Abstract
M3 muscarinic receptors mediate cholinergic-induced contraction in most smooth muscles. However, in the denervated rat bladder, M2 receptors participate in contraction because M3-selective antagonists [para-fluoro-hexahydro-sila-diphenidol (p-F-HHSiD) and 4-DAMP] have low affinities. However, the affinity of the M2-selective antagonist methoctramine in the denervated bladder is consistent with M3 receptor mediating contraction. It is possible that two pathways interact to mediate contraction: one mediated by the M2 receptor and one by the M3 receptor. To determine whether an interaction exists, the inhibitory potencies of combinations of methoctramine and p-F-HHSiD for reversing cholinergic contractions were measured. In normal bladders, all combinations gave additive effects. In denervated bladders, synergistic effects were seen with the 10:1 and 1:1 (methoctramine:p-F-HHSiD wt/wt) combinations. After application of the sarcoplasmic reticulum ATPase inhibitor thapsigargin to normal tissue, the 10:1 and 1:1 ratios became synergistic, mimicking denervated tissue. Thus in normal bladders both M2 and M3 receptors can induce contraction. In the denervated bladder, the M2 and the M3 receptors interact in a facilitatory manner to mediate contraction.
Keywords: urinary bladder, synergy, denervation, second messengers
Pharmacological data, based on the actions of subtype-selective antimuscarinic agents, can distinguish four subtypes of muscarinic acetylcholine receptors (M1–M4). Molecular techniques have identified five muscarinic receptor subtypes (M1–M5) arising from five separate genes (6). Both M2 and M3 muscarinic receptor subtypes are found in most smooth muscles. The M2 receptor preferentially couples to the inhibition of adenylyl cyclase through the Gi family of proteins, while the M3 receptor preferentially couples to inositol triphosphate (IP3) generation and calcium mobilization through the Gq family of proteins (6). Pertussis toxin (PTX), which ADP ribosylates and therefore inactivates the Gi family of proteins, has no apparent effect on contraction (14). Even though the M2 muscarinic receptor density is greater than the M3 receptor density in bladder and other smooth muscles, the affinity of subtype-selective muscarinic receptor antagonist drugs indicates that contraction is mediated by the M3 receptor in most smooth muscles under normal conditions.
A number of studies have shown that under certain conditions the M2 receptor subtype can contribute to the contractile response. This includes selective alkylation of M3 receptors in an environment of increased intracellular levels of cAMP in the rat urinary bladder (5, 11), guinea pig ileum (8), and trachea (19) or after alkylation without having to raise intracellular cAMP levels in other tissues such as the guinea pig gallbladder (2) and colon (13). Other studies of smooth muscle contraction after experimentally induced pathologies, for example in a cat model of experimentally induced esophagitis (16), in the denervated rat bladder (3), and in a model of acute cholecystitis in the guinea pig gallbladder (2), also suggest that the M2 receptor participates in mediation of contraction. In addition, in otherwise normal tissues, the M2 receptor appears to mediate contraction after inhibition of the sarcoplasmic reticulum calcium ATPase, Gq (16), phosphatidyl-inositol (PI)-specific phospholipase C (PI-PLC), phosphatidylcholine-specific phospholipase C (PC-PLC), or protein kinase C (PKC; Ref. 4). Treatment with 4-DAMP mustard causes a greater decrease in muscarinic agonist affinity for stimulating contraction in guinea pig colon from animals exposed to PTX for 3 days than in non-PTX-treated animals. However, 4-DAMP mustard is equally effective in decreasing agonist affinity for stimulating PI turnover in PTX-treated animals compared with normal animals. This suggests that signal transduction mechanisms unrelated to PI turnover such as those activated by the M2 subtype participate in the contractile response (14). Taken together, these findings suggest that the M2 and M3 receptors, through different second messenger pathways, interact to result in smooth muscle contraction.
