Abstract
A practical multinuclear transceiver RF volume coil with improved efficiency for in-vivo small animal 1H/13C/23Na MR applications at the ultrahigh magnetic field of 7T is reported. In the proposed design, the coil’s resonance frequencies for 1H and 13C are realized by using a traditional double-tuned approach while the resonant frequency for 23Na, which is only some 4-MHz away from the 13C frequency, is tuned based upon 13C channel by easy-operating capacitive “frequency switches”. In contrast to the traditional triple-tuned volume coil, the volume coil with the proposed design possesses less number of resonances, which helps improve the coil efficiency and alleviate the design and operation difficulties. This coil design strategy is advantageous and well-suitable for multinuclear MR imaging and spectroscopy studies, particularly in the case where Larmor frequencies of nuclei in question are not separate enough. The prototype multinuclear coil was demonstrated in the desired unshielded design for easy construction and experiment implementation at 7T. The design method may provide a practical and robust solution to designing multinuclear RF volume coils for in-vivo MR imaging and spectroscopy at ultrahigh fields. FDTD simulations for evaluating the design and 7T MR experiment results acquired using the prototype coil are presented.
INTRODUCTION
In vivo MR imaging (MRI) and spectroscopic imaging (MRSI) at ultrahigh static magnetic fields (7T and above) is proven to be advantageous due to its intrinsically high sensitivity and improved spectral dispersion [1–10]. 13C and 23Na MRSI combined with proton (1H) imaging is a promising tool to depict metabolism process and intercellular information [11–18]. To implement this multinuclear MR technology to ultrahigh field MR for improved sensitivity, a multinuclear RF coil is essential and critical for efficient MR signal excitation and reception of multinuclei involved, ultimately realizing the ultrahigh field advantages and ensuring correct spatial co-registration of signals from different nuclei without changing coils during imaging and spectroscopy examinations.
Birdcage coil is a mature and well-established volume coil structure for in vivo MR applications at relatively low field strengths (1.5T or below) [19]. Based on this design, a variety of design approaches of double-tuned birdcage coils have been proposed for multinuclear MRI and MRSI studies. These approaches include inserting band-pass/band-stop filters [20] or LC trap circuit [21–23] into a the rungs and/or the end rings of the birdcage coil. A transformer coupled birdcage coil which consists of two coaxial birdcages has been developed as a quadrature double-tuned birdcage [24], which provides nearly ideal quadrature performance in the low frequency mode. In order to achieve simultaneous, quadrature operation for both low and high frequencies, two four-ring birdcage configurations have been presented [25]. Another method to double-tuned birdcage coil design is to tune the alternate rungs to alternate frequencies [26–28], which can be treated as two imbricate coupled low-pass birdcages.
At ultrahigh magnetic fields, the required high frequency for proton imaging poses considerable technical challenges [2,7,8,29–33] in designing RF coils based on the conventional birdcage-coil technology. Despite the cumbersome structure and some degree of design/construction difficulties, RF shielding is a commonly used approach to alleviate the low quality factors (Q-factors) and low B1 efficiency of volume coils due to the high resonance frequency. In the design of multiple-tuned volume coils required for multinuclear MR studies at ultrahigh fields, increased interaction between proton channel and non-proton channels, ultimately degrading the efficiency on MR sensitivity for both proton and non-proton nuclei, makes the design of such multi-modal volume coil more challenging. The design of multiple-tuned multi-modal volume coil becomes even more problematic in the case where Larmor frequencies of nuclei involved, e.g. 1H/19F and 13C/23Na, are not separate enough for the volume coil to establish clearly defined multi-modal resonance for each nucleus.
