Abstract
The extent to which bone tissue composition varies across anatomic sites in normal or pathologic tissue is largely unknown, although pathologic changes in bone tissue composition are typically assumed to occur throughout the skeleton. Our objective was to compare the composition of normal cortical and trabecular bone tissue across multiple anatomic sites. The composition of cadaveric bone tissue from three anatomic sites was analyzed using Fourier transform infrared imaging: iliac crest (IC), greater trochanter (GT), and subtrochanteric femur (ST). The mean mineral:matrix ratio was 20% greater in the subtrochanteric cortex than in the cortices of the iliac crest (p=0.004) and the greater trochanter (p=0.02). There were also trends toward 30% narrower crystallinity distributions in the subtrochanteric cortex than in the greater trochanter (p=0.10) and 30% wider crystallinity distributions in the subtrochanteric trabeculae than in the greater trochanter (p=0.054) and the iliac crest (p=0.11). Thus the average cortical tissue mineral content and the widths of the distributions of cortical crystal size/perfection differ at the subtrochanteric femur relative to the greater trochanter and the iliac crest. In particular, the cortex of the iliac crest has lower mineral content relative to that of the subtrochanteric femur and may have limited utility as a surrogate for subtrochanteric bone.
Keywords: iliac crest, subtrochanteric femur, Fourier transform infrared imaging, cortical bone, material properties
INTRODUCTION
Although osteoporotic fractures are primarily localized to specific anatomic sites in the hip, spine, and forearm, osteoporosis is generally considered to be a systemic disease that affects the entire skeleton. Investigations of areal bone mineral density (aBMD) assessed by dual energy x-ray absorptiometry (DEXA) across multiple skeletal sites tend to support the view of osteoporosis as a generalized condition. Aeral BMD assessed at non-fracture sites and fracture sites correlates moderately to strongly with future fracture risk.6; 15; 26 Furthermore, a first fracture at one site is associated with increased risk of subsequent fractures at other sites.11; 22; 25 For example, a proximal humeral fracture independently quintuples the risk of a subsequent hip fracture in the first year after the humeral fracture.5 These data suggest that bone loss with osteoporosis occurs at multiple sites throughout the skeleton.
However, two-dimensional imaging modalities like DEXA cannot distinguish between changes in aBMD arising from alterations in bone thickness from those arising from alterations in tissue mineral density. In contrast, three-dimensional high resolution quantitative computed tomographiy (HR-pQCT) is capable of such a distinction. HR-pQCT assessment of volumetric bone mineral density (vBMD) revealed lower trabecular and cortical vBMD values for the distal radius and distal tibia in osteoporotic women with fragility fractures compared to healthy women without fractures.24 Spectroscopic characterization of bone tissue also showed that the material properties of bone tissue were altered with osteoporosis. Iliac crest biopsies from osteoporotic patients differ from controls in mineral and collagen properties assessed by Fourier transform infrared imaging (FTIRI) 4; 18 and Raman imaging.14 Moreover, these compositional changes at the iliac crest predict fracture at the hip and spine independently of aBMD,10 suggesting that pathologic changes in bone tissue properties occur throughout the skeleton, and not only at sites that fracture. Furthermore, in normal cancellous bone, the tissue-level bone mineral density distributions were found to be similar at multiple skeletal sites, including iliac crest, lumbar vertebra, patella, femoral head, and femoral neck.21 These findings suggest that cancellous tissue composition is similar across skeletal sites in the axial and appendicular skeleton.
However, bone tissue exhibits substantial heterogeneity within the skeleton, and pathologic changes occur nonuniformly across skeletal sites. In a study examining aBMD at the spine, hip, radius, and calcaneus in women with an average age of 67 years, 24% of women were classified in the lower aBMD tertile at all four sites, while 15% of women had one or more lower aBMD tertile sites and one or more upper aBMD tertile sites, respectively suggesting that osteoporosis may occur as a generalized or a regional disorder.8 Furthermore, postmenopausal bone loss is only moderately correlated across anatomic sites in the axial and appendicular skeleton, and correlation coefficients between hip and spine T-scores decrease with age from ~0.7 in 19-29-year-olds to ~0.5 for 80+-year-olds.9 At the tissue level, systematic variation in ash weight, total collagen, and noncollagenous proteins was observed across multiple anatomic sites in autopsy samples from the lumbar vertebra, iliac crest, calcaneus, tibial midshaft, and femoral neck,2; 17 in contrast to the findings of Roschger et al.,21 who observed similar cancellous bone mineral density distributions across skeletal sites. Thus, although variation in aBMD across skeletal sites has been extensively documented, much less is known about the variation in bone tissue composition within the skeleton, and the few studies that have examined bone composition at different skeletal sites2; 17; 21 have produced varying results.
