Abstract
Local cortical circuit activity in vivo comprises a complex and flexible series of interactions between excitatory and inhibitory neurons. Our understanding of the functional interactions between these different neural populations has been limited by the difficulty of identifying and selectively manipulating the diverse and sparsely represented inhibitory interneuron classes in the intact brain. The integration of recently developed optical tools with traditional electrophysiological techniques provides a powerful window into the role of inhibition in regulating the activity of excitatory neurons. In particular, optogenetic targeting of specific cell classes reveals the distinct impacts of local inhibitory populations on other neurons in the surrounding local network. In addition to providing the ability to activate or suppress spiking in target cells, optogenetic activation identifies extracellularly recorded neurons by class, even when naturally occurring spike rates are extremely low. However, there are several important limitations on the use of these tools and the interpretation of resulting data. The purpose of this article is to outline the uses and limitations of optogenetic tools, along with current methods for achieving cell type-specific expression, and to highlight the advantages of an experimental approach combining optogenetics and electrophysiology to explore the role of inhibition in active networks. To illustrate the efficacy of these combined approaches, I present data comparing targeted manipulations of cortical fast-spiking, parvalbumin-expressing and low threshold-spiking, somatostatin-expressing interneurons in vivo.
Keywords: interneuron, inhibition, fast-spiking, somatostatin, optogenetics, channelrhodopsin, halorhodopsin, archaerhodopsin, electrophysiology, tetrode, cortex
1. Introduction
Neural circuits in the cortex are comprised of extensively interconnected populations of excitatory glutamatergic neurons and inhibitory GABAergic neurons. These two broad classes can be further divided into distinct subpopulations. This is particularly true of inhibitory interneurons, which can be grouped based on their morphology, molecular expression profiles, intrinsic firing properties, and synaptic connectivity (Ascoli et al., 2008; Markram et al., 2004; Moore et al., 2010). The availability of diverse inhibitory cell types provides a critical degree of functional flexibility to the network and allows for a wide range of computational operations within the local circuit. In an active network in vivo, each neuron receives a continuous barrage of excitatory and inhibitory synaptic inputs that result in a high-conductance state (Destexhe et al., 2003). In turn, that neuron contributes spike output to the surrounding local network. This continuous reciprocal exchange poses a considerable challenge to determining the roles of specific classes of neurons in contributing to information processing and generating ongoing patterns of cortical activity. The distributed and diverse nature of inhibitory populations increases this difficulty.
Considerable recent interest has focused on the roles of different interneuron populations in local cortical circuit operations. In particular, the relative impact of somatic and dendritic synaptic inhibition in regulating input integration and sensory processing remains unclear. In vitro evidence suggests that the intrinsic properties and synaptic positions of soma- and dendrite-targeting interneurons may cause them to be differentially recruited by excitatory inputs during sensory stimulation or other active conditions (Moore et al., 2010). In addition, previous work suggests that these two sources of inhibition may affect their postsynaptic targets on different time scales. However, these hypotheses have been difficult to test due to the difficulty of identifying and directly manipulating specific interneurons in vivo. The functional roles of some extremely sparse populations of inhibitory cells have likewise been difficult to elucidate in the intact brain. The development of technologies that allow targeted manipulation of specific cell types is thus critical to understanding interneuron participation in local circuit activity and ultimately in behavior and cognition.
Optogenetics, which provides tools for bidirectional optical control of neural activity by incorporation of artificially generated light-sensitive proteins into the cell membrane, represents a significant advance in our ability to dissect neural circuits in vivo. Optical manipulations via light-activated channels and pumps represent a highly reliable and physiologically appropriate method for activating and suppressing the firing of specific neural populations both in vitro (Adesnik and Scanziani, 2010; Boyden et al., 2005; Kuhlman and Huang, 2008; Petreanu et al., 2007; Sohal et al., 2009) and in vivo (Cardin et al., 2009; Gradinaru et al., 2009; Han et al., 2009; Huber et al., 2008; Tsai et al., 2009). Considerable recent work has highlighted the use of cell type-specific expression of the light-activated nonspecific cation channel Channelrhodopsin-2 (ChR2) and the light-activated chloride pump Halorhodopsin (eNpHR). The purpose of this review is to provide a detailed overview of the advantages and drawbacks of an experimental approach that integrates traditional electrophysiology and optogenetics and to present data illustrating the power of cell type-specificity in causally testing hypotheses about the functions of discrete populations of inhibitory neurons in vivo.