In this work, we explored the feasibility of designing a multinuclear volume coil based on the birdcage-coil design for rat 1H/13C/23Na MR studies at the ultrahigh field of 7T. The proposed multinuclear volume coil was first designed to operate at 7T 1H and 13C frequencies based on the alternative rung double-tuned birdcage coil technology. The third frequency for 23Na is achieved by simply changing the capacitance on each 13C rung of the volume coil. This approach is feasible because Larmor frequency of 13C is close enough to the frequency of 23Na, only some 4 MHz difference at 7T, and therefore the third resonance frequency for 23Na is easy to be reached from 13C frequency by tuning commercial-available trimmer capacitors. The Finite difference time domain method (FDTD) was employed to predict the resonance characteristics and the B1 field distribution. Bench test, phantom MRI and MRSI experiments were carried out to investigate the coil performance. The results demonstrated the proposed strategy for designing multinuclear volume coils for in vivo MR is advantageous in terms of MR sensitivity because it diminishes the signal losses caused by conventional multiple-tuned coils. In addition, it makes the multimodal volume coil design convenient in multinuclear MR applications in which Larmor frequencies in question are not separate enough.
METHODS
1. Design and construction of the 7T multinuclear volume coil
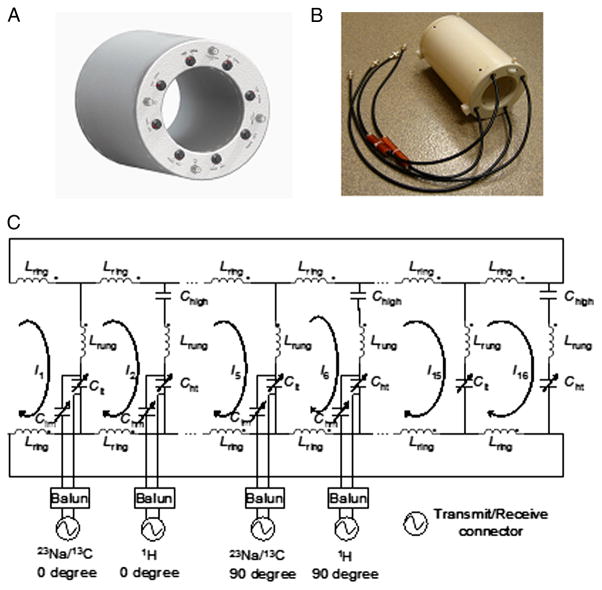
A multinuclear volume coil (Diameter: 10.16 cm, Length: 12.7 cm) showed in Fig. 1 was built with 16 copper tubes (OD: 6.35 mm, Length: 12.7 cm, 8 for each frequency). The inner diameter of the coil housing was 7.4 cm, which was the maximum usable diameter for the coil. Compared with copper foil, copper tubes would provide larger cross section area of the rungs in the limited space, which may potentially reduce the coil resistance and increase the Q factors of the coil. Whereas the copper tubes may cause certain distortions in B0 fields, the previous works showed the distortions may not be observable in practice [2,26,34–36]. Trimmer capacitors (Voltronics, Denville, NJ) were placed between copper tubes and end-rings to facilitate fine tuning of the coil. Fixed capacitors (American Technical Ceramics, Huntington Station, NY) were placed on the other end of each element. Alternate struts were tuned to the alternate coil frequencies. The coil was driven in quadrature at both frequencies. Instead of inductive driving, in which magnetic fields generated by driving loops can disturb the B1 field generated by RF volume coil, capacitive driving was employed in this design. Each port was matched to 50Ω while loaded with a cylindrical-shaped corn oil phantom (Diameter: 6.35 cm, Length: 15.24 cm) by matching capacitors. Because the cable braids current caused the difficulty to achieve good quadrature operation, baluns were used to suppress the cable braid current. The proposed multinuclear RF volume coil has two resonance frequencies at the same time (1H&13C or 1H&23Na). Adjustable capacitors at the low frequency rungs of the coil were tuned to switch between different working modes, as shown in Fig. 1(a). The photo and electrical diagram of the prototype coil were shown in Fig. 1(b) and (c) respectively. In Fig. 1(c), the Lring and Lrung denoted the inductances of the portions of the rings and the rungs respectively. Clt and Cht denoted the tuning capacitors of the 13C/23Na and 1H channels respectively. Clm and Chm denoted matching capacitors. In this coil design, Cht, Clm and Chm were implemented by using trimmer capacitors (NMAP19, Voltronics, Denville, NJ) while Clt was a variable capacitor (with a measured capacitance range of 1-20pF) connected in parallel with a 38.2-pF fixed capacitor. Chigh denoted the fixed capacitors connected between copper tubes and end-rings. The capacitance of Chigh was 10 pF in the prototype coil. Resonant modes for each nucleus were measured with an E5070B Network Analyzer (Agilent, Santa Clara, CA) equipped with N4431-60003 electronic calibration module.