Recently, identification of an atypical subtrochanteric fracture pattern associated with long-term bisphosphonate use has highlighted the subtrochanteric cortex as a region potentially preferentially affected by treatment-induced changes in bone tissue composition and microstructure.23 However, minimal compositional data are available for this site, which fractures rarely in comparison with the intertrochanteric or femoral neck regions typically associated with osteoporotic fractures of the proximal femur.1; 23 Although the extent to which bone tissue composition varies across anatomic sites in normal or pathologic tissue is largely unknown, the assumption of invariance in tissue properties across anatomic sites underlies the clinical use of diagnostic biopsies of the iliac crest, a site that rarely fractures. Our objective was to compare the composition of normal cortical and cancellous bone tissue at multiple sites in the iliac crest and proximal femur. We hypothesized that the tissue composition of the iliac crest is representative of that of sites in the proximal femur.
METHODS
Cadaveric tissue was collected from 10 donors (3 male, 7 female) ranging in age from 53 to 90 years (69 ± 12 y, mean ± SD) with no history of metabolic bone disease. From each cadaver one 8-mm-diameter cortico-cancellous bone biopsy was excised with a trephine from each of three sites: iliac crest (IC, 2 cm posterior to the anterior superior iliac spine), greater trochanter (GT), and subtrochanteric femur (ST, 1 cm distal to the lesser trochanter on the lateral aspect of the femur). All procedures were approved by the Institutional Review Board of the Hospital for Special Surgery.
The specimens were dehydrated in graded ethanols and embedded in poly methyl methacrylate (PMMA). Undecalcified sections were cut in the transverse plane to facilitate analysis of both cortical and trabecular tissue in the same sections. While most biopsies contained both cortical and trabecular bone, several specimens had no trabeculae, particularly at the subtrochanteric site, due to natural variation in the extent to which cancellous bone extends distally to the femoral diaphysis. Specifically, 7 donors (1, 3, 5, 6, 8, 9,10) lacked trabeculae at ST; 1 donor (8) lacked trabeculae at GT; and 1 donor (4) lacked trabeculae at IC. Additionally, 1 donor (4) had minimal detectable cortex at ST and GT. Thus, the sample numbers for each tissue type and site are as follows: Cortical IC n=10, Cortical ST n=9, Cortical GT n=9; Trabecular IC n=9, Trabecular GT n=9, and Trabecular ST n=3. For each of the 30 biopsies, 3 nonconsecutive, 1-μm-thick sections spaced a minimum of 10 μm apart were microtomed from the center of the core and placed on BaF2 windows (Spectral Systems, Hopewell Junction, NY, USA) for infrared imaging.
