes, the m1, m4 and m5 subtypes could not. The m2:m3 ratio was 9:1 in rat bladder but was only 3:1 in the other species examined. Immunoprecipitation of the m2 subtype correlated with the relative levels of high-affinity agonist binding sites measured by competition of carbachol for [3H]N-methylscopolamine binding or measured directly using [3H]oxotremorine-M. In the presence of agonist, but not antagonist, GTP binding proteins could be immunoprecipitated in concert with the m2 or m3 receptors using anti-receptor antibodies. These proteins were members of the Gi and Gq/11 subfamilies for both the m2 and the m3 receptor subtypes. In spite of the preponderance of the m2 receptor in all species studied, Schild analysis using somewhat selective antagonists showed that the pharmacologically defined m3 receptor mediated contractility in strips of rat and rabbit bladder. Thus acetylcholine activates bladder smooth muscle via the m3 receptor subtype, and subsequent contractility may be transduced by guanine nucleotide binding proteins such as the Gi and Gq/11 subfamilies.
Acetylcholine, through its action at muscarinic receptors on smooth muscle cells, is the primary neurotransmitter controlling bladder voiding (Wein et al., 1987, 1988; Yalla et al., 1988). Muscarinic receptor density and the sensitivity of bladder smooth muscle to muscarinic stimulation are greatest in the dome and lowest in the base, allowing efficient bladder emptying (Levin et al., 1980). Pharmacological, biochemical and genetic data provide ample evidence that muscarinic receptors are heterogeneous in nature (Hammer et al., 1980; Watson et al., 1986; Bonner et al., 1987). At least three subtypes of muscarinic receptors (M1, M2, and M3) are pharmacologically distinguishable. The M1 muscarinic receptor has high affinity for PZ but low affinity for AF-DX116, whereas the M2 muscarinic receptor has low affinity for PZ but high affinity for AF-DX116 (Hammer et al., 1980; Watson et al., 1986). A third type of muscarinic receptor is characterized by low affinity for both PZ and AF-DX116 and by high affinity for 4-DAMP (Kore et al., 1987). Recent advances in molecular biology have resulted in the cloning of a family of five (m1–m5) genes that code for muscarinic receptor subtypes and share the same proposed overall structure and a large degree of protein sequence homology (Bonner et al., 1987). The use of immunological and molecular reagents to study muscarinic receptor distribution has made it clear that most tissues present a mixture of receptor subtypes. Previous pharmacological characterization of muscarinic receptor subtypes must therefore be interpreted in the light of our understanding that the affinity being measured for any drug may represent composite properties of the drug’s interaction with a heterogeneous mixture of receptor subtypes in any given tissue.
Attempts to clarify the pharmacology associated with muscarinic receptor subtypes have used several approaches. One approach has been to transfect clonal cell lines with cDNA coding for individual muscarinic receptor subtypes and then to establish their biochemical properties (Buckley et al., 1989; Fukuda et al., 1989). These studies have established that m1, m3 and m5 muscarinic receptor subtypes efficiently promote PI breakdown and that the m2 and m4 subtypes interact with adenylyl cyclase to inhibit enzyme activity, the latter types being relatively inactive in promoting PI breakdown. These studies suggest a degree of specificity in the ability of individual receptor subtypes to interact with cellular effector systems. On the other hand, Dai et al. (1991) have reported an apparent interaction of the PI-linked m3 receptor with both pertussis-sensitive and -insensitive G proteins in rat parotid gland. Thus, factors influencing the interaction of muscarinic receptors with proximal G protein effectors remain to be clarified in most tissues.
In functional studies, stimulation of bladder muscarinic receptors causes smooth muscle contraction. Because bladder appears to contain more than one muscarinic receptor subtype (Maeda et al., 1988; Ruggieri et al., 1990; Wall et al., 1991), it is possible that one muscarinic receptor subtype is responsible for bladder smooth muscle contraction whereas the other is coupled to another parasympathetic associated function, such as influencing neuronal acetylcholine release. It remains to be discovered exactly what factors determine how the specificity of this receptor-effector coupling occurs. It could occur at the level of the coupling of one receptor subtype, through multiple G proteins, to several responses in bladder. This is one possibility investigated in these studies.
Previous studies have resulted in the following observations regarding bladder muscarinic receptor subtypes. High-affinity PZ binding sites are not present in the urinary bladder of the rat (Monferini et al., 1988), guinea-pig (Nilvebrant et al., 1983), rabbit (Lepor et al., 1984; Ruggieri et al., 1987) or human (Ruggieri et al., 1987; Batra et al., 1987). In addition, agonist stimulation of muscarinic receptors modestly inhibited adenylyl cyclase but failed to stimulate PI breakdown to any significant extent in the rabbit urinary bladder (Ruggieri et al., 1987). On the other hand, PI breakdown has been described in the guinea pig (Noronha-Blob et al., 1989) and recently in human urinary bladder (Anderson et al., 1991). Thus there are discrepancies in the literature as to which muscarinic receptor mediates contractility and whether this is conserved across species. In this study, we investigated the distribution of muscarinic receptor subtypes in rat, rabbit, guinea pig and human urinary bladder by subtype-specific immunoprecipitation. Agonist binding and receptor-G protein coupling in bladder membranes were determined as a measure of distinguishing the functional difference among bladder tissue from different species. Schild analysis using available subtype-selective antagonists was performed to determine the subtype responsible for bladder contractility in rat and rabbit.
Materials and Methods
Materials
All frozen rat, rabbit and guinea pig tissue was purchased from Pel Freeze Biologicals (Rogers, AR). Human bladder tissue obtained at autopsy within 2 h after cessation of circulation was either frozen in liquid nitrogen and transported to the laboratory on dry ice or placed in Eagle’s minimal essential medium and transported to the laboratory on wet ice within 12 h (National Disease Research Interchange, Philadelphia, PA, and the International Institute for the Advancement of Medicine, Exton, PA). The following drugs and chemicals were kindly provided by or obtained from the sources indicated: carbachol, goat anti-mouse IgG1-agarose, sodium cholate, GTP, protease inhibitors and atropine (Sigma Chemical Company, St. Louis, MO), methoctramine, 4-DAMP and p-F-HHSiD (Research Biochemicals International, Nantick, MA), AFDX-116 (Dr. Barry Wolfe, Georgetown University, Washington, DC), [3H]N-methylscopolamine (79 Ci/mmol) (Amersham Inc., Arlington Heights, IL), [3H]quinuclidinyl benzilate (39 Ci/mmol) and [3H]oxotremorine-M (89 Ci/mmol) (DuPont-New England Nuclear Research Products, Wilmington, DE), pansorbin (Calbiochem Inc., La Jolla CA), digitonin (Gallard-Schlesinger Industries Inc., Carle Place, NY). WGA (Vector Laboratories) and recombinant protein A (Calbiochem) were coupled to Affigel 10 (BioRad) at 10 and 2 mg/ml, respectively, as described by the manufacturer (Matesic et al., 1989).