Figure 1.
The multinuclear volume coil with quadrature-drive capability for 7T applications (a) Sketch map. (b) Prototype coil. The coil measured 10.16 cm in diameter and 12.7 cm in length and had no RF shielding utilized. (c) Electrical circuit diagram of the prototype coil.
2. Numerical modeling and FDTD simulation
FDTD was normally used to calculate RF fields generated by different RF coils including double-tuned birdcage coils[37,38]. In order to evaluate the efficiency of traditional multiple-tuned volume coil and proposed multinuclear volume coil with two resonance frequencies at the same time, both coils were modeled and simulated by using FDTD method. For comparison, the traditional triple-tuned volume coil had 24 rungs (8 for each frequency) with the same coil dimensions (Diameter: 10.16 cm, Length: 12.7 cm) as the proposed multinuclear volume coil. Different capacitors were placed at the center of each rung. The resonance frequencies of the coils were found by using a Gaussian excitation and a Fourier transform of the time domain response. Tuning was performed by changing the capacitance. All RF magnetic fields (B1) generated by both RF coils were calculated by using the commercially available software package XFDTD (Remcom, Inc., State College, PA). The size of Yee cells, which are the basic elements of 3D meshes in the FDTD method, was 3 mm in each dimension. The simulation was run with 100,000 time-steps to ensure that the steady state was reached.
In order to confirm the simulation result of the B1 field distribution, a bench test based on S21 measurement was implemented. The proposed coil was driven by a network analyzer and a magnetic field probe (sniffer) was employed to measure the B1 field directly. The diameter of the sniffer’s tip was kept as small as 6.35 mm to ensure accuracy and resolution of the measurement. The B1 field in the center slice (with 74 mm diameter) of the coil was measured with 2 mm × 2 mm resolution. A 2-axis mechanical moving table was used for accurate positioning control in two directions.
3. 7T MR experiments
A cylindrical-shaped corn oil phantom with 6.35 cm diameter and 15.24 cm length was employed for MR experiments. The gap between phantom and coil was approximately 0.5 cm on each side. This gap was needed for accessories, e.g. animal holder, animal physiological monitoring system and anesthesia system when the coil is used for in-vivo imaging. The proton MR imaging and 13C spectroscopy experiments with the proposed multinuclear volume coil were performed on a GE 7T/90cm MRI system (GE Healthcare, Waukesha, WI). Proton image was acquired using a gradient echo (GRE) sequence with TE = 7.3 ms, TR = 500 ms, Flip angle = 30°, FOV = 10 cm x 10 cm, slice thickness=3 mm, matrix size = 256 × 256, number of excitation (NEX) =1, and bandwidth=15.63 kHz. 13C spectrum of corn oil was acquired with hard pulse by single shot acquisition and TR = 2 sec.