1.1 Advantages
An integrative approach utilizing optical and electrophysiological methods provides two major advantages for in vivo exploration of network dynamics. Traditional extracellular recording techniques suffer from a lack of ability to identify the recorded neurons by class. Some cell types, such as fast-spiking interneurons, can be putatively identified by their characteristic waveform shape. However, this characterization has proven to be less than uniformly accurate. Most other types of inhibitory interneurons have broad spike waveforms and are indistinguishable from excitatory neurons in extracellular recordings. Furthermore, inhibitory cells are sparsely represented in the cortex and often have very low spontaneous firing rates, making them difficult to find. Inhibitory cells can be identified post-hoc by recovering and staining intracellularly recorded neurons, but in vivo yields from these experiments are relatively low (Cardin et al., 2007; Hirsch et al., 2003). The reliability and precision of optically evoked firing, in.combination with cell type-specific expression of ChR2, offers an unambiguous method for extracellular identification of cell type. Cells expressing ChR2 respond to brief light pulses with spikes at short latency and a high degree of reliability, allowing the experimenter to rigorously identify the subpopulation of recorded neurons belonging to the targeted cell class (Cardin et al., 2009, 2010; Lima et al., 2009). Multielectrode recordings can thus generate a higher yield of identified recordings. In addition, a major advantage for array recordings of large populations is the ability to artificially evoke many thousands of spikes from cells with normally low spontaneous firing rates, thereby enhancing spike waveform discrimination. A second, more obvious, advantage of the combined experimental approach is the ability to directly manipulate the level of activity of a specific class of cell in the context of observing ongoing network activity or behavior.
1.2 Caveats
There are several inherent limitations on currently available optogenetic approaches and on their incorporation with traditional electrophysiological techniques. As discussed in section 2.1, cell type specificity is limited by the choice of promoters, mouse lines, and vectors, making some cell types difficult to target. In addition, because ChR2 is distributed across the entire cell, light-evoked activation of ChR2 often causes conductance changes and depolarization simultaneously across entire targeted neurons, including the dendrites, cell body, and axon. It remains unclear whether calcium influx and synaptic vesicle release in response to this large, cell-wide depolarization is equivalent to that observed in response to naturally occurring spiking (Zhang et al., 2008). An additional concern is the relatively slow temporal kinetics of the onset and offset of ChR2, leading to light-evoked conductances and depolarization events that are significantly longer than naturally occurring events (Boyden et al., 2005; Gradinaru et al., 2010). Current optogenetic methods may thus be suboptimal for studies of synaptic transmission either in vitro or in vivo.
Because ChR2 activation generates robust current flow through ion channels, it can also produce misleading local field potential signals. For instance, activation of large layer 5 pyramidal neurons by light pulses at the cortical surface will preferentially depolarize the apical dendritic tufts of these cells. This localized current flow produces a superficial current sink, easily observed in the local field potential but unrelated to synaptic activity. Optoelectric artifacts, discussed below, can further obscure local field potential signals (Cardin et al., 2010; Han et al., 2009). An additional potential caveat to the use of optogenetic tools is the lack of any data on the cellular impact of long-term expression of these exogenous proteins. The potential for cell health to be compromised by high levels of expression of artificial rhodopsins over long periods remains unexplored.
2. Practical Concerns
2.1 Expression of optogenetic tools
In utero electroporation in rats and mice (Huber et al., 2008), constitutive and inducible expression in transgenic mice (Arenkiel et al., 2007), and viral approaches either alone or in association with the well-characterized Cre-loxP system (Cardin et al., 2009; Sohal et al., 2009; Tsai et al., 2009) have all been used to achieve expression of light-activated channels and pumps. Each method has advantages and disadvantages for achieving functional levels of cell type-specific expression. Generation of transgenic mice expressing ChR2 under the control of a cell type-specific promoter is highly effective, but relatively expensive and time-intensive. In addition, little is known about potential toxicity due to long-term expression of optogenetic tools at high levels. In utero electroporation can be targeted to general cell classes with relative ease by restricting the treatment to particular sites and specific prenatal days (Marin and Rubenstein, 2003; Saito and Nakatsuji, 2001; Tabata and Nakajima, 2001, 2002, 2003; Wonders and Anderson, 2005). This approach confers the advantage of whole-brain expression with potentially high levels of cell type specificity. However, cell-type specificity using in utero electroporation is somewhat limited to the resolution provided by cell birth date and broad localization within the germinal zones (Borrell et al., 2005). Combining this approach with appropriate cell type-specific promoters may potentially yield a higher degree of specificity (LoTurco et al., 2009; Matsuda and Cepko, 2007).
One frequently used approach to the expression of optogenetic tools in neurons has been the use of lentivirus or adeno-associated virus (AAV) for viral transduction in vivo. Viral vector production is relatively inexpensive, and virus can be injected directly into target brain regions with limited tissue damage and a high degree of resulting transduction efficacy. Lentiviral vectors expressing optogenetic tools have been used in rodent and primate cortex, but do not spread substantially around the initial site of delivery (Boyden et al., 2005; Gradinaru et al., 2009; Han et al., 2009; Huber et al., 2008; Jasnow et al., 2009). Lentiviruses also exhibit significant tropism for excitatory neurons (Nathanson et al., 2009) and may be suboptimal for targeting inhibitory interneurons. AAVs, which have been used in human gene therapy trials (Fu et al., 2003; Taymans et al., 2007; Van Vliet et al., 2008), provide extensive spatial spread and high expression levels in cortex and hippocampus when used with appropriate promoters. Like lentivirus, AAV can be used effectively in a number of model systems, including rats, mice, and primates.