Fourier transform infrared imaging (FTIRI) was used to analyze three sections from each biopsy. Within each section, three cortical and three trabecular FTIR images (64 × 80 pixels2, 400 × 500 μm2) over the spectral range 800-2000 cm−1 were collected at a spectral resolution of 4 cm−1 and a spatial resolution of 6.25 μm using an infrared imaging system (Spotlight 300, PerkinElmer Instruments, Waltham, MA, USA). Background (BaF2 window only) and PMMA spectra were also collected for each section and used to correct the sample spectra. Spectra were baseline corrected, and the PMMA spectral contribution was subtracted using chemical imaging software (ISys, Malvern Instruments, Worcestershire, UK). The infrared spectrum at each pixel was analyzed to determine 4 FTIR parameters:3 the mineral:matrix ratio (area ratio of the phosphate ν1/amide I peaks), which characterizes tissue mineral content; the carbonate:phosphate ratio (area ratio of the carbonate/phosphate ν1 peaks), which characterizes the extent of carbonate substitution into the mineral lattice; the collagen maturity (XLR, intensity ratio of 1660 cm−1/ 1690 cm−1 bands), which is related to the ratio of nonreducible to reducible collagen crosslinks;19 and the mineral crystallinity (XST, intensity ratio of 1030 cm−1/1020 cm−1 bands), which is related to crystal size assessed by x-ray diffraction.20
The FTIR parameter values calculated at each of the bone pixels within each image yielded a distribution of values for each FTIR parameter. Each distribution was characterized by two outcome variables: (1) the mean of the distribution and (2) the full width at half maximum (FWHM) of the Gaussian curve fit to the distribution, which provided a measure of tissue compositional heterogeneity (Fig. 1). For each parameter, the mean and FWHM values for each of the images were averaged to obtain mean and FWHM values for each specimen. Thus, for each sample the following 8 outcomes were assessed separately for cortical and trabecular bone: mean mineral:matrix, FWHM mineral:matrix, mean carbonate:phosphate, FWHM carbonate:phosphate, mean XLR, FWHM XLR, mean XST, and FWHM XST.
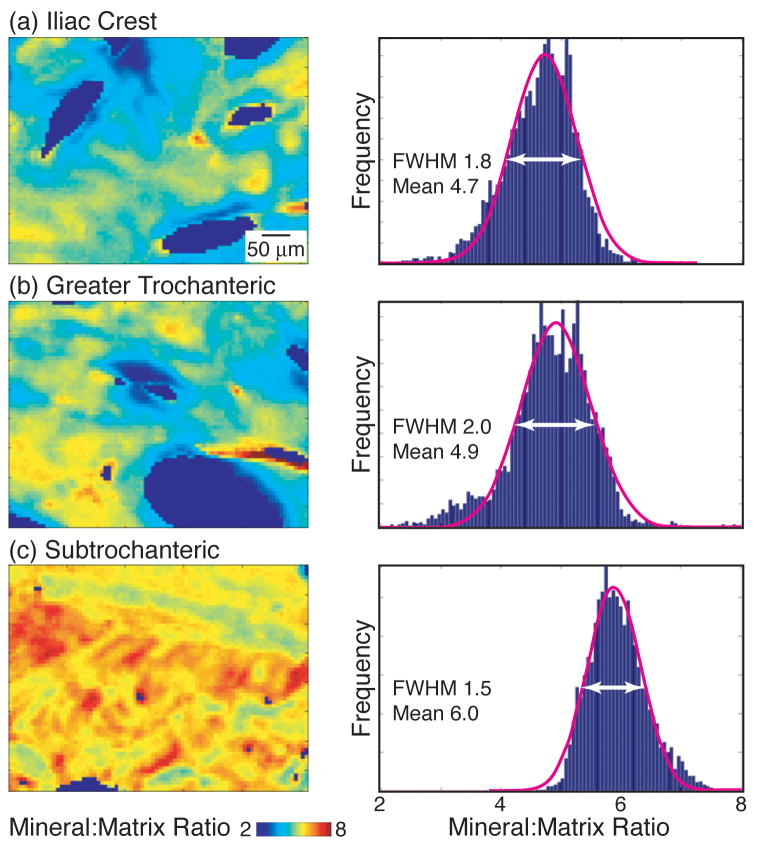
Figure 1.
Representative FTIRI mineral:matrix ratio images and corresponding pixel histograms listed with mean and full width at half maximum (FWHM) values for the Gaussian curves fit to each distribution for cortical bone at the (a) iliac crest, (b) greater trochanter, and (c) subtrochanteric femur.