RESULTS AND DISCUSSION
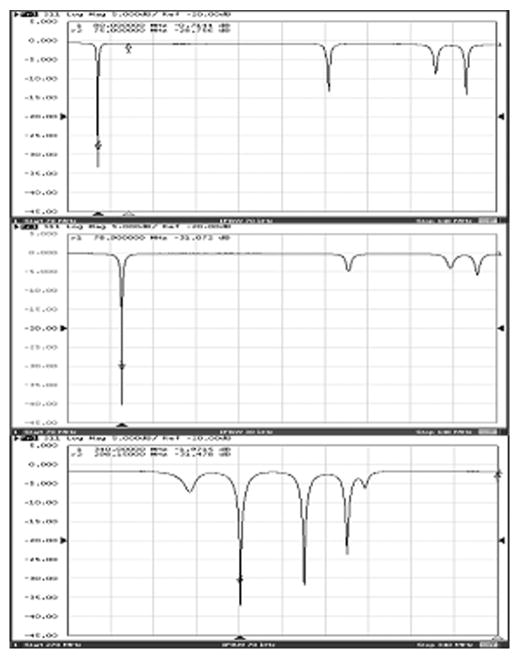
The measured frequency responses (reflection coefficient S11) of the multinuclear volume resonator were illustrated in Fig. 2 by using network analyzer E5070B (Agilent, Santa Clara, CA). All driving ports have been in good matching condition (S11 < −29 dB). The unloaded Q is ~273 for 13C and ~198 for 1H by S11 measurement. Because the loading is corn oil, the loaded Q almost the same as the unloaded Q. The imaging mode (mode 1) for 13C MRS and 1H MRI were tuned to 75MHz and 298MHz respectively. Well-defined resonant mode peaks for 13C MRS and 1H MRI were easily identified in Fig. 2. For 13C channel, the frequency gap between mode 1 and mode 2 is larger than 35MHz, and for 1H channel, the frequency gap between mode 1 and mode 2 is above 10MHz. The isolation between 13C channel and 1H channel is better than −17 dB. Because the resonant frequency of 23Na is 78.9MHz at 7T, only 3.9MHz different from the resonant frequency of 13C at 7T (75 MHz), it is expected to tune the 13C channel to resonant frequency of 23Na easily. Because the current at lower frequency is also flowed on the rungs of higher frequency, the tuning of lower frequency will affect the higher resonant frequency too. In our case, the higher resonant frequency (for proton) increases from 298 MHz to 298.5 MHz if the lower frequency was tuned from 75MHz (13C) to 78.9MHz (23Na). This kind of small resonant frequency shift can be tuned back by changing the tuning capacitor at the driving ports. The frequency response of 23Na channel was shown in Fig. 2 (middle) and the unloaded Q is ~267. On the other hand, because high resonant frequency rungs and low resonant frequency rungs were placed alternatively, this type of symmetric design can generate relatively identical B1 field distribution for both high resonant frequency and low resonant frequency. This kind of characteristics can gain benefit from shimming procedure for both nuclei.
Figure 2.
Measured frequency response of the multinuclear volume coil for 13C (top), 23Na(middle) and 1H (bottom) with the frequency span of 70MHz. Well-defined resonance modes for each nucleus demonstrate proposing behavior of the proposed multinuclear volume coil.
The B1 fields of the traditional triple-tuned coil and the proposed multinuclear coil for proton frequency have been calculated by using XFDTD. All the B1 fields were normalized to unit input power. The results (Fig. 3) shown that the proposed multinuclear RF coil, which has two resonant frequencies at the same time, has 8.8% higher efficiency than the traditional triple-tuned RF Coil.
Figure 3.