2.2 Achieving cell type-specificity with viral vectors
The well-characterized Cre-loxP system provides a robust method for targeting genes of interest to specific cell types. A more recent innovation, the FLEX (Atasoy et al., 2008) or ‘Double-floxed’ inverted (DIO) (Cardin et al., 2009; Sohal et al., 2009; Tsai et al., 2009) construct, has been used to produce enhanced specificity and high expression levels. In the frequently used AAV DIO ChR2-mCherry vector, for example, two incompatible loxP variants flank an inverted version of ChR2 fused to the fluorescent marker mCherry. In the presence of Cre recombinase, a stochastic recombination of either loxP variant occurs, resulting in inversion of ChR2-mCherry into the sense direction and expression of light-activated channels under the control of the strong promoter EF1a (Elongation Factor 1a) (Livet et al., 2007). Cre-dependent expression of light-activated proteins such as ChR2 and eNpHR is particularly well suited to targeting cell types that lack comprehensively identified promoter sequences. Cellular promoters specific for neural subtypes, such as parvalbumin and somatostatin, often exceed the size limitation imposed by using a viral vector. Cellular promoters alone may also lack the high degree of specificity or expression levels necessary for physiologically useful optogenetic manipulations. Effective use of optogenetic tools in vitro and in vivo depends on a minimal effective level of expression of the light-activated proteins, thus limiting the use of a small or weak promoter element (Sohal et al., 2009). This problem can be overcome by inserting a strong general promoter into the viral vector and utilizing the high degree of cell type specificity conferred by the Cre expression pattern of a transgenic line.
Most often, Cre-dependent vectors are constructed with a loxP-flanked transcriptional stop cassette preceding the gene of interest. However, the intrinsic size limitations of viral vectors preclude the use of large stop cassettes. Small stop cassettes frequently result in a low but consistent rate of expression of the gene of interest in the absence of Cre (Kuhlman and Huang, 2008; Sohal et al., 2009). The use of an inverted sequence in a double-floxed vector eliminates the need for a polyadenylation stop cassette in the viral construct, as expression is dependent on both Cre-dependent recombination and inversion events. The FLEX/DIO construct allows expression of light-activated channels specifically in a subpopulation of neurons that expresses Cre recombinase, with a low rate of erroneous expression. A large number of commercially available transgenic, BAC, and knock-in mouse lines express Cre in specific populations of excitatory neurons (e.g. CW2, T29-1, T29-2), inhibitory neurons (e.g. PV-Cre), or in a layer-specific manner (e.g., Six3-Cre, Ntsr1-Cre). The FLEX/DIO construct confers a high degree of specificity of expression of the gene of interest, but individual mouse lines exhibit varied degrees of specificity of Cre expression. A nonspecific Cre line will thus result in expression of light-activated channels in a varied population of cells, regardless of the construct used.
2.3 Hardware choices for optical stimulation
Stimulation via a laser or LED light source focused into an optical fiber is one of the simplest and most effective methods for light stimulation and is readily integrated with traditional electrophysiological approaches. Light from the point source at the tip of an optical fiber spreads in an approximate cone through neural tissue, although this can be modified by the inclusion of lenses at the fiber tip. Light power from a fiber at the surface of the brain decreases with tissue depth, resulting in variability of the effective light drive with distance from the light source (Aravanis et al., 2007). Surface positioning of an optical fiber provides effective stimulation of cells in the supragranular and granular layers of cortex. Light stimulation can also be delivered through mouse dura or thinned skull. Alternatively, optical fibers of small core diameter (10–100μm) can be placed in the brain tissue to stimulate deeper cells. Light intensity is measured in mW/mm2, and a safe stimulation range for in vivo stimulation is up to ~75mW/mm2 for short pulses (0.25 to 50ms). Sustained stimulation (>1s) at levels above 100mW/mm2 can result in tissue damage immediately under the site of optical stimulation. Very long or continuous stimulation can also cause abnormal activity patterns and excitotoxicity.
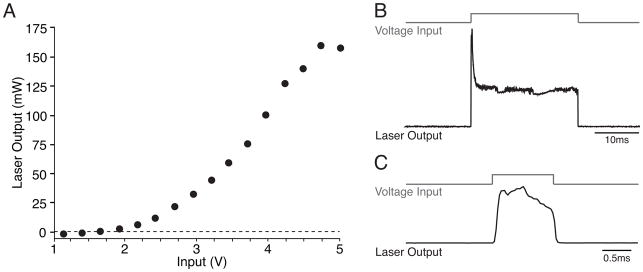
The pulsed output of a small laser can be significantly nonlinear, with an initial peak followed by a drop to a steady level (Fig. 1). In such a case, short pulses take advantage of the reliability of the initial peak and provide a stimulation waveform approximating a step function (Fig. 1b,c). For experiments requiring long pulses of stimulation, it may be advantageous to use a steady-state light source and either a mechanical or liquid crystal shutter or a Pockels cell to deliver timed pulses of light stimulation. Alternatively, LEDs provide a stable light source capable of generating a square pulse output. However, because LEDs produce uncollimated light, lenses are necessary to achieve a collimated beam for experimental use.