When two drugs with overtly similar actions are administered together, the combination may produce effects that are significantly greater (superadditive or synergistic) or less (subadditive) than the simple additive effects calculated from the individual drug potencies. One explanation for a departure from additivity is an interaction between the drug targets involved in the response. To determine whether an interaction exists between the M2 and M3 receptor subtypes in normal urinary bladder tissue, the inhibitory potencies of combinations of methoctramine (M2-selective antagonist) and para-fluoro-hexahydro-sila-diphenidol ( p-F-HH-SiD; M3-selective antagonist) for reversing carbachol-induced contractions by competitive antagonism (inhibition) were measured. Similar experiments using 3-day denervated rat bladder tissue were performed for comparison. Using the inhibitory potency of each drug alone, the theoretical additive potency of each combination of methoctramine and p-F-HHSiD (i.e., 10:1, 1:1, and 1:10 wt:wt of methoctramine to p-F-HHSiD) was determined. Comparison of the actual inhibitory potencies of these drug combinations with those predicted from simple additivity allowed identification of certain antagonist combinations with inhibitory potencies that were significantly different from those predicted by simple additivity. These comparisons were made using both normal and denervated rat bladder. Because Sohn et al. (16) have shown that treatment of lower esophageal sphincter smooth muscle cells with thapsigargin (sarcoplasmic reticulum calcium ATPase inhibitor) alters the contractile mechanism such that the M2 muscarinic receptor is involved in mediation of contraction, these comparisons were also made in normal bladder exposed to thapsigargin. Revealing an interaction between the second messenger systems activated by the M2 and M3 receptor subtypes resulting in smooth muscle contraction may allow for the development of superior drugs for the treatment of overactive bladder or urinary incontinence and may help explain the clinical efficacy of the currently available antimuscarinic agents for the treatment of overactive bladder.
METHODS
Materials
The following drugs or chemicals were obtained from the sources indicated: carbachol and thapsigargin were from Sigma Chemical (St. Louis, MO), and methoctramine and p-F-HHSiD were from Research Biochemicals International (Natick, MA).
Surgery
Rats (200 to 250-g female Sprague-Dawley rats from Ace Animals, Boyertown, PA) were anesthetized with 2% isoflurane in oxygen, and a midline incision was made in the lower abdomen. The pelvic plexus was exposed. For bilateral denervation, both the left and right major pelvic ganglion were electrocauterized with a Valleylab (Boulder, CO) hand stitching pencil attached to a model SSE 2 solid-state electrosurgery device (Valleylab). For sham-operated animals, the plexus was exposed but left intact. After surgery, because the denervated animals were unable to micturate, urine was expressed by manual pressure on the lower abdomen of the denervated and sham-operated animals twice daily for 3 days.
Muscle strips
Urinary bladders were removed from rats euthanized by decapitation. The urinary bladder body (tissue above the ureteral orifices) was dissected free of the serosa and surrounding fat; the epithelium was left in place. The bladder was divided in the midsagittal plane, then cut into longitudinal smooth muscle strips (approximately 4 × 10 mm). The muscle strips were then suspended with 1 g of tension in tissue baths containing 15 ml of modified Tyrode solution (in mM: 125 NaCl, 2.7 KCl, 0.4 NaH2PO4, 1.8 CaCl2, 0.5 MgCl2, 23.8 NaHCO3, and 5.6 glucose) and equilibrated with 95% O2-5% CO2 at 37°C. The muscle strips were tested for their ability to contract in response to electric field stimulation of 8 V, 30 Hz, and 1-ms duration. To determine cross-sectional area, at the conclusion of the experiment, we measured the length and weight of each muscle strip between the suspension clips. Cross-sectional area was determined by weight/length, which assumes a density of 1.0 for all muscle strips.
Inhibition of carbachol response
After equilibration of the muscle strips to the bath solution for 60 min, 10 μM carbachol (~10 times greater than the measured EC50 value) was added to each bath. Once a stable baseline was reached (~10 min), increasing concentrations of antagonist or combinations of antagonist were added every 5 min. For meaningful display of drug combinations, all combinations of antagonists are expressed as weight/weight ratios of methoctramine:p-F-HHSiD. For example, an 11 μg/ml concentration of a 10:1 combination contains 10 μg/ml of methoctramine and 1 μg/ml of p-F-HHSiD, resulting in a molar ratio of 5.2 mol of methoctramine per 1 mol of p-F-HHSiD. Every experiment contained time control strips where Tyrode solution was added in place of antagonist. The data are presented as the percent contraction at each antagonist concentration normalized to the contraction at 10 μM carbachol for 6–10 muscle strips per condition. During the course of the experiment, the tension in the time control strips gradually declined. As a result, the data were normalized by the percent decline of the time control strips such that the contractile response of the control strips always equaled 100% contraction.