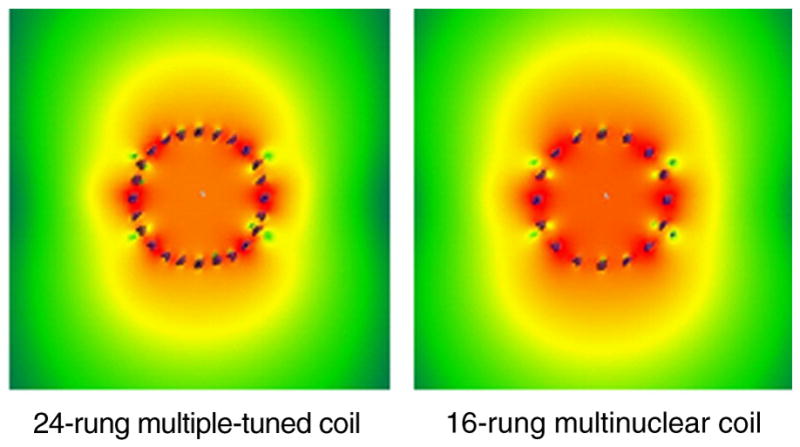
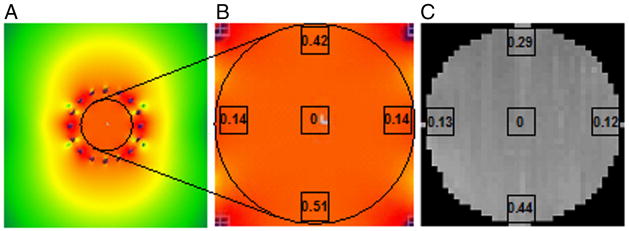
B1 field distributions at proton frequency for traditional 24-rung multiple-tuned volume coil (left) and proposed 16-rung multinuclear volume coil with two resonance frequencies at the same time (right). Both cases were normalized to 1W net input power. The B1 field at the center of coil increased from 6.57 μT (24-rung coil) to 7.15μT (16-rung coil).
The direct measurement of B1 field distribution was shown and compared with simulation result in Fig. 4. Fig. 4(a) and (b) showed the simulation result and zoom-in result within 74 mm diameter circle respectively. Fig. 4(c) showed the actual measurement result. Both the B1 simulation and measurement results were normalized to 0 dB at the center of the coil. The comparison indicated good consistency between the simulation and measurement results, which confirmed the B1 field distribution.
Figure 4.
Simulation and actual measurement results comparison. (a) simulation result; (b) zoom-in simulation result with 74 mm diameter (unit in dB); (c) actual measurement result (unit in dB). The B1 field distribution was normalized by the B1 at the center of the coil, which was indicated as 0 dB.
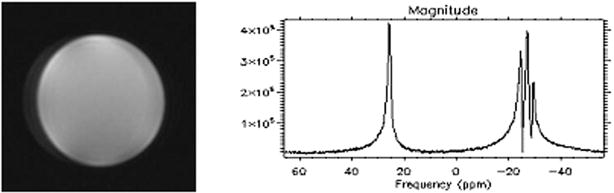
Axial GRE proton image of the cylindrical corn oil phantom acquired using the prototype multinuclear volume coil was shown in Fig. 5 (left). Because the loading of corn oil phantom was very light and the relative permittivity of corn oil was less than 3, the phantom image reflected the intrinsic B1 field pattern generated by the prototype coil. The relatively homogeneous axial GRE phantom image in Fig. 5 (left) demonstrated the relatively homogeneous intrinsic B1 field pattern generated by the prototype coil. Fig. 5 (right) illustrated high SNR for 13C spectroscopy with single shot acquisition. Based on the bench test and MRI/S experiment, the multinuclear RF volume coil was used successfully at 7T.
Figure 5.
Proton image (left) and 13C spectra (right) of a cylindrical corn oil phantom acquired using the multinuclear volume coil on a GE whole body 7T MR scanner. Artifacts in the proton image (left) were caused by chemical shift.
CONCLUSIONS
A practical and easy-implementing multinuclear volume coil for 7T small animal 1H/13C/23Na MR studies was designed and validated. The multi-tuned design strategy provided a simple and efficient way to overcoming the technical challenges, reducing interference between nuclear channels encountered in multinuclear volume coil designs for ultrahigh field MR. The method is also advantageous to volume coil designs for nuclei of which Larmor frequencies are not separate enough to establish a clear multi-modal performance. Although the design method proposed in this work was demonstrated for 1H/13C/23Na applications, it can also be adopted for MR applications with other nucleus combinations, e.g. 1H/19F.
Acknowledgments
This work was supported by NIH grants EB004453, UL1 RR024131-01, EB008699 and EB007588, and a QB3 Research Award and UC ITL-Bio04-10148.