Figure 1. Nonlinear laser power output.
A. Many small lasers (50mW–1W) exhibit a nonlinear relationship between input voltage (V) and output power (mW). In this case, above a threshold value (~2V) output power rises sigmoidally as a function of voltage and then saturates. B. Laser output can also be nonlinear in the time domain. In many cases, a step function voltage input results in an initial transient peak in laser power output, followed by a drop to steady state and variable ongoing noise. C. Very short pulses take advantage of the repeatability of the initial peak and approximate a step function.
Power transmitted by an optical fiber will vary with fiber type (multimode or single mode) and numerical aperture (NA), with a small NA giving a narrow angle of light spread from the fiber and a high NA giving a broader angle of spread. Power will also vary as a function of the radius (r) of the fiber, as the area of the initial cross-section of the light at the tip of the fiber increases as πr2.
2.4 Optoelectric artifacts
Illumination of metal by a laser beam, either directly or via indirect reflection, results in electrical artifacts in recorded signals. These effects can be elicited by light hitting the tip of a metal electrode, a ground electrode, or a silver wire in a glass pipette (Cardin et al., 2010; Han et al., 2009). Optoelectric artifacts have been demonstrated in recordings from tungsten, platinum/iridium, nichrome, steel, gold, and silver electrodes and are proportional in amplitude to the total laser power and the amount of exposed metal. The onset of the artifact signal is aligned with the onset of the light stimulus, rather than showing the several millisecond delay typical of evoked neural activity. Light-evoked artifacts exhibit both an onset ramp that continues for the duration of the light if the pulse is brief (~1ms) and a slower offset time constant (~2–10ms decay time for a 1ms pulse) that can appear as an overshoot followed by a return to baseline. Optoelectric artifacts can contaminate both LFP and intracellular recordings and are independent of the presence of optogenetic tools. These artifacts have little impact on extracellular recordings of action potentials.
Several straightforward approaches have been successfully used to minimize the contribution of light-evoked artifacts to electrophysiological recordings. One effective method is to reduce the total exposure of metal to the light by limiting the duration of the light pulse and decreasing the area of the exposed metal surface. For field potential or intracellular recordings, glass electrodes with a distal metal wire insert that is not contacted by direct or reflected light are relatively free of artifacts. For field potential recordings made with glass electrodes, adding a non-reflective, opaque coating to the glass (e.g., acrylic paint or lacquer) further reduces light transmission to the silver wire. Tungsten electrodes with small exposed tip surfaces, such as twisted wire stereotrodes or tetrodes (diameter <20μm), exhibit smaller optoelectric artifacts than do electrodes with larger exposed surfaces, such as standard prefabricated tungsten electrodes (FHC, Inc.) or laminar silicon probes (Neuronexus, Inc). Minimization of light-evoked artifacts can be accomplished by 1) using thin wire electrodes and 2) adjusting the electrode angle so that minimal exposed metal lies directly in the cone of laser light beneath the tip of the optical fiber. Bipolar electrode recordings eliminate small amplitude optical artifacts by subtracting locally generated signals, but may not completely eradicate larger artifacts. Future development of recording technologies using alternative metals may also provide substrates that are less prone to optoelectric effects.
3. Integrated electrophysiological and optogenetic approaches
3.1 Activation vs suppression
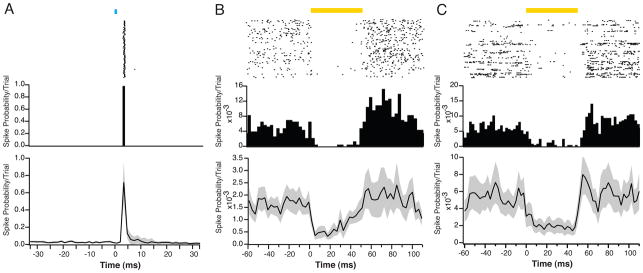
Recently developed optogenetic tools provide the means to activate or suppress neural activity in a cell type-specific manner with a high degree of temporal and spatial fidelity. Channelrhodopsin-2 is a nonspecific cation channel activated by blue light (~473nm) that passes Na+ and K+ and has some Ca2+ permeability. Opening of these ion channels causes conductance increases across the targeted neuron, and therefore alters biophysical membrane properties, such as the cellular input resistance and membrane time constant, for the duration of the activation. The recently released version of ChR2, ChETA, has a faster onset and offset and may be more appropriate for experiments requiring extremely accurate spike times (Gunaydin et al., 2010). However, even the original ChR2 can evoke repeated spikes from targeted cells with <1ms precision. This is particularly true of fast-spiking interneurons, which have a short membrane time constant and are easily activated by brief, strong depolarization (Fig. 2a).
Figure 2. Optogenetic tools for activation and suppression in vivo.