Calculation of potency for inhibition levels
The potency of methoctramine, p-F-HHSiD, and the combinations for the different inhibitory levels was calculated as described by Tallarida (18). Briefly, these potencies were determined using nonlinear regression of the individual dose-effect curves. The predicted inhibitory potency of the combination is based on the inhibitory potencies of the individual antagonists, expressed as doses A and B, and the combination doses a and b, which together produce the same effect (50% inhibition of contraction) as dose A alone or dose B alone. An additive interaction relates these as a/A + b/B = 1, while superadditivity and subadditivity lead to the fraction sums being less than or greater than one, respectively. In other words, a subadditive action is one that requires greater doses (a, b), whereas a superadditive action requires lower doses (a, b) to get the same degree of inhibition (50%). For example, in normal animals the IC50 for methoctramine was 4.57 μg/ml and the IC50 for p-F-HHSiD was 0.29 μg/ml. Following simple additivity, a combination of these two compounds that contains one-half the IC50 of methoctramine (2.28 μg/ml) and one-half the IC50 of p-F-HHSiD (0.15 μg/ml) would be expected to inhibit 50% of contraction. If this combination produces significantly less than 50% inhibition, then this combination is subadditive. Conversely, if this combination produces significantly greater than 50% inhibition, this combination is synergistic. Significance between actual and predicted IC50 values was determined by the Student’s t-test with significance level P < 0.05.
RESULTS
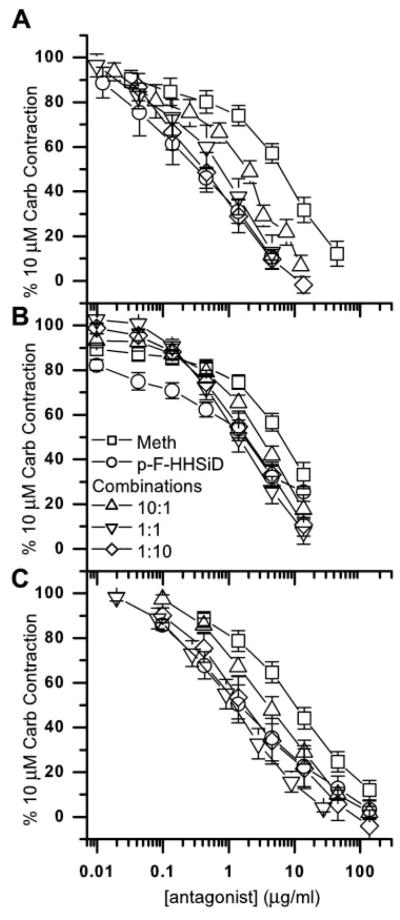
Denervated bladders weighed significantly more (P < 0.05) than sham-operated bladders (240 ± 15 vs. 96 ± 3 mg). However, muscle strips from denervated bladders contracted significantly less (P < 0.05) to electric field stimulation (8 V, 30 Hz, 1 ms) than control strips (3.7 ± 0.4 and 18.9 ± 1.5 mN/mm2, respectively). No differences in the maximal carbachol contraction were seen (sham operated, 33.9 ± 2.9 mN/mm2; denervated, 28.2 ± 2.2 mN/mm2). There were no significant differences in the contractile responses between normal bladders and bladders from sham-operated controls (data not shown); therefore, the data for normal and sham-operated controls were pooled (normal). As seen in Fig. 1, the addition of each of these muscarinic antagonists reversed the carbachol (10 μM)-induced contraction in a concentration-dependent manner. As shown in Fig. 2A, in normal rat bladder muscle strips, p-F-HHSiD (IC50 0.75 μM or 0.29 μg/ml) was just over eight times more potent in reversing contraction than methoctramine (IC50 6.2 μM or 4.57 μg/ml). However, in muscle strips from denervated urinary bladders (Fig. 2B), p-F-HHSiD (IC50 4.3 μM or 1.67 μg/ml) was only two times more potent in reversing contraction than methoctramine (IC50 8.8 μM or 6.51 μg/ml). These results are consistent with our previous study showing that the affinity of p-F-HHSiD for inhibiting contraction decreased after denervation (3). Addition of 1 μM thapsigargin to normal tissue had no effect on the maximal carbachol-induced contraction (43.7 ± 7.4 mN/mm2 for thapsigargin treated vs. 44.4 ± 4.8 mN/mm2 for normal) or the potency of carbachol for inducing contraction (EC50 = 2.25 ± 0.3 μM for thapsigargin treated vs. 2.10 ± 0.3 μM for normal). However, thapsigargin treatment increased the IC50 for methoctramine approximately twofold, from 4.57 μg/ml in normal to 9.7 μg/ml (13.3 μM). The IC50 for p-F-HHSiD increased more than fivefold, from 0.29 μg/ml in normal to 1.58 μg/ml (4.1 μM).