Appendix
This appendix shows a brief mathematical physics theory of the proposed coil. Based on the Kirchoff’s law, the mesh currents in the coil, as shown in Fig 1(c), satisfy
| [A1] |
in which I = [I1, I2, …, I16], Z is the impedance matrix following
| [A2] |
where ω is angular frequency, Lring and Lrung are the inductances of the portions of the rings and the rungs respectively, is the mutual inductance between rung i and rung j, and denote the mutual inductances between ith and jth ring segments of the top ring and the bottom ring respectively, is the mutual inductance between ith ring segment in the top ring and jth ring segment in the bottom ring, and Clow and Chigh are the equivalent capacitances of the tuning capacitors for 23Na/13C channel and 1H channel respectively. In the cases of i = j −1, the terms in equation A2 should be modified respectively as
| [A3] |
In the case of i−1 = j, the terms in equation A2 should be modified respectively as
| [A4] |
Because of the periodicity structure of the proposed coil, the mutual inductance M(i, j) in equation A2 to A4 satisfies
| [A5] |
The equation A2 can be modified to
| [A6] |
where λ =1/ω2. The equations A3 and A4 are then written respectively as
| [A7] |
| [A8] |
The equation A6 can be viewed as an eigenvalue problem with the variable λ. The eigenvalues λ of this problem represent the resonant frequencies of different modes and the eigenvectors I corresponding to λ represent the mesh currents in each rung. There will be two eigenvectors satisfy A9 respectively
| [A9] |
in which Al and Ah are constants. These two eigenvectors I and I′ would provide homogeneity B1 distribution desired by MRIS and MRI applications. The resonant frequencies for 23Na/13C and 1H can be calculated by the corresponding eigenvalues λl and λh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abduljalil AM, Kangarlu A, Zhang X, Burgess RE, Robitaille PM. Acquisition of human multislice MR images at 8 Tesla. J Comput Assist Tomogr. 1999;23(3):335–40. doi: 10.1097/00004728-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan JT, Garwood M, Collins CM, Liu W, DelaBarre L, Adriany G, et al. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med. 2001;46(1):24–30. doi: 10.1002/mrm.1156. [DOI] [PubMed] [Google Scholar]
- 3.Yacoub E, Shmuel A, Pfeuffer J, Van De Moortele PF, Adriany G, Andersen P, et al. Imaging brain function in humans at 7 Tesla. Magn Reson Med. 2001;45(4):588–94. doi: 10.1002/mrm.1080. [DOI] [PubMed] [Google Scholar]
- 4.Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, et al. Development of (17)O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci U S A. 2002;99(20):13194–9. doi: 10.1073/pnas.202471399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Natl Acad Sci U S A. 2007;104(28):11796–801. doi: 10.1073/pnas.0610821104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelinski AC, Wald LL, Setsompop K, Alagappan V, Gagoski BA, Goyal VK, et al. Fast slice-selective radio-frequency excitation pulses for mitigating B+1 inhomogeneity in the human brain at 7 Tesla. Magn Reson Med. 2008;59(6):1355–64. doi: 10.1002/mrm.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Ugurbil K, Chen W. Microstrip RF surface coil design for extremely high-field MRI and spectroscopy. Magn Reson Med. 2001;46(3):443–50. doi: 10.1002/mrm.1212. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Ugurbil K, Sainati R, Chen W. An inverted-microstrip resonator for human head proton MR imaging at 7 tesla. IEEE Trans Biomed Eng. 2005;52(3):495–504. doi: 10.1109/TBME.2004.842968. [DOI] [PubMed] [Google Scholar]