A. Raster plot (upper panel) and PSTH (middle panel) of the light-evoked activity of an FS-PV+, ChR2-expressing interneuron in layer 2/3 of visual cortex in a Parvalbumin-Cre mouse after injection of AAV-DIO-ChR2-mCherry. This cell produced one spike in response to each light pulse (1ms duration) with a latency of ~3.5ms and a reliability of ~100%. A population average (lower panel) shows a high degree of repeatability of this type of response (n = 62 cells). Black line indicates mean response, gray shading indicates SEM. All data in this and other figures were recorded using custom-built dense tetrode arrays and an optical fiber. B. Raster plot (upper panel) and PSTH (middle panel) of the activity of an example RS, eNpHR v3.0-expressing excitatory neuron in layer 2/3 of visual cortex in an Emx1-Cre mouse after injection of AAV-DIO-eNpHRv3.0-eYFP. On each trial, the neuron’s spiking was suppressed in response to a pulse of yellow light. The PSTH also shows a small increase in firing immediately after the light stimulus ended. Across the population of recorded neurons (n = 34, lower panel), eNpHR v3.0 activation eliminated ~65% of spikes, but demonstrated a slow decrease in efficacy during the light pulse. C. Raster plot (upper panel) and PSTH (middle panel) of an example RS, Arch-expressing excitatory neuron in layer 2/3 of visual cortex in an Emx1-Cre mouse after injection of AAV-FLEX-Arch-eGFP. On each trial, the neuron’s spiking was suppressed during the light pulse. Across the population of recorded neurons (n = 28, lower panel), Arch activation eliminated ~70% of spikes. PSTH bins are 2.5ms.
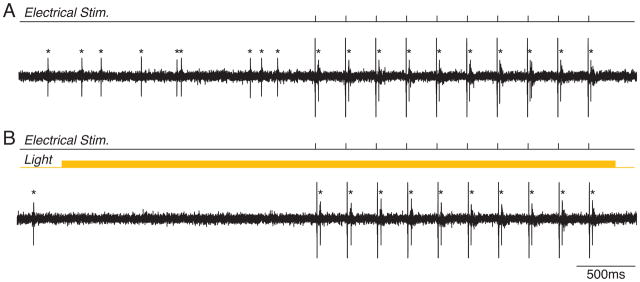
Two main tools for activity suppression are currently available: Halorhodopsin V3.0 and Archaerhodopsin (Arch) (Fig. 2b,c) (Chow et al., 2010; Han and Boyden, 2007; Zhao et al., 2008). eNpHR v3.0 is a Cl− pump activated by yellow (~593nm) light. In contrast, Arch is a proton pump activated by green-yellow light in the same overall wavelength range. In both cases, in vitro results suggest a complete suppression of spiking, but the temporal dynamics of suppressor activation are slower than those for ChR2. In particular, eNpHR exhibits a relatively slow ramp to peak efficacy, followed by a gradual decrease. Due to the significant elevation of spike rates in active networks in vivo, along with an increased tendency towards bursting during sleep and anesthesia, it remains unclear whether the ~5–15mV hyperpolarization caused by eNpHR or Arch activation is sufficient to suppress all spiking. Indeed, our data suggest that under in vivo conditions, eNpHR and Arch suppress only 65–80% of all spikes. Spikes in response to large bursts of synchronous excitatory synaptic input (Fig. 2b,c) or electrical stimulation (Fig. 3) remain even in the presence of maximal eNpHR or Arch activation. Low efficacy of spike suppression may also result from difficulties in achieving high levels of expression of eNpHR and Arch or from inefficient cellular trafficking of these proteins. Improved trafficking has mitigated some expression issues of early versions of eNpHR (Gradinaru et al., 2010) In addition, the newer form of Arch, ArchT, demonstrates an increased degree of light sensitivity and may therefore be a more effective suppressor of activity in vivo (Han et al., 2011).
Figure 3. Optogenetic suppression of spontaneous but not evoked spiking.
A. Single unit recording of an LTS-SOM+ inhibitory interneuron in the hippocampus of a Somatostatin-Cre mouse after injection of AAV-FLEX-Arch-eGFP. The cell shows a relatively low rate of spontaneous activity. Electrical stimuli given to the alveolar pathway evoked a single spike per stimulus. B. Sustained activation of Arch by yellow light eliminated spontaneous firing, but did not affect firing in response to electrical stimulation. Each spike is marked by an asterisk.