Initial analyses of the differences between outcomes by site were performed using one-way analysis of variance with Bonferroni post hoc adjustment for multiple pairwise comparisons. To account for the clustering effect of the variables contained in each donor, generalized estimating equation models were created to identify predictors for each of the eight outcomes in both cortical and trabecular bone. In addition to donor site, donor age and sex were also included in the analysis to determine their effect on the each outcome. Two-sided pairwise comparisons were performed, and variables that achieved a p-value of 0.05 or less were considered statistically significant. All analyses were performed using SAS software version 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
When the FTIR parameter distribution means were examined within donors, the mineral:matrix ratio exhibited the greatest variability across anatomic sites, whereas the carbonate:phosphate ratio, collagen maturity, and crystallinity varied minimally from site to site in cortical bone (Fig. 2a) and trabecular bone (Fig. 3a). When the FTIR parameter distribution means were examined across all donors, the cortical mean mineral:matrix ratio was greater in the subtrochanteric femur (5.7 ± 0.58) than in the iliac crest (4.7 ± 0.66, p=0.004) and the greater trochanter (4.7 ± 0.57, p=0.019) (Fig. 1, 4a). The means of all other cortical and trabecular FTIR parameter distributions were similar across anatomic sites (Fig. 4a).
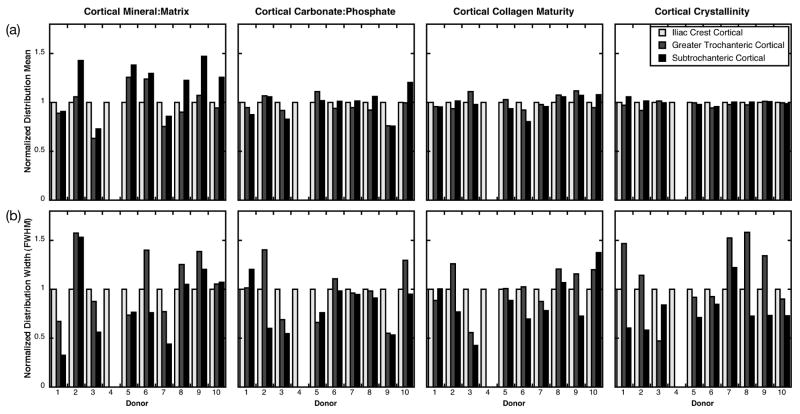
Figure 2.
FTIR parameter distribution (a) means and (b) full widths at half maximum (FWHM) for cortical bone from each of the 10 donors. Values have been normalized to those at the iliac crest for each donor to facilitate visual comparison across sites. Missing bars indicate that the tissue type was not present in the biopsy.
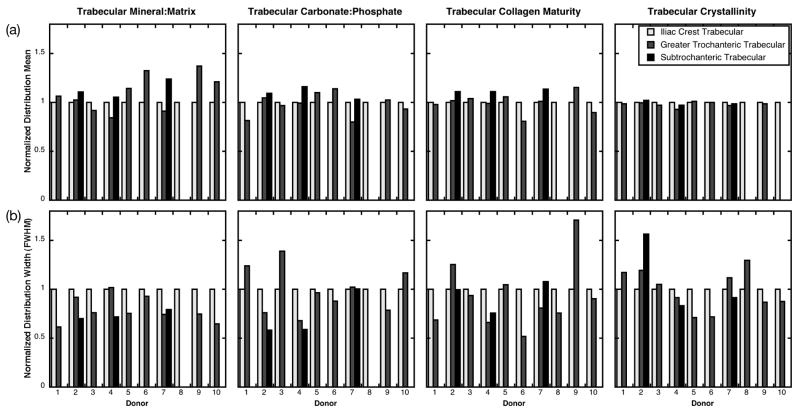
Figure 3.
FTIR parameter distribution (a) means and (b) full widths at half maximum (FWHM) for trabecular bone from each of the 10 donors. Values have been normalized to those at the iliac crest for each donor to facilitate visual comparison across sites. Missing bars indicate that the tissue type was not present in the biopsy.
Figure 4.
FTIR parameter distribution (a) means and (b) full widths at half maximum averaged over the 10 donors for 3 anatomic sites: iliac crest (IC) greater trochanter (GT), and subtrochanteric femur (ST). Error bars indicate standard deviations. * p < 0.05 vs. IC, ** p < 0.05 vs. GT, ^ p < 0.1 vs. IC, ^^ p < 0.1 vs. GT. (Sample sizes for each tissue type and site: Cortical IC 10, ST 9, GT 9; Trabecular IC 9, GT 9, ST 3.)