Fig. 1.
Inhibition of carbachol (Carb; 10 μM)-induced contraction of normal (A), denervated (B), and thapsigargin-treated rat urinary bladder muscle strips (C) in the presence of methoctramine (Meth; □), para-fluoro-hexahydro-sila-diphenidol (p-F-HHSiD; ○), and methoctramine:p-F-HHSiD wt/wt combinations [10:1 (△), 1:1 (▽), and 1:10 (◇)]. Data are presented as means ± SE of 6–10 muscle strips from at least 4 different animals per condition. A: normal. Hill slopes: 1.06 ± 0.09 (methoctramine), 0.76 ± 0.08* (p-F-HHSiD), 1.09 ± 0.03 (10:1 combination), 0.93 ± 0.09 (1:1 combination), and 0.76 ± 0.06* (1:10 combination). B: denervated. Hill slopes: 0.65 ± 0.08* (methoctramine), 0.64 ± 0.05* (p-F-HHSiD), 0.96 ± 0.07 (10:1 combination), 1.00 ± 0.04 (1:1 combination), and 0.88 ± 0.04* (1:10 combination). C: thapsigargin treated. Hill slopes: 0.82 ± 0.09 (methoctramine), 0.70 ± 0.07* (p-F-HHSiD), 0.79 ± 0.03 (10:1 combination), 0.96 ± 0.07 (1:1 combination), and 0.81 ± 0.06* (1:10 combination). *Hill slope significantly different from 1.0 (P < 0.05).
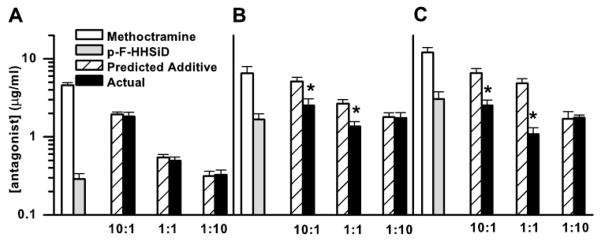
Fig. 2.
Average IC50 for inhibition of carbachol (10 μM)-induced contraction of normal (A), denervated (B), and thapsigargin-treated (C) rat urinary bladder muscle strips in the presence of methoctramine, p-F-HHSiD, and combinations of methoctramine and p-F-HHSiD along with the predicted additive inhibitory potencies of the combinations (wt:wt ratios of methoctramine:p-F-HHSiD). Data were derived from Fig. 1 and display means ± SE for doses that cause 50% inhibition (IC50) of contraction induced by 10 μM carbachol. *Significant difference (P < 0.05) between the predicted inhibitory potency based on simple additivity and the actual inhibitory potency of the combination (Student’s t-test).
Based on the individual inhibitory potencies of these antagonists, theoretical additive potencies for 50% inhibition of contraction for the three fixed-ratio combinations of methoctramine:p-F-HHSiD (10:1, 1:1, and 1:10 wt/wt) were calculated. The character of the interaction is most easily seen by comparing the actual and calculated doses based on simple additivity (Fig. 2). If the actual combination dose is lower than the calculated additive dose, then there is superadditivity (synergism); if it is higher, then there is subadditivity. In bladder strips from normal animals, all combinations were additive. The results from denervated animals were quite different. The 10:1 and 1:1 combinations were synergistic while the 1:10 combination was additive. Likewise, in bladder strips from normal animals treated with 1 μM thapsigargin, both the 10:1 and the 1:1 combinations were superadditive, while the 1:10 combination was additive, thus appearing more similar to the denervated preparation than the normal preparation.