3.2 Spontaneous activity of identified excitatory and inhibitory cell types
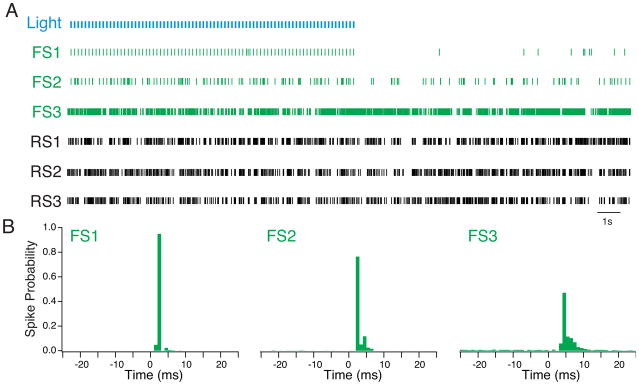
As noted above, one advantage of the combined electrophysiology-optogenetics approach is an enhanced ability to rigorously identify extracellularly recorded neurons in vivo. ChR2 functions as a physiological tag whose distribution is determined by the expression profile of the targeted cells. Under conditions of highly cell type-specific expression, light-evoked spikes identify individual neurons as belonging to a cellular class defined by its molecular expression profile. Using a double-floxed ChR2 construct carried by an AAV vector, we have expressed ChR2 specifically in populations of regular spiking excitatory neurons that express the homeobox gene Emx1 (Emx1+), low-threshold spiking interneurons that coexpress the peptide somatostatin (LTS-SOM+), and fast-spiking interneurons that coexpress the calcium binding protein parvalbumin (FS-PV+) in layers 2/3 of mouse primary visual cortex. In each case, repeated activation of ChR2-expressing cells by short (0.25ms) pulses of blue light evoked reliable spiking, allowing identification of a subpopulation of recorded neurons (Fig. 4a, 5a). This extracellular identification relies on the short latency of ChR2-evoked spikes and the very high degree of temporal precision of the evoked spikes, neither of which is observed for spikes evoked by synaptic input (Fig. 4b). One consideration in evaluating light-evoked spikes is that the efficacy of light drive in vivo depends critically on several factors, including ChR2 expression levels, anesthesia level, cell type, and effective light power delivered. Cells that have low ChR2 levels, are very hyperpolarized, or are far away from the light source will exhibit more variable spike responses to light stimuli.
Figure 4. Extracellular identification of cortical FS-PV+ interneurons by repeated activation of ChR2.
A. Raster plot of simultaneous recordings made from 3 FS-PV+ interneurons (green) and 3 regular spiking, putative excitatory neurons (black) in layer 2/3 of primary visual cortex of a Parvalbumin-Cre mouse after injection of AAV-DIO-ChR2-mCherry. The interneurons show different levels of spontaneous activity, but all three respond to light pulses (blue). B. Histograms of the spike responses of each FS-PV+ cell to light pulses. The light pulse is at 0ms. In each case, the interneurons show a peak in firing between 3 and 5 ms after the light pulse. However, FS3 has a lower spike probability (~50%) and a wider distribution of spike times, suggesting that it expresses a lower level of ChR2 or is more distant from the light source than FS1 and FS2. PSTH bins are 1 ms.
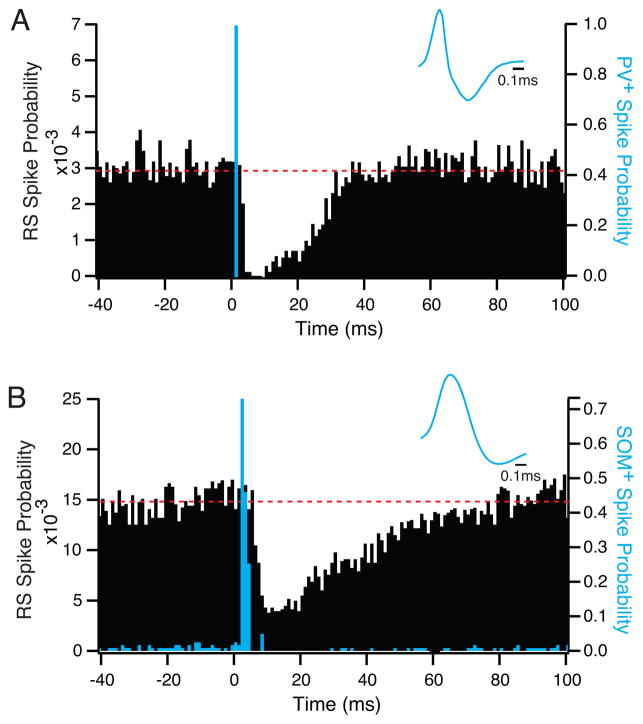
Figure 5. Identification of somatostatin-expressing interneurons in vivo.
A. Raster plots of data recorded simultaneously from 2 LTS-SOM+ interneurons (green) and 16 regular spiking, putative excitatory neurons (black) in layer 2/3 of primary visual cortex in a Somatostatin-Cre mouse after injection of AAV-DIO-ChR2-mCherry. The two LTS-SOM+ interneurons have low spontaneous activity but are robustly driven by intermittent light pulses (blue). B. Expanded portion of data denoted by box in A. Activation of LTS-SOM+ interneurons by light pulses cased a suppression of activity in the local regular spiking cells that outlasted the light stimulus by almost 100ms.