Development of peptide antisera
The peptides CXKQQYQQRQSVISHKR (X = norleucine) and KKTFRHLLLCQYRNIGTAR were synthesized and coupled to bovine thyroglobulin using glutar-aldehyde. These peptides represent C-terminal sequences of the m3 and m4 muscarinic receptor subtypes, respectively. All injection and bleed protocols were performed at Lampire Biological Laboratories (Coopersberg, PA) as previously described (Luthin et al., 1988; Wall et al., 1991). Blood was collected monthly and screened in a solid phase immunoassay against both antigen and unrelated peptide. Positive bleeds were further characterized by an immunoprecipitation assay (see “Results”). Antibodies to the m1 and m2 receptor subtypes have been previously described (Luthin et al., 1988). The 31–1D1 anti-m2 receptor antibody was a generous gift from Dr. Neil M. Nathanson, University of Washington (Seattle). The m5 antiserum has been described (Yasuda et al., 1993) and was obtained as a gift from Dr. Barry B. Wolfe (Georgetown University, Washington, DC). The anti-G protein antibodies were a generous gift of Dr. David Manning and have the following specificities: 8730, Gi1α and Gi2α; 1521, Gi2α; 2918, Goα; 2921, Gzα; 0946, Gq/11α (Matesic et al., 1991, Mumby et al., 1991). An antibody to an epitope common to members of the Giα, Goα, Gq/11α and Gzα subfamilies of G proteins was also used in some studies (antibody 8646).
Tissue preparation
All operations were performed at 4°C. Frozen bladders and parotids were thawed, minced and homogenized at 10 ml/g tissue in a buffer containing 10 mM Tris, 1 mM EDTA (pH 7.5), with 10 μg/ml of the following protease inhibitors: soybean and lima bean trypsin inhibitors, aprotinin, leupeptin, pepstatin, and α2-macroglobulin. Homogenates were centrifuged at 20,000 × g for 15 min. Membranes were resuspended in the same buffer and used immediately for either radioligand binding assays or receptor solubilization as described (Matesic et al., 1989). When present, GTP was included at a concentration of 0.1 mM, and membranes were supplemented with 3 mM MgCl2.
Radioligand binding assay
[3H]Oxo-M or [3H]NMS (5 nM) was incubated with bladder membranes for 3 h at 4°C in a final volume of 0.5 ml of buffer containing 10 mM Tris, 3 mM MgCl2 and 1 mM EDTA. Reactions were terminated by dilution with 5 ml of a buffer containing 10 mM Tris and 150 mM NaCl and by vacuum filtration over glass-fiber filters, followed by a wash with 5 ml of buffer. Filters were combined with 4 ml of BioSafe II scintillation cocktail and measured for radioactivity.
Receptor labeling and solubilization
Membranes were incubated with [3H]QNB (1.5 nM) in buffer at 25°C for 30 min, followed by centrifugation at 20,000 × g for 12 min at 4°C. The supernatant was discarded, and the pellet was solubilized in the original volume of buffer containing 1% digitonin and 0.2% cholic acid, followed by a 45 to 60-min incubation at 4°C. After centrifugation at 30,000 × g for 30 min, the supernatant was removed, filtered through a 0.45-μl membrane and used as the solubilized receptor preparation.
Immunoprecipitation
All immunoprecipitations were performed as described (Luthin et al., 1988). Solubilized receptor (0.5 ml) and antibody were incubated overnight at 4°C, desalted using Sephadex G-50 minicolumns and precipitated using pansorbin (m1, m3, m4 and m5 receptors) or goat anti-mouse IgG1 beads (m2 receptors). Tissue and clonal cell lines expressing the respective receptor subtypes were used in parallel experiments as positive controls. Specifically, m1-transfected mouse L cells (gift from Dr. Josephine Lai, University of Arizona, Tucson), rat heart (m2), m3-cDNA-transformed CHO cells, and m4-cDNA-transfected A9 cells (gift from Dr. Donna Flynn, University of Miami, Miami) were used for specific antisera. Under these conditions, the relative efficiencies of m1 to m4 antibodies at precipitating receptors are 100%, 75%, 85% and 100%, respectively. All data presented are corrected for antibody efficiency for each precipitation.
Agonist binding and immunoprecipitation
The conditions for agonist binding, receptor solubilization and WGA-affigel chromatography have been described in detail (Matesic et al., 1989, 1990). In brief, membranes were incubated with labeled or unlabeled agonist or antagonist, solubilized in digitonin/cholate and either used directly or chromatographed over WGA-affigel 10. Fractions eluted with N-acetylglucosamine were concentrated and were incubated as indicated with anti-receptor or anti-G protein antibodies. Bound proteins were precipitated using goat anti-mouse IgG1 agarose (m2 antibodies) or protein A-affigel (m3 receptor and G protein antibodies). As indicated, proteins were identified in the pellets either by direct counting ([3H]Oxo-M) or by subsequent incubation with [3H]NMS or [36S]GTPγS. Conditions for GTPγS binding were essentially as described by Northup et al. (1982). Beads containing bound proteins were incubated in a final volume of 50 μl containing 10 mM Tris (pH 8), 1 mM EDTA, 100 mM NaCl, 1 mM DTT, 2 mM MgCl2 and 200 nM GTPγS (10,000–15,000 cpm/pmol). GTP (1 mM) was used to define nonspecific binding. Samples were incubated for 30 min at 30°C, quenched with 5 volumes of ice-cold buffer containing 0.1% NP40, 50 μM GTP and 10 mM MgSO4 and then filtered over BA83 nitrocellulose filters. Identical results were obtained if the beads were removed from the assay tubes by centrifugation, and supernatant fractions were desalted. For [3H]NMS binding, precipitated proteins were released from the beads by incubation in the presence of 0.1 mM GTP for 30 min at 25°C, conditions that totally reverse agonist (e.g., carbachol) binding to the m2 receptor in heart and bladder (Matesic and Luthin, 1991). Beads were removed by centrifugation, and samples were incubated with [3H]NMS (1 nM) for 30 min at 25°C; then bound ligand was determined after samples were desalted (Matesic et al., 1989). So that we could determine nonspecific trapping of proteins by the precipitating agents, controls contained identical samples but without the primary antibody.