DISCUSSION
Additivity of two antagonists implies independence, i.e., one agent may be substituted for the other in an amount that is calculated from their relative inhibitory potencies. A finding of subadditivity is consistent with a mechanism in which the two antagonists inhibit two pathways that are activated by the agonist and that attenuate each other. Alternatively, a finding of subadditivity could also be seen with two compounds that compete for a common receptor. Conversely, a finding of superadditivity is consistent with the two antagonists inhibiting two pathways that inhibit each other. In the normal rat bladder, the finding that all combinations of the two antagonists displayed simple additivity suggests that the contractile pathways activated by the M2 and M3 receptor subtypes appear to be functioning independently and that the two antagonists are sufficiently selective such that competition for the same receptor subtype (subadditivity) is not seen under these conditions.
Evidence for the existence of an interaction between the signal transduction pathways activated by the M2 and the M3 receptor subtypes is seen with normal rat bladder strips in the presence of thapsigargin. Thapsigargin treatment had no effect on the maximal carbachol-induced contraction or the potency of carbachol for inducing contraction. However, thapsigargin treatment altered the mechanism of contraction such that the 10:1 and the 1:1 combinations of methoctramine:p-F-HHSiD that were additive in normal (Fig. 2A) became superadditive after treatment (Fig. 2C), a change similar to the effects of denervation. We performed immunoprecipitation assays to confirm that thapsigargin exposure for 30 min did not change the density of muscarinic receptor subtypes, unlike denervation, where the density of M2 receptors increased and the density of M3 receptors decreased (3, 4). Thus, at least for this pharmacological manipulation (in vitro thapsigargin exposure), the change in the mechanism of smooth muscle contraction mediated by M2 and M3 muscarinic receptors is not related to changes in receptor densities. The results of this series of experiments support the hypothesis that two interacting contractile mechanisms exist in normal tissue, one mediated by the M2 receptor subtype and the other mediated by the M3 receptor subtype, which may interact through calcium mobilization. While the interaction of the contractile pathways activated by the M2 and the M3 receptor subtypes is apparently masked in normal tissue, treatment with thapsigargin reveals the interaction. These results provide functional evidence that both the M2 and the M3 receptor subtypes are found on smooth muscle cells and mediate contraction.
M2 receptors are traditionally thought to preferentially couple to PTX-sensitive G proteins such as the Gi subfamily, resulting in inhibition of adenylyl cyclase. An M2-mediated contractile response in bladder muscle can be demonstrated after the majority of M3 receptors are inactivated in an environment of increased intracellular cAMP, such as during stimulation with a β-adrenergic agonist (5, 11). This pathway has been proposed to mediate contraction indirectly, merely by blocking β-adrenergic agonist-induced relaxation via increased cAMP (7). However, M2 receptors acting through Gi may also stimulate bladder contraction directly via PKC activation (17). As found in the cat lower esophageal sphincter, a low degree of muscarinic stimulation, and consequently a low degree of calcium mobilization, results in activation of PKC, whereas PKC activation is inhibited at higher intracellular calcium concentrations (15). Thus, in the face of normal calcium mobilization mediated by the M3 receptor sub-type, the signal transduction pathway mediated by the M2 subtype may be inhibited. This may explain the finding that in the presence of thapsigargin, which interferes with calcium signaling, the signal transduction pathways activated by the M2 and the M3 receptor subtype interact in a facilitatory manner to induce contraction.
The denervated preparation gave results markedly different from normal tissue. Here the combinations with the highest proportion of the M2-selective antagonist methoctramine (Fig. 2B) showed a synergistic inhibitory effect that reverted to simple additivity when its proportion in the mixture was reduced to one-tenth that of the M3-selective antagonist p-F-HH-SiD. These results in the denervated preparation support an interaction between subtypes in the model reported by Sawyer and Ehlert (14), which predicts that the M2 and M3 receptor subtypes interact in a facilitatory manner to mediate contraction of the guinea pig colon. However, our results in the normal rat bladder do not support a facilitatory interaction between muscarinic receptor subtypes for inducing contraction. Only after either denervation or thapsigargin treatment does this interaction become facilitatory. It is possible that the interaction between subtypes is different in the guinea pig colon, or that after 3 days of in vivo PTX treatment, the interaction between subtypes becomes altered. Denervation results in a twofold increase in the density of M2 receptors while the density of M3 receptors decreases ~50% (3, 4). It is possible that the alteration in the mechanism of contraction in the denervated preparation is related to changes in receptor density.