When the FTIR parameter distribution widths were examined within donors, they were generally more variable across anatomic sites than the distribution means (Fig. 2, 3). When the FTIR distribution widths were examined across all donors, there was a trend in the cortical tissue that did not reach statistical significance toward reduced width of the crystallinity distribution of the subtrochanteric femur (0.10 ± 0.037) relative to that of the greater trochanter (0.14 ± 0.53, p =0.10) (Fig. 4b). In the trabecular tissue, there was a trend that did not reach statistical significance toward greater width of the crystallinity distribution of the subtrochanteric femur (0.17 ± 0.032) relative to that of the greater trochanter (0.13 ± 0.025, p=0.054) and the iliac crest (0.13 ± 0.015, p=0.11) (Fig. 4b). The distribution widths for all other cortical and trabecular FTIR parameters were similar across anatomic sites (Fig. 4b).
When the effects of donor age and sex on the FTIR properties were examined, age and sex were not significant factors in predicting any of the outcomes. Furthermore, the interaction terms between age, sex, and site were not significant for any of the outcomes.
DISCUSSION
The objective of this study was to compare the compositional properties of normal cortical and cancellous bone tissue across three anatomic sites: iliac crest, greater trochanter, and subtrochanteric femur. Contrary to our hypothesis, cortical and trabecular material properties varied across anatomic sites within donors. Within donors, with the exception of the cortical mineral:matrix ratio, the mean properties were similar across sites, with each parameter typically within 10–20% of the value at the iliac crest (Fig. 2a, 3a); but the distribution widths were more variable across sites (Fig. 2b, 3b). When properties were examined across all donors, the mean cortical tissue mineral content was 20% greater in the subtrochanteric femur than in the iliac crest or the greater trochanter; the other cortical and trabecular FTIR parameter distribution means were not different across sites (Fig. 4a). In addition, despite the site-to-site variability in distribution widths observed within donors (Fig 2b, 3b), this variation did not reach statistical significance when examined across all donors (Fig. 4b). In the cortical tissue, there was a trend toward 30% narrower crystallinity distributions of the subtrochanteric femur than those of the greater trochanter. In the trabecular tissue, there was also a trend toward 30% wider crystallinity distributions of the subtrochanteric femur versus those of the iliac crest and the greater trochanter, although the limited number of biopsies at with trabeculae at the subtrochanteric femur (n=3 ST vs. 9 GT and 9 IC) limit the generalizability of this observation. In summary, these findings indicate that the average cortical tissue mineral content as well as the width of the distribution of cortical crystal size/perfection at the subtrochanteric femur differ from those at the greater trochanter and the iliac crest.
Studies examining trabecular bone composition at multiple anatomic sites in the axial and appendicular skeleton have yielded varying results. The trend observed in the current study toward 7% greater mineral:matrix ratio in trabeculae at the femoral sites versus those at the iliac crest is consistent with the 2.5% greater ash fraction of femoral neck trabeculae relative to iliac crest trabeculae observed previously.2 In contrast, the mean, peak, and width of the bone mineral density distribution assessed by quantitative backscattered electron imaging were constant in trabeculae of the iliac crest, L4 vertebra, patella, femoral neck, and femoral head.21 Neither previous study examined the subtrochanteric femur, hindering direct comparison with the data of the current study. To our knowledge no similar data exist for cortical bone mineral and collagen properties across anatomic sites.
Nevertheless, quantitative computed tomographic approaches for assessment of vBMD at peripheral and central skeletal sites can give insight into mineral density variations across anatomic sites. A correlation coefficient of 0.68 was observed when relationships between lumbar spine and hip vBMD assessed by central quantitative computed tomography (cQCT) were examined in premenopausal osteoporotic women.13 However, cQCT is limited by its inability to resolve trabeculae and to distinguish cortical and trabecular bone. With higher resolution at peripheral sites, correlation coefficients of 0.73–0.74 for trabecular bone and 0.40–0.75 for cortical bone were observed when relationships between distal radial and distal tibial vBMD assessed by HR-pQCT were examined in normal, osteopenic, and osteoporotic women.13; 24 Thus, moderate-to-strong correlations in mineral density have been observed between the lumbar spine and hip and between the distal radius and distal tibia using computed tomographic techniques; however, the larger length scale of CT assessment of mineral density (spatial resolution: ~940 um for cQCT and 82 um for HR-pQCT) compared with FTIRI (7 um) hinders comparison of mineral density measures across these techniques.