Contractility studies
Full-thickness longitudinal bladder body strips (approximately 10 × 3 mm) were sectioned from rats euthanized by decapitation and rabbits euthanized by cervical dislocation. Strips were suspended in 15-ml tissue baths (Radnotti Glass Technology, Monrovia CA) with 1 g of isometric tension in Tyrodes solution (125 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 1.8 mM CaCl2, 0.5 mM MgCl2, 23.8 mM NaHCO3 and 5.6 mM glucose) and equilibrated with 95%/5% oxygen/carbon dioxide at 37°C for 30 min. Previous length-tension studies demonstrated that this procedure results in maximal active force production by the strips. Isometric force was monitored with force displacement transducers. Dose-response curves were generated by cumulative addition of 9 to 10 concentrations of carbachol at half-log concentration intervals (range, 10−8 to 10−3 M) dissolved in deionized water at 0.01 volume of the bathing solution. For Schild analysis, baths were equilibrated in the presence of antagonist for 30 min before addition of the agonist. Each concentration of antagonist was tested on 4 to 8 separate muscle strip preparations from each species, and dose ratios were determined on the basis of the responses of four control (antagonist-free) strips for any given experimental day. The contractile response of bladder tissue to muscarinic stimulation is biphasic, consisting of a peak response followed by a plateau phase of lower, sustained increase in tension. The dose response was determined for carbachol to produce both peak response (maximal tension) and sustained contractions (operationally defined as the increase in tension remaining 5 min after addition of the agonist). Agonist concentrations were added in a cumulative fashion at 5-min intervals, and each curve was transformed to an EC50 value via a Hill transformation of the data. The EC50 values determined in the presence of antagonist were used to generate the Schild plots for determination of pA2 values.
Results
Immunoprecipitation of muscarinic receptor subtypes from urinary bladder
Our laboratory has obtained antisera that can immunoprecipitate the m1 to m4 subtypes of muscarinic receptor. The m1, m3, m4 and m5 selective antisera were developed to C-terminal or fusion protein determinants of receptor subtypes as described (Luthin et al., 1988; Yasuda et al., 1993). The anti-m2 receptor antibody (31–1D1) is a monoclonal antibody raised against the purified porcine cardiac m2 receptor (Luetje et al., 1987). Receptors from clonal cells expressing individual receptor subtypes (m1, m3 and m4) or rat heart (m2) were labeled with [3H]QNB and solubilized and then were precipitated using the specific antisera. Results shown in table 1 are the average cpm [3H]QNB binding in individual experiments. Total added receptor was measured by desalting samples after labeling and solubilization. This varied with tissue type, reflecting the levels of expression of the plasmids encoding receptors. This means that absolute levels of receptor precipitated cannot be directly compared. For clarity these data are not shown, but the percentage of total receptor precipitated by each antibody is given in the legend. The specificity of the antibodies to the m5 receptor has been demonstrated in the results of similar studies (Yasuda et al., 1993) and is not shown here. These results demonstrate not only that each antibody precipitates receptors solubilized from cells expressing the appropriate receptor subtype but also that none of the antibodies is cross-reactive with other receptor subtypes.
TABLE 1.
Specificity of anti-muscarinic receptor antibodies
| Antibodya | m1 cellsb | Heart | m3 cells | m4 cells |
|---|---|---|---|---|
| m1 + | 2435 | 507 | 756 | N.D.c |
| − | 380 | 461 | 786 | |
| m2 + | 88 | 5348 | 584 | 246 |
| − | 95 | 149 | 501 | 252 |
| m3 + | 356 | 444 | 13876 | 355 |
| − | 382 | 458 | 289 | 526 |
| m4 + | 271 | 482 | 473 | 1322 |
| − | 208 | 458 | 403 | 526 |
Solubilized receptors were incubated with (+) or without (−) the indicated antibody, at a concentration optimized to produce immunoprecipitation. Blank values reflect the differences in receptor concentration, amount of tissue, precipitating agent and assay volume in each assay. Similar data for the m5 receptor have been published (Yasuda et al., 1993).
Data shown are the average cpm [3H]QNB precipitated using receptors solubilized from each tissue in a representative experiment. The standard error of triplicate determinations was generally less than 10% of the sample mean and for clarity is not shown.
N.D. = not determined.
In order to identify the subtypes of muscarinic receptors in bladder, receptors from rat, rabbit, guinea pig and human bladder were labeled with [3H]QNB, solubilized and immunoprecipitated with antisera (or monoclonal antibodies) recognizing the m1 to m5 receptor subtypes. Significant precipitation of m2 and m3 receptor subtypes was observed in the urinary bladder under these conditions, whereas no detectable precipitation was measured for m1, m4 or m5 receptors in these tissues. During the course of preliminary experiments, we found that if receptors were solubilized before labeling with [3H]QNB (Luthin et al., 1988), only m2 receptors could be precipitated and that this subtype accounted for all measurable bladder receptors. This result indicates an apparent conditional instability of at least the m3 receptor subtype and points to this as a potential artifact using the immunoprecipitation assay. This apparent instability was tissue-dependent; in parotid gland or clonal cells, prelabeling was not a prerequisite for immunoprecipitation of the m3 receptor (data not shown). The distribution of m2 and m3 receptors in urinary bladder was investigated by comparing the density of each receptor subtype in the species studied. The total solubilized muscarinic receptor density in the bladder was also measured by [3H]QNB binding to determine whether the m2 and m3 antibodies together precipitate all the solubilized receptors. We found that the m2 receptor was, in general, abundant in bladder tissue from all species tested and made up 61% to 78% of the total muscarinic receptor density (fig. 1). The density of m3 receptors varied among species, from 8% in rat to 32% in human. The combined density of m2 and m3 was very close to the density of total receptors in each species (95%–110%). The m2:m3 ratio was relatively stable among species (3.3, 2.4 and 3.4 for rabbit, human and guinea pig, respectively) except for the rat, which had a much higher m2:m3 ratio (9.8) than the other species tested.
Fig. 1.
Precipitation of m2 and m3 muscarinic receptor subtypes from bladder of different species. Receptors were labeled with [3H]QNB and solubilized as described in the text. Data shown are converted to percent of total solubilized receptors and are the average ± S.E.M. from 3 to 6 determinations in each species. Open, m2 receptors; hatched, m3 receptors. The levels of m1, m4 and m5 receptors were undetectable.