3.3 Discrete populations of interneurons have distinct impacts on local network activity
While fast-spiking inhibitory interneurons can be putatively identified in extracellular recordings on the basis of their action potential waveforms, other classes of interneuron are largely indistinguishable from regular spiking excitatory cells. This overlap in spike waveform profiles has made it difficult to determine the activity or function of the regular spiking interneuron populations in vivo, including the somatostatin-expressing (SOM), vasoactive intestinal peptide-expressing (VIP), and neuropeptide Y-expressing (NPY) inhibitory cells. However, the combination of Cre-expressing mouse lines and virally targeted optogenetic tools have made identification and manipulation of these sparsely represented cell types possible. Dense tetrode or stereotrode recordings allow isolation of the spike waveforms of many regular spiking cells simultaneously. As shown in Figure 5, activation of ChR2-expressing cells can identify the subgroup of recorded regular spiking cells that belong to a specific regular spiking interneuron population. This kind of optical activation also allows rigorous identification of cell types that have very little or no spontaneous firing, and would not otherwise produce sufficient spikes to permit rigorous post-hoc identification by waveform analysis (Fig. 5a).
One advantage of this combined approach is the ability to compare the impact of discrete sources of inhibition on the surrounding local network. This is of particular interest in comparing soma-targeting cells, such as FS-PV+ interneurons, and dendrite-targeting cells, such as LTS-SOM+ interneurons, whose roles in regulating excitatory neuron activity are hypothesized to differ. The impacts of different interneuron populations on local excitatory neurons can be tested by simultaneously recording a large number of closely spaced neurons and activating local cells belonging to a specific interneuron population. Indeed, data from this type of experiment suggest that whereas FS-PV+ interneurons exert a brief but powerful suppressive effect on local regular spiking neurons (Fig. 6a), LTS-SOM+ interneurons may have a longer suppressive effect on the spiking of nearby cells, with a slower onset and a long tail (Fig. 6b). This long-lasting inhibitory impact can also be observed in Figure 5b. A complementary method for assessing the role of different sources of inhibition in the local network is to selectively suppress the activity of an interneuron population by light activation of cell type-specific eNpHR or Arch. However, long-term suppression of inhibition can lead to runaway excitation and pathological activity patterns.
Figure 6. Different interneuron populations may have distinct impacts on local activity.
A. Overlaid PSTHs of the activity of a simultaneously recorded FS-PV+ interneuron expressing ChR2 (blue) and a nearby (75μm) RS, putative excitatory cell (black). The RS cell showed significant but brief suppression of spiking following FS-PV+ activation. B. Overlaid PSTHs of a simultaneously recorded SOM+ interneuron expressing ChR2 and a nearby (60μm) RS, putative excitatory cell. In this case, the RS cell showed longer suppression following interneuron activation, with a slower onset and a tail that continued for up to 80ms. Average spike waveforms for the two interneurons are shown in blue in each panel. Dashed lines indicate mean spontaneous firing rates. PSTH bins are 1ms.
4. Conclusion
Combined optogenetic and electrophysiological approaches provide multilevel access to cellular interactions and local network operations in vivo. In particular, the ability to target sparsely represented cell types with a high degree of fidelity permits the identification and manipulation of many inhibitory interneuron classes not previously available for in vivo study. One remaining limitation on this integrated experimental approach is the lack of cell type-specific promoters and mouse lines for many neural populations of interest. However, a greater depth of knowledge at the level of in vitro classification and molecular expression profiles continues to produce new tools. A further limitation is the difficulty of targeting light stimulation to individual cells or many specific areas in sequence. Such manipulations are possible using 2-photon microscopy (Andrasfalvy et al., 2010; Papagiakoumou et al., 2010; Rickgauer and Tank, 2009), but present a significant technical challenge. Multisite stimulation probes and optrodes currently in development may offer a more intermediate avenue for precise spatial patterns of stimulation across neighboring and distant brain areas (Zorzos et al., 2010).
Recent work has successfully used optogenetics in combination with diverse behavioral assays (Kravitz et al., 2010; Tye et al., 2011; Witten et al., 2010). However, a detailed understanding of the relationship between local circuit activity and behavior may require the combination of dense extra- and intracellular recordings in vivo with optogenetics in awake behaving animals. Such work could potentially provide a direct link between the activity of specific excitatory and inhibitory cell types and higher-order cognitive and perceptual processes.
Inhibitory interneurons play key roles in regulating cortical activity.
Integrated techniques allow targeting of specific interneuron classes.
Optogenetic tools provide direct identification and manipulation of inhibitory cells.
Promoter specificity, expression levels, and vector choice constrain this approach.
FS-PV+ and LTS-SOM+ interneurons differentially affect local circuit activity.