- 9.Qiao H, Zhang X, Zhu XH, Du F, Chen W. In vivo 31P MRS of human brain at high/ultrahigh fields: a quantitative comparison of NMR detection sensitivity and spectral resolution between 4 T and 7 T. Magn Reson Imaging. 2006;24(10):1281–6. doi: 10.1016/j.mri.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei H, Zhu XH, Zhang XL, Ugurbil K, Chen W. In vivo 31P magnetic resonance spectroscopy of human brain at 7 T: an initial experience. Magn Reson Med. 2003;49(2):199–205. doi: 10.1002/mrm.10379. [DOI] [PubMed] [Google Scholar]
- 11.Chen AP, Kurhanewicz J, Bok R, Xu D, Joun D, Zhang V, et al. Feasibility of using hyperpolarized [1–13C]lactate as a substrate for in vivo metabolic 13C MRSI studies. Magn Reson Imaging. 2008;26(6):721–6. doi: 10.1016/j.mri.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100(18):10158–63. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler SJ, Yen Y, Wolber J, Chen AP, Albers MJ, Bok R, et al. In vivo 13 carbon metabolic imaging at 3T with hyperpolarized 13C-1-pyruvate. Magn Reson Med. 2007;58(1):65–9. doi: 10.1002/mrm.21253. [DOI] [PubMed] [Google Scholar]
- 14.Boada FE, Gillen JS, Shen GX, Chang SY, Thulborn KR. Fast three dimensional sodium imaging. Magn Reson Med. 1997;37(5):706–15. doi: 10.1002/mrm.1910370512. [DOI] [PubMed] [Google Scholar]
- 15.Thulborn KR, Davis D, Adams H, Gindin T, Zhou J. Quantitative tissue sodium concentration mapping of the growth of focal cerebral tumors with sodium magnetic resonance imaging. Magn Reson Med. 1999;41(2):351–9. doi: 10.1002/(sici)1522-2594(199902)41:2<351::aid-mrm20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Jucker BM, Dufour S, Ren J, Cao X, Previs SF, Underhill B, et al. Assessment of mitochondrial energy coupling in vivo by 13C/31P NMR. Proc Natl Acad Sci U S A. 2000;97(12):6880–4. doi: 10.1073/pnas.120131997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen JD, Barrere BJ, Boada FE, Vevea JM, Thulborn KR. Quantitative tissue sodium concentration mapping of normal rat brain. Magn Reson Med. 1996;36(1):83–9. doi: 10.1002/mrm.1910360115. [DOI] [PubMed] [Google Scholar]
- 18.Towner RA, Janzen EG, Chu SC, Rath A. Use of H-1/Na-23 and H-1/P-31 Double Frequency Tuned Birdcage Coils to Study Invivo Carbon Tetrachloride-Induced Hepatotoxicity in Rats. Magnetic Resonance Imaging. 1992;10(4):679–88. doi: 10.1016/0730-725x(92)90020-z. [DOI] [PubMed] [Google Scholar]
- 19.Hayes CE, Edelstein WA, Schenck JF, Mueller OM, Eash M. An Efficient, Highly Homogeneous Radiofrequency Coil for Whole-Body Nmr Imaging at 1. 5-T. J Magn Reson. 1985;63(3):622–28. [Google Scholar]
- 20.Rath AR. Design and Performance of a Double-Tuned Bird-Cage Coil. J Magn Reson. 1990;86(3):488–95. [Google Scholar]
- 21.Isaac G, Schnall MD, Lenkinski RE, Vogele K. A Design for a Double-Tuned Birdcage Coil for Use in an Integrated MRI/MRS Examination. J Magn Reson. 1990;89(1):41–50. [Google Scholar]
- 22.Shen GX, Boada FE, Thulborn KR. Dual-frequency, dual-quadrature, birdcage RF coil design with identical B1 pattern for sodium and proton imaging of the human brain at 1.5 T. Magn Reson Med. 1997;38(5):717–25. doi: 10.1002/mrm.1910380507. [DOI] [PubMed] [Google Scholar]
- 23.Shen GX, Wu JF, Boada FE, Thulborn KR. Experimentally verified, theoretical design of dual-tuned, low-pass birdcage radiofrequency resonators for magnetic resonance imaging and magnetic resonance spectroscopy of human brain at 3.0. Tesla Magn Reson Med. 1999;41(2):268–75. doi: 10.1002/(sici)1522-2594(199902)41:2<268::aid-mrm9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Fitzsimmons JR, Beck BL, Brooker HR. Double resonant quadrature birdcage. Magn Reson Med. 1993;30(1):107–14. doi: 10.1002/mrm.1910300116. [DOI] [PubMed] [Google Scholar]