Agonist inhibition of [3H]NMS binding to bladder membranes
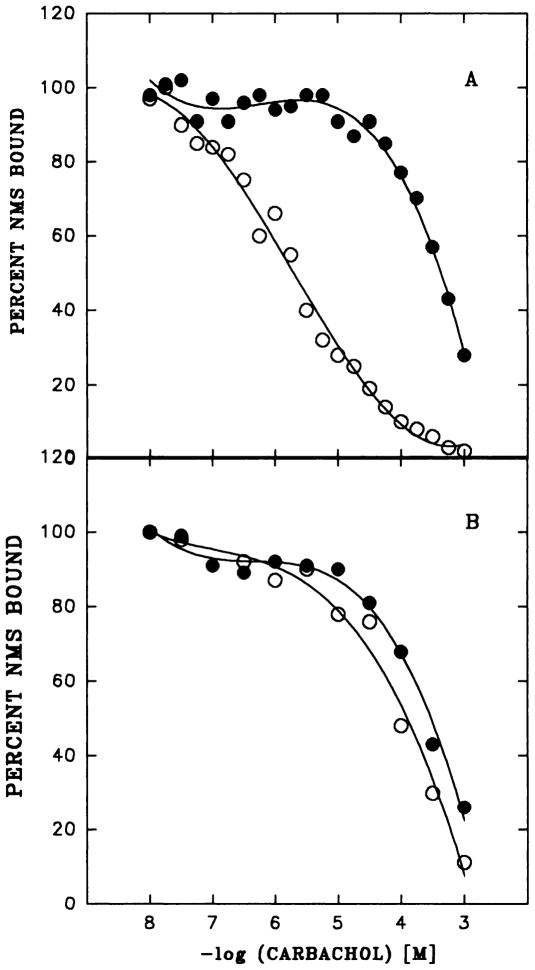
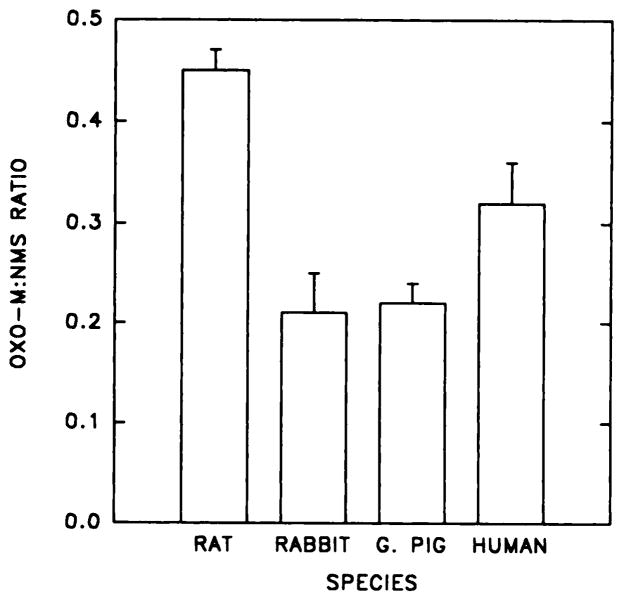
The muscarinic agonist carbachol inhibits specific [3H]NMS binding to bladder membranes in a concentration-dependent manner. The conditions for this assay (e.g., extended incubation at 4°C) were chosen to permit comparison of direct and indirect measurements of agonist binding (Matesic et al., 1989). A high concentration of [3H]NMS was used to occupy membrane receptors fully, so as not to exclude populations of a given subtype. Dose-response curves for inhibition of [3H]NMS binding to rat bladder and parotid membranes in the absence of GTP were generated and are shown in figure 2. In the presence of GTP, the dose-response curves in bladder were displaced to the right (fig. 2A), reflecting only the low-affinity component of binding. In contrast, GTP had no effect on the affinity of the receptors in rat parotid, a predominantly m3 tissue, and this tissue did not exhibit a high-affinity component for agonist binding (fig. 2B). In the absence of GTP, the Hill coefficient derived from the carbachol inhibition curve was lower in rat bladder than in bladder from any other species (data not shown), which suggests a larger proportion of the high-affinity component of binding. To quantitate directly the proportion of high-affinity agonist binding sites, we measured the [3H]Oxo-M:[3H]NMS binding ratio in bladder membranes from different species (fig. 3). In agreement with the dose-response curve, the Oxo-M:NMS binding ratio was highest in rat bladder when compared with the other species. No detectable binding of [3H]Oxo-M to rat parotid could be demonstrated (data not shown). Together, these data suggest that the agonist binding characteristics seen in bladder reflect the relative ratio of m2:m3 receptor present in the tissue, with higher levels of agonist binding indicating higher levels of m2 receptor.
Fig. 2.
A) Dose-response for carbachol inhibition of [3H]NMS binding to membranes from rat bladder without (○) and with GTP (●). Data shown are the averages of duplicate determinations in a representative experiment. These results have been replicated at least three times. B) The same experiment as above performed in rat parotid gland.
Fig. 3.
Comparison of [3H]Oxo-M and [3H]NMS binding to bladder membranes from different species. Binding assays were as described in the text and are expressed as the ratio of total Oxo-M to total NMS binding determined in three separate experiments.
Coupling of the m2 and m3 receptors to G proteins
We have developed a technique for measuring interactions of the m2 receptor with G proteins in heart, and we applied this technique to the study of the same receptor subtype in bladder. It relies on co-precipitation of G proteins with the m2 receptor using either anti-receptor or anti-G protein antibodies. The original protocol relied on Western blotting with anti-Gα antibodies to identify co-purifying G proteins, and modifications of this protocol have been used to confirm the results obtained with the Western blot assay in the present study.