The differences in bone tissue composition observed across sites in this study may correspond to differences in mechanical behavior. Mean compositional parameters assessed by FTIRI are indicators of bone quality that contribute to fracture risk independently of aBMD. Specifically, increased cortical mean mineral:matrix ratio, crystallinity, and collagen maturity are associated with increased fracture risk.10 In the current study, the subtrochanteric cortex had a 20% greater mineral:matrix ratio than the cortices of the greater trochanter or the iliac crest. While increased tissue mineral content strengthens and stiffens bone, it is also associated with reduced postyield work to failure.7; 12 Thus, the tissue of the subtrochanteric femur may be stronger and stiffer but also more brittle relative to that of the greater trochanter and iliac crest. The naturally high mineral content may also make the subtrochanteric cortex especially susceptible to atypical fractures when subject to the tissue embrittlement processes associated with bisphosphonate treatment.23
This investigation was the first to our knowledge to examine the composition of human cortical and trabecular tissue together across multiple anatomic sites and to examine the composition of the subtrochanteric femur. One limitation of the study is that it examined tissue properties of donors without metabolic bone disease, and the results may not be generalizable to patients with metabolic bone diseases such as osteoporosis. Several trends that did not reach statistical significance were observed across anatomic sites, and the sample size of the current study (10 donors) may have limited our ability to detect subtle differences in tissue properties. Nevertheless, the sample size was sufficient to detect significant site-to site differences in mean mineral:matrix ratio, a parameter significantly associated with increased fracture risk in a multiple logistic regression analysis of iliac crest tissue from postmenopausal women.10 In addition, post-hoc power analyses indicated that 60–110 donors would be needed, depending on the outcome, to find statistical significance of the effects of age and sex and the associated interaction terms tested with at least 80% power. However, the statistical tools we used in the study allowed us to obtain a snapshot of the effects of all variables that we wanted to account for in the models. Furthermore, the clustered study design allowed characterization of tissue properties across multiple sites within individual donors.
Taken together, our results demonstrate variability in tissue compositional properties across anatomic sites in the iliac crest, greater trochanter, and subtrochanteric femur. In non-pathologic cadaveric bone, the cortices of the iliac crest and greater trochanter have lower mineral content relative to the cortex of the subtrochanteric femur, suggesting that the iliac crest may have limited utility as a surrogate for subtrochanteric bone. The observed differences in bone tissue properties across anatomic sites are consistent with previously observed variation in bone tissue composition and mechanical properties across multiple anatomic sites.2; 16 These findings suggest that the use of tissue from an easily accessible site such as the iliac crest to estimate the material properties of a site of clinical interest such as the subtrochanteric femur should be approached with caution and prior validation.
Acknowledgments
This work was supported by NIH F32 AR561482 to ED and P30 AR046121 and R01 AR043125 to ALB. JN was partially supported by NIH Clinical Translational Science Center UL1-RR024996. We thank Dr. Brian Gladnick, Dr. Aasis Unnanuntana, Bradley Jensen, Jennifer Hammann, and Dr. Andrew Pearle for assistance with obtaining cadaveric specimens.