- 25.Murphyboesch J, Srinivasan R, Carvajal L, Brown TR. 2 Configurations of the 4-Ring Birdcage Coil for H-1 Imaging and H-1-Decoupled P-31 Spectroscopy of the Human Head. J Magn Reson Ser B. 1994;103(2):103–14. doi: 10.1006/jmrb.1994.1017. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan JT, Hetherington HP, Otu JO, Pan JW, Pohost GM. High frequency volume coils for clinical NMR imaging and spectroscopy. Magn Reson Med. 1994;32(2):206–18. doi: 10.1002/mrm.1910320209. [DOI] [PubMed] [Google Scholar]
- 27.Amari S, Ulug AM, Bornemann J, van Zijl PC, Barker PB. Multiple tuning of birdcage resonators. Magn Reson Med. 1997;37(2):243–51. doi: 10.1002/mrm.1910370217. [DOI] [PubMed] [Google Scholar]
- 28.Matson GB, Vermathen P, Hill TC. A practical double-tuned 1H/31P quadrature birdcage headcoil optimized for 31P operation. Magn Reson Med. 1999;42(1):173–82. doi: 10.1002/(sici)1522-2594(199907)42:1<173::aid-mrm23>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Ugurbil K, Chen W. A microstrip transmission line volume coil for human head MR imaging at 4T. J Magn Reson. 2003;161(2):242–51. doi: 10.1016/s1090-7807(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Zhu XH, Chen W. Higher-order harmonic transmission-line RF coil design for MR applications. Magn Reson Med. 2005;53(5):1234–9. doi: 10.1002/mrm.20462. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan JT, Snyder C, DelaBarre L, Bolinger L, Tian J, Andersen P, et al. 7T body imaging: first results. Seattle, WA: 2006. p. 213. [Google Scholar]
- 32.Wu B, Wang C, Kelley DA, Xu D, Vigneron DB, Nelson SJ, et al. Shielded microstrip array for 7T human MR imaging. IEEE Trans Med Imaging. 2010;29(1):179–84. doi: 10.1109/TMI.2009.2033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu B, Wang C, Krug R, Kelley DA, Xu D, Pang Y, et al. 7T human spine imaging arrays with adjustable inductive decoupling. IEEE Trans Biomed Eng. 2010;57(2):397–403. doi: 10.1109/TBME.2009.2030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pimmel P, Briguet A. A hybrid bird cage resonator for sodium observation at 4.7 T. Magn Reson Med. 1992;24(1):158–62. doi: 10.1002/mrm.1910240116. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Zhu X, Qiao H, Liu H, Vaughan T, Ugurbil K, et al. A circular-polarized double-tuned (31P and 1H) TEM coil for human head MRI/MRS at 7T. Proceedings of the 11th Annual Meeting of the International Society of Magnetic Resonance in Medicine; Toronto, ON, Canada. 2003. p. 423. [Google Scholar]
- 36.Avdievich NI, Hetherington HP. 4 T actively detunable transmit/receive transverse electromagnetic coil and 4-channel receive-only phased array for (1)H human brain studies. Magn Reson Med. 2004;52(6):1459–64. doi: 10.1002/mrm.20264. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim TS, Lee R, Baertlein BA, Kangarlu A, Robitaille PL. Application of finite difference time domain method for the design of birdcage RF head coils using multi-port excitations. Magn Reson Imaging. 2000;18(6):733–42. doi: 10.1016/s0730-725x(00)00143-0. [DOI] [PubMed] [Google Scholar]
- 38.Ibrahim TS, Lee R, Baertlein BA, Yu Y, Robitaille PM. Computational analysis of the high pass birdcage resonator: finite difference time domain simulations for high-field MRI. Magn Reson Imaging. 2000;18(7):835–43. doi: 10.1016/s0730-725x(00)00161-2. [DOI] [PubMed] [Google Scholar]