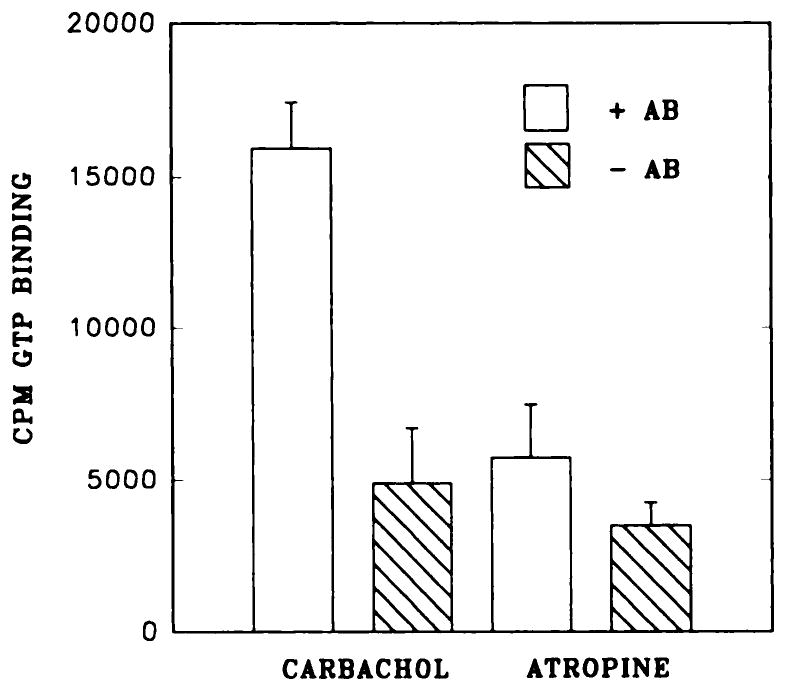
Membranes from rat bladder were incubated with the agonist carbachol (1 mM) or the antagonist atropine (1 μM) on ice. Next they were solubilized and chromatographed over WGA-affigel 10 as described in “Materials and Methods” and then concentrated using centricon 30 ultrafilters (Matesic et al., 1991). Concentrated receptor preparations were incubated with an anti-Gαcommon antibody (8646) and precipitated using protein-A beads. After warming and desalting of the precipitated fractions to remove residual bound drug, [3H]NMS could be used to label receptors from fractions preincubated with carbachol but not atropine (fig. 4). In the absence of antibody, insignificant levels of receptor were precipitated, which demonstrates the specificity of the carbachol effect. WGA-purified receptors were also precipitated using an anti-m2 receptor antibody, and G protein α subunits present in the precipitates could be identified using [35S]GTPγS (fig. 5). Thus, in the presence of carbachol but not of atropine, GTPγS binding activity co-precipitated with the m2 muscarinic receptor. This was not seen if the precipitating antibody was excluded from the incubation (fig. 5). To identify more specifically those G proteins associated with agonist-labeled receptors, we used [3H]Oxo-M to label membranes from rat bladder. Receptors were then solubilized and immunoprecipitated using antibodies specific to anti-Gαcommon, anti-Gαi1,2, anti-Gαi2, anti-Gαo, anti-Gαq/11 and anti-Gαz. Antibodies to Gαcommon and Giα, and to some extent antibodies to Gq/11α, were capable of precipitating [3H]Oxo-M binding activity under these conditions (fig. 6).
Fig. 4.
Precipitation of [3H]NMS binding activity using anti-G protein antibodies. Membranes from rat bladder were incubated with carbachol or atropine and processed as described in the text, and WGA-purified proteins were concentrated and incubated with or without anti-G protein antibodies (8646) and then precipitated using protein A-affigel beads. Precipitated proteins were eluted from the beads using GTP and then were incubated with [3H]NMS (1 nM, 30 min at 25°C) to determine levels of muscarinic receptor in the pellets. +Ab, proteins incubated with antibody 8646; −Ab, proteins incubated without inclusion of antibody 8646.
Fig. 5.
Precipitation of GTPγS binding activity using anti-m2 muscarinic receptor antibodies. Membranes from rat bladder were incubated with carbachol or atropine as indicated, solubilized and purified over WGA-Affigel. Eluted fractions were concentrated and incubated with (+Ab) or without (−Ab) anti-m2 receptor antibody and then were precipitated using anti-mouse IgG beads. Precipitated proteins were incubated with GTPγS to determine total binding activity. Data shown are the average ± S.E.M. of triplicate determinations in a representative experiment; these results have been repeated at least three times.
Fig. 6.
Precipitation of [3H]oxo-M-labeled muscarinic receptors using specific anti-Gα antibodies. Membranes from rat bladder were incubated with [3H]Oxo-M, solubilized and precipitated with the specific anti-Gα antibodies shown (lanes B–G; see “Materials and Methods”). Results shown are the average ± S.E.M. of triplicate determinations (±S.E.M.) in a representative experiment; these results have been repeated at least three times. A: added receptor, determined by desalting parallel fractions; B: Gcommon; C: Gi1,2; D: Gi2; E: Go; F: Gq/11; G: Gz; H: no antibody added.
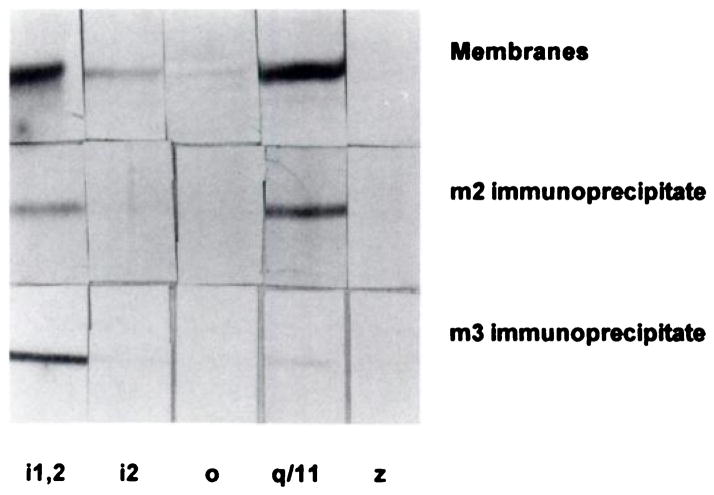
It is possible that antigenic determinants were masked in these experiments, which prevented the identification of interactions between the receptors and G proteins by means of the described immunoprecipitation assays. Also, G proteins such as Gq/11 α bind GTP only weakly and would not be measurable using GTPγS binding as described. As shown above, the agonist [3H]Oxo-M cannot label the m3 receptor subtype, and the precipitation assays described would measure only the association of G proteins with the m2 receptor subtype labeled with this agonist. Therefore, the following experiments were used to clarify further the interactions between bladder muscarinic receptors and G proteins. Receptors were preincubated with carbachol or atropine in membranes from rat and human bladders and then solubilized. Human bladder was chosen as a source of the m3 receptor, because levels in rat bladder are too low to permit use of this technique. We have found that the m3 receptor subtype is refractory to chromatography over WGA resins and that it remains tightly bound to the resin even after attempted elution with N-acetylglucosamine. Receptors from human bladder and rat parotid were therefore processed with and without WGA chromatography before immunoprecipitation using m2 and m3 antibodies, respectively. Fractions precipitated using the m2 or m3 antibodies were subjected to SDS-PAGE and Western blotting, and blots were developed using selective anti-G protein antibodies. A representative experiment is shown in figure 7, and the data obtained in other tissue preparations are summarized in table 2. In the presence of carbachol, both Giα and Gq/11α subunits could be identified as present in the precipitates containing the m2 and m3 receptor subtypes. Both Giα and Gq/11α were precipitated from rat parotid gland using the same m3 antibodies. The Giα subunits were identified as belonging to Gi1α and not Gi2α. This is in spite of the observation that high levels of Gi1α, Gi2α and Gq/11α and lower levels of Goα are present in rat and human bladder membranes. Gzα was undetectable in both rat and human bladder membranes and in immunoprecipitated preparations.