References
- 1.Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res. 2009;24:1095–1102. doi: 10.1359/jbmr.081247. [DOI] [PubMed] [Google Scholar]
- 2.Aerssens J, Boonen S, Joly J, Dequeker J. Variations in trabecular bone composition with anatomical site and age: potential implications for bone quality assessment. J Endocrinol. 1997;155:411–421. doi: 10.1677/joe.0.1550411. [DOI] [PubMed] [Google Scholar]
- 3.Boskey A, Mendelsohn R. Infrared analysis of bone in health and disease. J Biomed Opt. 2005;10:031102. doi: 10.1117/1.1922927. [DOI] [PubMed] [Google Scholar]
- 4.Boskey AL, Spevak L, Weinstein RS. Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int. 2009;20:793–800. doi: 10.1007/s00198-008-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinton J, Franta A, Polissar NL, et al. Proximal humeral fracture as a risk factor for subsequent hip fractures. J Bone Joint Surg Am. 2009;91:503–511. doi: 10.2106/JBJS.G.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–668. [PubMed] [Google Scholar]
- 7.Currey JD. Tensile yield in compact bone is determined by strain, post-yield behaviour by mineral content. J Biomech. 2004;37:549–556. doi: 10.1016/j.jbiomech.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Davis JW, Ross PD, Wasnich RD. Evidence for both generalized and regional low bone mass among elderly women. J Bone Miner Res. 1994;9:305–309. doi: 10.1002/jbmr.5650090303. [DOI] [PubMed] [Google Scholar]
- 9.Deng HW, Li JL, Li J, et al. Heterogeneity of bone mineral density across skeletal sites and its clinical implications. J Clin Densitom. 1998;1:339–353. doi: 10.1385/jcd:1:4:339. [DOI] [PubMed] [Google Scholar]
- 10.Gourion-Arsiquaud S, Faibish D, Myers E, et al. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res. 2009;24:1565–1571. doi: 10.1359/JBMR.090414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 12.Les CM, Stover SM, Keyak JH, et al. Stiff and strong compressive properties are associated with brittle post-yield behavior in equine compact bone material. J Orthop Res. 2002;20:607–614. doi: 10.1016/S0736-0266(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 13.Liu XS, Cohen A, Shane E, et al. Bone density, geometry, microstructure, and stiffness: Relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res. 2010;25:2229–2238. doi: 10.1002/jbmr.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCreadie BR, Morris MD, Chen TC, et al. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone. 2006;39:1190–1195. doi: 10.1016/j.bone.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, et al. Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res. 1993;8:1227–1233. doi: 10.1002/jbmr.5650081010. [DOI] [PubMed] [Google Scholar]
- 16.Morgan EF, Bayraktar HH, Keaveny TM. Trabecular bone modulus-density relationships depend on anatomic site. J Biomech. 2003;36:897–904. doi: 10.1016/s0021-9290(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 17.Ninomiya JT, Tracy RP, Calore JD, et al. Heterogeneity of human bone. J Bone Miner Res. 1990;5:933–938. doi: 10.1002/jbmr.5650050906. [DOI] [PubMed] [Google Scholar]
- 18.Paschalis EP, Betts F, DiCarlo E, et al. FTIR microspectroscopic analysis of human iliac crest biopsies from untreated osteoporotic bone. Calcif Tissue Int. 1997;61:487–492. doi: 10.1007/s002239900372. [DOI] [PubMed] [Google Scholar]
- 19.Paschalis EP, Verdelis K, Doty SB, et al. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- 20.Pleshko N, Boskey A, Mendelsohn R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys J. 1991;60:786–793. doi: 10.1016/S0006-3495(91)82113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roschger P, Gupta HS, Berzlanovich A, et al. Constant mineralization density distribution in cancellous human bone. Bone. 2003;32:316–323. doi: 10.1016/s8756-3282(02)00973-0. [DOI] [PubMed] [Google Scholar]
- 22.Ross PD, Genant HK, Davis JW, et al. Predicting vertebral fracture incidence from prevalent fractures and bone density among non-black, osteoporotic women. Osteoporos Int. 1993;3:120–126. doi: 10.1007/BF01623272. [DOI] [PubMed] [Google Scholar]
- 23.Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 24.Vico L, Zouch M, Amirouche A, et al. High-resolution pQCT analysis at the distal radius and tibia discriminates patients with recent wrist and femoral neck fractures. J Bone Miner Res. 2008;23:1741–1750. doi: 10.1359/jbmr.080704. [DOI] [PubMed] [Google Scholar]
- 25.Wasnich RD, Davis JW, Ross PD. Spine fracture risk is predicted by non- spine fractures. Osteoporos Int. 1994;4:1–5. doi: 10.1007/BF02352253. [DOI] [PubMed] [Google Scholar]
- 26.Wasnich RD, Ross PD, Heilbrun LK, Vogel JM. Selection of the optimal skeletal site for fracture risk prediction. Clin Orthop Relat Res. 1987:262–269. [PubMed] [Google Scholar]