Does the M3 pathway inhibit the M2 pathway; does the M2 pathway inhibit the M3 pathway; or do both pathways inhibit each other? Synergistic analysis as performed in this study does not distinguish between these scenarios. However, some insight may be derived from the experiments with normal rat bladder in the presence of thapsigargin. In the presence of thapsigargin, the interaction of the subtypes not only becomes superadditive, but subtype-selective antagonist affinities suggest that the M2 receptor subtype is involved in mediation of contraction (4). Our interpretation is that thapsigargin inhibits some component of the M3 contractile pathway, and consequently the M2 pathway is not inhibited and thus mediates contraction. If activation of the M2 pathway inhibited the M3 pathway to a significant extent, one would predict that the M2 pathway would mediate contraction after selective alkylation of M3 receptor subtypes. However, when the majority of the M3 receptor subtypes in the rat bladder are inactivated by alkylation, and intracellular cAMP is not elevated, antagonist affinities remain consistent with M3 receptor-mediated contraction, suggesting that relatively low levels of M3 receptor activation are sufficient to inhibit the M2-mediated response. Consequently, the data presented here for normal tissue without thapsigargin suggest that no interaction between subtypes or their signal transduction systems exists. When analyzed in context with the results of the denervated and thapsigargin-treated tissue, we speculate that in normal bladder smooth muscle, the M3 contractile pathway inhibits the M2 contractile pathway such that only the M3 pathway is evoked.
A possible explanation for why the antagonists in combination did not show subadditive effects in normal tissue is because these experiments were performed with prestimulation of muscarinic receptors, which raised intracellular calcium and thus inactivated the M2 pathway before the addition of the antagonists. Because these antagonists are only ~10-fold selective, it may not be possible to lower intracellular calcium enough to allow reactivation of the M2 pathway without also inhibiting it at the receptor level. Our previous receptor inactivation studies with 4-DAMP-mustard alkylation in normal tissue demonstrated that very low levels of the M3 receptor subtype are sufficient to mediate contraction (5). This suggests that virtually all M3 receptor activation must be blocked in normal tissue, or a component of the signal transduction pathway mediated by the M3 receptor subtype must be blocked with an inhibitor such as thapsigargin, to reveal the contractile pathway activated by the M2 receptor sub-type.
It is possible that inhibition of the M2 pathway by the M3 pathway in normal bladders is the result of one subtype inducing the release of a relaxant factor and that after denervation or thapsigargin treatment, the release of this factor is blocked and once blocked, the M2 and M3 receptor subtypes cooperate in a positive manner to result in contraction. However, if one subtype mediates the release of a relaxant factor, while the other subtype induces smooth muscle contraction, then as the antagonist inhibits the release of this inhibitory factor an increase in the magnitude of contraction would be expected. Our data (collected with the epithelium in place) are not consistent with this scenario, since no increase in the magnitude of contraction with any doses of antagonist or antagonist combinations occurred either in normal, thapsigargin-treated, or de-nervated bladders. In the rat urinary bladder, no urothelial relaxant factor has been identified; however, this has been shown in the pig bladder (10). However, Fovaeus et al. (9) describe a relaxant factor derived from rat bladder that is not of epithelial origin.
The above results support the existence of two independent contractile pathways in normal bladder smooth muscle, and the interaction between these pathways changes significantly after denervation or inhibition of calcium mobilization. In the denervated rat bladder, the finding of a superadditive inhibition with the combinations suggests that the M2 and M3 receptors (or the second messenger systems activated) interact in a facilitatory manner to mediate contraction. Therefore, after denervation, the contractile pathways activated in response to muscarinic stimulation change from inhibitory to facilitatory. This finding, if confirmed in human bladder, has important implications regarding the design of antimuscarinic agents for urologic use and may help explain the clinical efficacy of the non-subtype-selective antimuscarinic drug tolterodine (12) recently approved by the Food and Drug Administration for treatment of an overactive bladder. This is based on the recently proposed concept that bladder overactivity results from varying degrees of functional or actual motoneuron denervation of the detrusor (1). Our application of synergistic analysis to antagonist inhibition, usually used in agonist combination analysis, has provided strong quantitative evidence for the joint roles of both M2 and M3 muscarinic receptor subtypes in smooth muscle contraction.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-43333 (to M. R. Ruggieri) and National Institute of Drug Abuse Grant RO1-DA-09793 (to R. J. Tallarida).