Fig. 7.
Identification of G proteins that co-precipitate with carbachol-labeled muscarinic receptors in human bladder. Membranes were incubated with (bottom 2 rows) or without (top row) carbachol and processed as described in fig. 4. WGA-purified proteins were concentrated and precipitated with anti-m2 receptor antibodies. The m3 receptors were precipitated without prior WGA chromatography. Proteins were subjected to SDS-PAGE and Western blotting using specific anti-G protein antibodies (see “Materials and Methods”). Lane 1, 8730; Lane 2, 1521; Lane 3, 2918; Lane 4, 0946; Lane 5, 2921. Gels show band patterns in the molecular weight range of 39 to 42 kD.
TABLE 2.
Identification of Gα subunits that co-precipitate with muscarinic receptors
| Tissue | Gα subunita |
|---|---|
| Rat bladder | |
| Membranes | Gi1,2,3, (Go)b, Gq, (Gz) |
| Precipitate (m2)c | Gi1, Gq |
| Human bladder | |
| Membranes | Gi1,2,3, (Go), Gq |
| Precipitate (m2) | Gi1, Gq |
| Precipitate (m3) | Gi1, Gqd |
| Rat parotid | |
| Membranes | Gi1,2,3, Gq |
| Precipitate (m3) | Gi1, Gq |
Gα subunits were identified by Western blotting using selective anti-Gα antisera. The proteins identified by strong banding at molecular masses of 39 to 41 kDa are listed for each tissue. SDS-solubilized membranes are compared to Gα subunits precipitated using anti-m2 or anti-m3 receptor antibodies. Control blots on purified Gi/Go were performed to verify the viability of each antibody.
Subunits listed in parentheses were present at very low levels but were detectable.
The parenthetic m2 and m3 refer to the anti-receptor antibody used to precipitate receptor-G protein complexes in each tissue.
Although Gq subunits were present on blots, they were present in low levels and were also present when membranes were incubated with atropine instead of carbachol.
Studies of bladder contractility
Initial studies were designed to determine the relationship between the presence of a given receptor subtype and its role in bladder contractility. Although none of the available muscarinic receptor antagonists is uniquely selective for a single receptor subtype, a panel of antagonists can be used to predict the involvement of a receptor subtype in functional studies. We used the antagonists AFDX-116, p-F-HHSiD, methoctramine and 4-DAMP to shift agonist dose-response curves in bladder contractility studies. Results from a representative experiment on rabbit bladder strips using 4-DAMP and peak contractile responses are shown in figure 8, and the data obtained using the described antagonists in both rabbit and rat bladder strips are summarized in table 3. Data were converted to pA2 values from Schild replots. Inhibition curves and Schild plots were constructed for both peak (maximal) contractile responses and plateau responses (operationally defined as the increase in tension remaining 5 min after addition of the agonist). The pA2 values obtained from peak responses were identical to those obtained from plateau responses, which indicates that the same muscarinic receptor subtype mediates both phases of the contractile response. Table 3 shows pK values for the potency of these antagonist drugs as reported in the literature (Caulfield, 1993). The pA2 values obtained from both rat and rabbit were most consistent with an m3-mediated response and were not consistent with an m2-mediated response.
Fig. 8.
Representative carbachol dose-response displacement curves and Schild plot (insert) for 4-DAMP effect on rabbit bladder strips in vitro. Each curve represents the average responses of four separate muscle strip preparations expressed as the percent of each individual strip’s maximal carbachol response. This maximal response (mean ± S.E.M.) in grams of tension were 2.8 ± 0.5, 4.3 ± 1.2, 3.7 ± 0.9, 3.7 ± 1.5 and 1.8 ± 0.3 for control (□), 0.0033 μM (○), 0.01 μM (△), 0.033 μM (▽) and 0.1 μM (◇) respectively. Similar curves were obtained for both rabbit and rat bladder strips, and the pA2 values for all the drugs used are displayed in table 3.
TABLE 3.
pA2 values for inhibition of carbachol-induced contractions in rabbit and rat bladdera
| m2b | m3b | Rabbit Bladder | Rat Bladder | |
|---|---|---|---|---|
| p-F-HHSiD | 6.0–6.9 | 7.8–7.9 | 7.1 | 7.8 |
| 4-DAMP | 8.0–8.4 | 8.9–9.3 | 9.2 | 10.6 |
| AFDX-116 | 7.1–7.2 | 5.9–6.6 | 5.5 | n.d.c |
| Methoctramine | 7.8–8.3 | 6.3–6.9 | 6.7 | 6.1 |
A series of agonist dose-response curves were generated in the presence of increasing levels of the indicated partially selective muscarinic antagonists as described in “Materials and Methods.” The x-intercept of Schild replots of the data was used to determine the pA2 value for each drug.
The affinity ranges for the various antagonists are taken from a recent review (Caulfield, 1993) and include data from functional studies, studies on cloned receptors expressed in cell lines and radioligand binding displacement studies.
N.D. = not determined.
Discussion
It has been reported that m2 mRNA is the predominant mRNA coding for muscarinic receptors in rat bladder, although m3 mRNA was also detected (Maeda et al., 1988). Previously reported immunoprecipitation studies indicate that in rat bladder, m2 and m3 receptors constitute 84% and 9% of the total receptor, respectively (Wall et al., 1991). In agreement with these findings, binding studies with a panel of seven antagonists have suggested that the predominant receptor expressed in rat bladder is of the cardiac (M2) type, although a smaller population of glandular M3 receptor was also present (Monferini et al., 1988). The results of our studies generally concur with these results. With a panel of m1 to m5 antisera, we were able to precipitate m2 and m3 receptors from rat bladder, whereas no detectable precipitation was observed when m1, m4 and m5 subtype-specific antisera were used.