Acknowledgments
This work is supported by NIH/NEI grant R00EY018407, a Whitehall Foundation grant, an Esther A. and Joseph Klingenstein Foundation fellowship, and a NARSAD Young Investigator award funded by the Fairfax Foundation to J.A.C. The SOM-ires-Cre mice were a gift of Dr. Z. Josh Huang, Cold Spring Harbor Laboratories. The ChR2 and eNpHR constructs were a gift of Dr. Karl Deisseroth, Stanford University. The Arch construct was developed by Dr. Edward Boyden, MIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464(7292):1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasfalvy BK, Zemelman BV, Tang J, Vaziri A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc Natl Acad Sci U S A. 2010;107(26):11981–11986. doi: 10.1073/pnas.1006620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4(3):S143–156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54(2):205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9(7):557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28(28):7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V, Yoshimura Y, Callaway EM. Targeted gene delivery to telencephalic inhibitory neurons by directional in utero electroporation. J Neurosci Methods. 2005;143(2):151–158. doi: 10.1016/j.jneumeth.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 2010;5(2):247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Palmer LA, Contreras D. Stimulus feature selectivity in excitatory and inhibitory neurons in primary visual cortex. J Neurosci. 2007;27(39):10333–10344. doi: 10.1523/JNEUROSCI.1692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463(7277):98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci. 2003;4(9):739–751. doi: 10.1038/nrn1198. [DOI] [PubMed] [Google Scholar]
- Fu H, Muenzer J, Samulski RJ, Breese G, Sifford J, Zeng X, McCarty DM. Self-complementary adeno-associated virus serotype 2 vector: global distribution and broad dispersion of AAV-mediated transgene expression in mouse brain. Mol Ther. 2003;8(6):911–917. doi: 10.1016/j.ymthe.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141(1):154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nat Neurosci. 2010;13(3):387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One. 2007;2(3):e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES. A high-light sensitivity optical neural silencer: development, and application to optogenetic control of nonhuman primate cortex. Frontiers in Systems Neuroscience. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron. 2009;62(2):191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Martinez LM, Pillai C, Alonso JM, Wang Q, Sommer FT. Functionally distinct inhibitory neurons at the first stage of visual cortical processing. Nat Neurosci. 2003;6(12):1300–1308. doi: 10.1038/nn1152. [DOI] [PubMed] [Google Scholar]
- Huber D, Petreanu L, Ghitani N, Ranade S, Hromadka T, Mainen Z, Svoboda K. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451(7174):61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Rainnie DG, Maguschak KA, Chhatwal JP, Ressler KJ. Construction of cell-type specific promoter lentiviruses for optically guiding electrophysiological recordings and for targeted gene delivery. Methods Mol Biol. 2009;515:199–213. doi: 10.1007/978-1-59745-559-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Huang ZJ. High-resolution labeling and functional manipulation of specific neuron types in mouse brain by Cre-activated viral gene expression. PLoS ONE. 2008;3(4):e2005. doi: 10.1371/journal.pone.0002005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One. 2009;4(7):e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450(7166):56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- LoTurco J, Manent JB, Sidiqi F. New and improved tools for in utero electroporation studies of developing cerebral cortex. Cereb Cortex. 2009;19(Suppl 1):i120–125. doi: 10.1093/cercor/bhp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Controlled expression of transgenes introduced by in vivo electroporation. Proc Natl Acad Sci U S A. 2007;104(3):1027–1032. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Carlen M, Knoblich U, Cardin JA. Neocortical interneurons: from diversity, strength. Cell. 2010;142(2):189–193. doi: 10.1016/j.cell.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience. 2009;161(2):441–450. doi: 10.1016/j.neuroscience.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiakoumou E, Anselmi F, Begue A, de Sars V, Gluckstad J, Isacoff EY, Emiliani V. Scanless two-photon excitation of channelrhodopsin-2. Nat Methods. 2010;7(10):848–854. doi: 10.1038/nmeth.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10(5):663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Rickgauer JP, Tank DW. Two-photon excitation of channelrhodopsin-2 at saturation. Proc Natl Acad Sci U S A. 2009;106(35):15025–15030. doi: 10.1073/pnas.0907084106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 2001;240(1):237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103(4):865–872. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Neurons tend to stop migration and differentiate along the cortical internal plexiform zones in the Reelin signal-deficient mice. J Neurosci Res. 2002;69(6):723–730. doi: 10.1002/jnr.10345. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 2003;23(31):9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, Wilson JM, Debyser Z, Baekelandt V. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18(3):195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471(7338):358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet KM, Blouin V, Brument N, Agbandje-McKenna M, Snyder RO. The role of the adeno-associated virus capsid in gene transfer. Methods Mol Biol. 2008;437:51–91. doi: 10.1007/978-1-59745-210-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330(6011):1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders C, Anderson SA. Cortical interneurons and their origins. Neuroscientist. 2005;11(3):199–205. doi: 10.1177/1073858404270968. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Holbro N, Oertner TG. Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci U S A. 2008;105(33):12039–12044. doi: 10.1073/pnas.0802940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Cunha C, Zhang F, Liu Q, Gloss B, Deisseroth K, Augustine GJ, Feng G. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol. 2008;36(1–4):141–154. doi: 10.1007/s11068-008-9034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzos AN, Boyden ES, Fonstad CG. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt Lett. 2010;35(24):4133–4135. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]