References
- 1.Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57–73. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- 2.Braverman AS, Bartula LL, Myers SI, Parkman HP, Ruggieri MR. Inflammation changes the muscarinic receptor subtype and signal transduction pathway that mediates gall-bladder contraction. Gastroenterology. 2000;118:A197. [Google Scholar]
- 3.Braverman AS, Luthin GR, Ruggieri MR. M2 muscarinic receptor contributes to contraction of the denervated rat urinary bladder. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1654–R1660. doi: 10.1152/ajpregu.1998.275.5.R1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braverman AS, Ruggieri MR. Alterations in muscarinic receptor subtype function and second messenger systems involved in contraction of the denervated rat urinary bladder. Pharmacologist. 1999;41:205. [Google Scholar]
- 5.Braverman AS, Ruggieri MR. Selective alkylation of rat urinary bladder muscarinic receptors with 4-DAMP mustard reveals a contractile function for the M2 muscarinic receptor. J Recept Signal Transduct Res. 1999;19:819–833. doi: 10.3109/10799899909042875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caulfield MP. Muscarinic receptors—characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 7.Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 8.Ehlert FJ, Thomas EA. Functional role of M2 muscarinic receptors in the guinea pig ileum. Life Sci. 1995;56:965–971. doi: 10.1016/0024-3205(94)00035-q. [DOI] [PubMed] [Google Scholar]
- 9.Fovaeus M, Fujiwara M, Hogestatt ED, Persson K, Andersson KE. A non-nitrergic smooth muscle relaxant factor released from rat urinary bladder by muscarinic receptor stimulation. J Urol. 1999;161:649–653. [PubMed] [Google Scholar]
- 10.Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–419. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilvebrant L, Hallen B, Larsson G. Tolterodine—a new bladder selective muscarinic receptor antagonist: preclinical pharmacological and clinical data. Life Sci. 1997;60:1129. doi: 10.1016/s0024-3205(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 13.Sawyer GW, Ehlert FJ. Contractile roles of the M2 and M3 muscarinic receptors in the guinea pig colon. J Pharmacol Exp Ther. 1998;284:269–277. [PubMed] [Google Scholar]
- 14.Sawyer GW, Ehlert FJ. Muscarinic M3 receptor inactivation reveals a pertussis toxin-sensitive contractile response in the guinea pig colon: evidence for M2/M3 receptor interactions. J Pharmacol Exp Ther. 1999;289:464–476. [PubMed] [Google Scholar]
- 15.Sohn UD, Chiu TT, Bitar KN, Hillemeier C, Behar J, Biancani P. Calcium requirements for acetylcholine-induced contraction of cat esophageal circular muscle cells. Am J Physiol Gastrointest Liver Physiol. 1994;266:G330–G338. doi: 10.1152/ajpgi.1994.266.2.G330. [DOI] [PubMed] [Google Scholar]
- 16.Sohn UD, Harnett KM, Cao W, Rich H, Kim N, Behar J, Biancani P. Acute experimental esophagitis activates a second signal transduction pathway in cat smooth muscle from the lower esophageal sphincter. J Pharmacol Exp Ther. 1997;283:1293–1304. [PubMed] [Google Scholar]
- 17.Sohn UD, Zoukhri D, Dartt D, Sergheraert C, Harnett KM, Behar J, Biancani P. Different protein kinase C isozymes mediate lower esophageal sphincter tone and phasic contraction of esophageal circular smooth muscle. Mol Pharmacol. 1997;51:462–470. [PubMed] [Google Scholar]
- 18.Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. Boca Raton, FL: Chapman Hall/CRC; 2000. [Google Scholar]
- 19.Thomas EA, Ehlert FJ. Involvement of the M2 muscarinic receptor in contractions of the guinea pig trachea, guinea pig esophagus, and rat fundus. Biochem Pharmacol. 1996;51:779–788. doi: 10.1016/0006-2952(95)02396-8. [DOI] [PubMed] [Google Scholar]