Investigation of the m2 and m3 receptor subtype distribution in bladder from different species demonstrated that the m2 subtype was always the predominant receptor subtype expressed. This observation is consistent with the low or absent PI response in this tissue. The binding of [3H]NMS to rat bladder was inhibited in a concentration-dependent manner by carbachol, and the high-affinity component of binding was eliminated in the presence of GTP. High-affinity agonist binding, as measured using carbachol or [3H]Oxo-M, correlated with the relative levels of the m2 receptor subtype. The results obtained with [3H]Oxo-M were confirmed by immunoprecipitation of agonist-labeled receptor using anti-m2, but not anti-m3, receptor antibody (data not shown). The affinity of muscarinic receptors for agonists in the rat urinary bladder is therefore most similar to heart, an m2 tissue, and not parotid, an m3 tissue; this may be functionally relevant.
In the rat parotid gland, the m3 receptor mediates both calcium mobilization and adenylyl cyclase inhibition (Dai et al., 1990). The cellular activity of muscarinic receptors is thought to be ultimately dependent on receptor-G protein interactions. In parotid, we confirmed that the m3 receptor subtype, as predicted by previous studies (Dai et al., 1990), couples through both pertussis toxin-sensitive (Gi) and pertussis toxin-insensitive (Gq/11) G proteins. We have now directly confirmed coupling of the bladder m2 and m3 receptor subtypes to members of the Giα and Gq/11α subfamilies of G proteins, representing pertussis-sensitive and -insensitive proteins, respectively. This was demonstrated using complementary techniques, including 1) precipitation of GTPγS binding activity using anti-m2 receptor antibodies, 2) precipitation of NMS and Oxo-M binding activity using anti-G protein antibodies and 3) precipitation of G protein immuno-reactivity using anti-m2 and anti-m3 receptor antibodies. All of these observed co-precipitations of receptor and G protein required prior incubation of receptor with agonist, as shown previously in rat heart and brain (Matesic et al., 1989; Matesic et al., 1991). The pharmacology of the contractility best matched that associated with the m3 receptor, and this receptor subtype was shown to be present in bladder from all species tested, though at low levels. Together these data suggest that the m3 receptor, through Giα, Gq/11α or a related G protein, mediates bladder contractility in rat and rabbit. Thus, selective interaction of bladder m2 and m3 receptors with the repertoire of bladder G proteins may define functionally the associated tissue response.
The inhibitory effect of muscarinic agonists on the contractile activity of the heart has been known for as many years as the stimulatory effect of muscarinic agonists on the contractile activity of the bladder. The majority of muscarinic receptors in the bladder from all species tested are of the m2 subtype, the predominant (if not exclusive) receptor subtype found in cardiac tissue. If the m2 receptor in the rat bladder is associated with postsynaptic mechanisms in a manner similar to cardiac m2 receptors, then stimulation of these receptors is predicted to cause inhibition of muscle contractility. It has been reported that muscarinic agonists, acting through presynaptic receptors, can induce a decrease in the release of acetylcholine in rat bladder (D’Agostine et al., 1986). Thus the m2 receptors of rat bladder are more likely to be located on the presynaptic cholinergic nerve terminals and, upon stimulation, may induce inhibition of acetylcholine release. Our data suggest that the m3 receptor is directly responsible for contractility in bladder. We therefore find it difficult to confirm a role for the m2 receptor in mediating this activity, leaving open the possibility of a different—and as yet uncharacterized—activity for this receptor subtype in urinary bladder. Further investigations of muscarinic receptor coupling mechanisms in the bladder are necessary so that comparisons may be made to similar mechanisms in heart and other smooth muscle and glandular tissues.
Acknowledgments
Supported by PHS NS23006 (G.R.L.), PHS DK43333 (M.R.R. and G.R.L.) and PHS DK40579 (M.R.R. and G.R.L.).
The authors wish to thank Neil M. Nathanson and David R. Manning for their generous gifts of antisera and to thank Barry B. Wolfe, Stephen J. Wall, Robert Yasuda and Min Li for their donations of antisera and peptides and their constant support.
ABBREVIATIONS
- AFDX-116
(11[[2-[diethylamino)methyl]-1-piperidinyl]acetyl]-5, 11-dihydro-6H-pyrido[2,3–6][1,4]benzodiazepine-6-)one
- 4-DAMP
4-diphenylacetoxy-N-methylpiperidine methiodide
- DTT
1,4-dithiothreitol
- GTPγS
guanosine 5′-(γ-thio)triphosphate
- NMS
N-methylscopolamine
- Oxo-M
oxotremorine-M
- p-F-HHSiD
para fluoro hexahydrosilodifenidol
- QNB
quinuclidinyl benzilate
- Tris
2-amino-2-hydroxymethyl-1,3-propanediol
- WGA
wheat germ agglutinin
- PZ
pirenzepine
- PI
polyphosphoinositide
References
- Anderson KE, Holmquist F, Fovaeus M, Hedlund H, Sundler R. Muscarinic receptor stimulation of phosphinositide hydrolysis in the human isolated urinary bladder. J Urol. 1991;146:1156–1159. doi: 10.1016/s0022-5347(17)38030-8. [DOI] [PubMed] [Google Scholar]
- Batra S, Bjorklund A, Hedlund H, Andersson KE. Identification and characterization of muscarinic cholinergic receptors in the human urinary bladder and parotid gland. J Auton Nervous Sys. 1987;20:129–135. doi: 10.1016/0165-1838(87)90110-x. [DOI] [PubMed] [Google Scholar]
- Bonner TI, Buckley NJ, Young AC, Brann MR. Identification of a family of muscarinic acetylcholine receptor genes. Science (Wash DC) 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Buckley CM, Brann MR. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989;35:469–476. [PubMed] [Google Scholar]
- Caulfield MP. Muscarinic receptors—characterization, coupling and function. Pharmac Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- D’Agostine G, Kilbinger H, Chiari MC, Garna E. Presynaptic inhibitory muscarinic receptors modulating [3H]acetylcholine release in the rat urinary bladder. J Pharmacol Exp Ther. 1986;239:522–528. [PubMed] [Google Scholar]
- Dai YS, Ambudkar IS, Horn VJ, Yeh CK, Kousvelari EE, Wall SJ, Li M, Yasuda RP, Wolfe BB, Baum BJ. Evidence that M3 muscarinic receptors in rat parotid gland couple to two second messenger systems. A J Physiol. 1991;261(6 Pt 1):C1063–1073. doi: 10.1152/ajpcell.1991.261.6.C1063. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Kubo T, Maeda A, Akiba I, Bujo H, Nakai J, Mishina M, Higashida H, Neher E, Marty A, Numa S. Selective effector coupling of muscarinic acetylcholine receptor subtypes. Trends Pharmacol Sci. 1989;10(Suppl IV):4–6. [PubMed] [Google Scholar]
- Hammer R, Berrie CP, Birdsall NJM, Burger ASV, Hulme EC. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature (Lond) 1980;283:90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Kore M, Ackerman MS, Roeske WR. A cholinergic antagonist identifies a subclass of muscarinic receptors in isolated rat pancreatic acini. J Pharmacol Exp Ther. 1987;240:118–122. [PubMed] [Google Scholar]
- Lepor H, Kuhar MJ. Characterization of muscarinic cholinergic receptor binding in the vas deferens, bladder, prostate and penis of the rabbit. J Urol. 1984;134:392–396. doi: 10.1016/s0022-5347(17)49635-2. [DOI] [PubMed] [Google Scholar]
- Levin RM, Shofer F, Wein AJ. Cholinergic, adrenergic, and purinergic response of sequential strips of rabbit urinary bladder. J Pharmacol Exp Ther. 1980;212:536–540. [PubMed] [Google Scholar]
- Luetje CW, Brunwell KC, Norman MG, Peterson GL, Schimerlik MI, Nathanson NM. Isolation and characterization of monoclonal anti-bodies specific for the cardiac muscarinic acetylcholine receptor. Biochemistry. 1987;26:6892–6896. doi: 10.1021/bi00396a003. [DOI] [PubMed] [Google Scholar]
- Luthin GR, Harkness J, Artymyshyn R, Wolfe BB. Antibodies to a synthetic peptide can be used to distinguish between muscarinic acetylcholine receptor binding sites in brain and heart. Mol Pharmacol. 1988;34:327–333. [PubMed] [Google Scholar]
- Maeda A, Kubo Y, Mishina M, Numa S. Tissue distribution of mRNAs encoding muscarinic acetylcholine receptor subtypes. FEBS Lett. 1988;239:339–342. doi: 10.1016/0014-5793(88)80947-5. [DOI] [PubMed] [Google Scholar]
- Matesic DF, Luthin GR. Atropine dissociates complexes of muscarinic acetylcholine receptor and guanine nucleotide binding protein in heart membranes. FEBS Lett. 1991;284:184–186. doi: 10.1016/0014-5793(91)80680-2. [DOI] [PubMed] [Google Scholar]
- Matestic DF, Manning DR, Luthin GR. Tissue-dependent association of muscarinic acetylcholine receptors with guanine nucleotide-binding regulatory protein. Mol Pharmacol. 1991;40:347–353. [PubMed] [Google Scholar]
- Matesic DF, Manning DR, Wolfe BB, Luthin GR. Pharmacological and biochemical characterization of complexes of muscarinic acetylcholine receptor and guanine nucleotide-binding protein. J Biol Chem. 1989;264:21638–21645. [PubMed] [Google Scholar]
- Monferini E, Giraldo E, Ladinski H. Characterization of the muscarinic receptor subtypes in the rat urinary bladder. Eur J Pharmacol. 1988;147:453–485. doi: 10.1016/0014-2999(88)90180-x. [DOI] [PubMed] [Google Scholar]
- Mumby SM, Gilman AG. Synthetic peptide antisera with determined specificity for G protein alpha or beta subunits. Methods Enzymol. 1991;195:215–233. doi: 10.1016/0076-6879(91)95168-j. [DOI] [PubMed] [Google Scholar]
- Nilvebrant L, Sparf B. Difference between binding affinities of some anti-muscarinic drugs in the parotid gland and those in the urinary bladder and ileum. Acta Pharmacol Toxicol. 1983;53:304–313. doi: 10.1111/j.1600-0773.1983.tb03427.x. [DOI] [PubMed] [Google Scholar]
- Noronha-Blob L, Lowe V, Hanson RC, U’Prichard DC. Heterogeneity of muscarinic receptors coupled to phosphoinositide breakdown in guinea-pig brain and peripheral tissues. Life Sci. 1987;41:967–975. doi: 10.1016/0024-3205(87)90684-9. [DOI] [PubMed] [Google Scholar]
- Noronha-Blob L, Lowe V, Patton A, Canning B, Costello D, Kinner WJ. Muscarinic receptors: Relationship among phosphoinositide breakdown, adenylate cyclase inhibition, in vitro detrusor muscle contractions and in vivo cystometrogram studies in guinea-pig bladder. J Pharmacol Exp Ther. 1989;249:843–851. [PubMed] [Google Scholar]
- Northup JK, Smigel MD, Gilman AG. The guanine nucleotide activating site of the regulatory component of adenylate cyclase: Identification by ligand binding. J Biol Chem. 1982;257:11416–11423. [PubMed] [Google Scholar]
- Ruggieri MR, Bode DC, Levin RM, Wein AJ. Muscarinic receptor subtypes in human and rabbit bladder. Neurourol Urodyn. 1987;6:119–128. [Google Scholar]
- Ruggieri MR, Luthin GR. Identification of muscarinic receptor subtypes in human and rabbit bladder. FASEB J. 1990;4:A1011. [Google Scholar]
- Wall SJ, Yasuda RP, Li M, Wolfe BB. Development of an antiserum against m3 muscarinic receptors: Distribution of m3 receptors in rat tissues and cloned cell lines. Mol Pharmacol. 1991;40:783–789. [PubMed] [Google Scholar]
- Watson M, Roeske WR, Vickroy TW, Smith TL, Akiyama K, Gulya K, Duckles SP, Serra M, Adem A, Nordberg A, Gehlert DR, Wamsley JK, Yamamura HI. Biochemical and functional basis of putative muscarinic receptor subtypes and its implications. Trends Pharmacol Sci. 1986;2:46–55. [Google Scholar]
- Wein AJ, Barrett DM. Voiding Function and Dysfunction: A Logical and Practical Approach. Year Book Medical Publishers Inc; Chicago: 1988. [Google Scholar]
- Wein AJ, Levin RM, Barrett DM. Voiding Function: Relevant Anatomy, Physiology, and Pharmacology. In: Gillenwater JY, Grayhack JT, Howards SS, Duckett JD, editors. Adult and Pediatric Urology. Year Book Medical Publishers Inc; Chicago: 1987. pp. 933–999. [Google Scholar]
- Yalla SV, Mcguire EJ, Elbadawi A, Blaivas JG. Neurourology and Urodynamics. Macmillan Publishing Co; New York: 1988. [Google Scholar]
- Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB. Development of antisera selective for m4 and m5 muscarinic cholinergic receptors: Distribution of m4 and m5 receptors in rat brain. Mol Pharmacol. 1993;43:49–57. [PubMed] [Google Scholar]