Abstract
MMP-9 enzyme recognizes a peptide sequence Lys-Gly-Pro-Arg-Ser-Leu-Ser-Gly-Lys and cleaves the peptide into two parts. We synthesized a dual fluorophore beacon consisting of 5-FAM and Cy5 dyes. The fluorescence emission of the fluorescein moiety is dramatically quenched by Cy5 molecule due to Förster Resonance Energy Transfer (FRET) and the fluorescence of Cy5 is strongly enhanced. Upon addition of MMP-9 enzyme, the fluorescence of 5-FAM intensifies and Cy5 decreases. The control MMP-2 enzyme does not cause any changes in either 5-FAM or Cy5 fluorescence. We believe that our observation will help in early detection of elevated MMP-9 levels under disease conditions.
Keywords: MMP-9, fluorescence, FRET, labeled peptide
INTRODUCTION
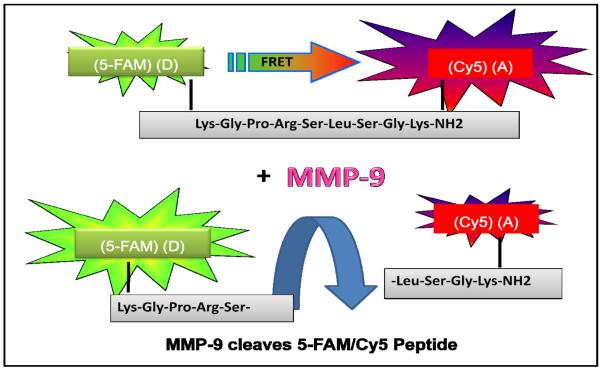
Forstere resonance energy transfer (FRET), also often referred to as “Fluorescence resonance energy transfer”, is a distance- dependent interaction between the electronic excited states of two dye molecules in which excitation is non-radiatively transferred from a donor molecule to an acceptor molecule. This energy transfer mechanism results in the quenching of the donor fluorescence and increase of the acceptor emission. FRET occurs when donor and acceptor molecules are in close proximity (1-10 nm) and the emission spectrum of the donor partially overlaps with the absorption spectrum of the acceptor. FRET can be used for the detection of various biomolecular interactions, in particular DNA hybridization or various enzymatic reactions [1-4]. Here we report a spectroscopic FRET based detection strategy for detection of enzymatic activity of MMP-9. Studies by Kridel et al. arrive with optimal substrate (peptide sequence) consensus for MMP-9 (Pro-Arg-Ser/Thr-↓*-Leu/Ile-Ser/Thr). [*Scissile bonds are identified by↓] [5]. The substrate for our bioassays was custom modified sequences of this peptide mainly by the incorporation of specific fluorophores. The peptide was labeled with a fluorescein derivative, 5-FAM, on the one end and Cy5 dye on the other end. This 5-FAM/Cy5 peptide forms a fluorescence resonance energy transfer (FRET) pair where a donor (5-FAM) is strongly quenched by a Cy5 acceptor. The cleavage of the peptide results in the disappearance of FRET Fig. (1). In the presented manuscript we are studying spectral properties of 5-FAM/Cy5 peptide in the absence and presence of MMP-9 enzyme.
Fig. (1).
Schematic of the cleavage of 5-FAM/Cy5 peptide by MMP-9 enzyme. After the cleavage reaction the fluorophores are separated and 5-FAM donor is no longer quenched by Cy5 acceptor. The color of the observed fluorescence changes from week red to strong green.
Proteases play various important roles in tumor angio-genesis, invasion and metastasis. Matrix metalloproteinases (MMPs) are a family of zinc-dependent neutral endopepti-dases capable of cleaving almost all extracellular matrix components. Up to date 26 MMPs have been described [6-8]. Information on substrate selection by the MMPs helps explain their unique biological roles. MMPs can influence action of growth factors by activating and releasing them for their function. All functions are presumed to involve a relatively high degree of selectivity among the MMPs for their physiologic substrates. MMPs play also an important role in many pathological conditions such as rheumatoid arthritis, osteoarthritis, autoimmune disorders of skin and in tumor invasion and metastasis [9-12]. One of the most important hallmarks of the tumor progression, invasion, and finally metastasis formation is ability to disassembly of the basement membrane by MMPs [13].
MMP-9 is named as gelatinase B, or 92 kDa type IV collagenase, because of its ability to degrade gelatin in vitro. MMP-9 contains three fibronectin type II repeats, unique among others MMPs, that have high binding affinity for collagen [14, 15]. MMP-9 is related to tumor invasion and metastasis by its capacity for tissue remodeling via extracellular matrix as well as basement membrane degradation and induction of angiogenesis [13, 16]. This gelatinase is secreted as zymogen and cleaved to the active form, and their function is tightly regulated by several different mechanisms. To date many reports suggest that increased expression MMP-9 correlates with worse prognosis for cancer patients. Over expression of MMP-9 in tumor tissue and stroma can result in increased activity of this enzyme in various body fluids. Increased presence of MMP-9 has been detected in the serum and plasma of tumor bearing rats and in humans with malignant tumors [17-22].
Optical imaging of proteases, in particular with fluorescence, using activatable probes is considered as a one of most accurate and appropriate means of measuring proteases activity. The reliable detection of MMP-9 enzyme will greatly benefit early detection of cancer and in other pathological conditions. Here we describe the possibility of a sensitive fluorescence-based detection of MMP-9.
MATERIALS AND METHODS
Reagents
Lys(5-FAM)-Gly-Pro-Arg-Ser-Leu-Ser-Gly-Lys(Cy5)-NH2 peptide was synthesized by AnaSpec (AnaSpec, Fremont, CA). After cleavage, the peptide was purified by preparative HPLC. Molecular weight (MW=2266.8 Da) and purity (>96%) of the peptide was verified by mass spectrometry and analytical HPLC, respectively. Deionized water used for the experiments was from Millipore distillation system. Sodium chloride (NaCl) and calcium chloride (CaCl2) were purchased from Fisher Scientific (Pittsburgh, PA), APMA 4-aminophenylmercuric acetate (APMA) and (hydroxymethyl)aminomethane (Tris) were from Sigma-Aldrich (St. Louis, MO)
Activation of MMP-9 and MMP-2
Monomeric, native MMP-9 from stimulated human neutrophils and pro-MMP-2 from cultured, human rheumatoid synovial fibroblasts were purchased from Calbiochem (Calbiochem, La Jola, CA). Enzymes were activated by incubation with 20mM APMA (4-aminophenylmercuric acetate) in accordance to manufacturer’s protocols. Briefly, to initiate activation proenzyme solution was mixed with APMA solution (APMA in 0.1M NaOH) at 10:1 volume ratio (MMPs:APMA) and incubated at 37°C for 3h.
Fluorescence Measurements of Peptide Hydrolysis
5-FAM/Cy5 labeled peptide (1μM) was incubated with active-MMP-9 or active-MMP-2 at a concentration of 2.5 nM, in 50 mM Tris, pH 7.5, 100 mM NaCl, 10 mM CaCl2, 50 μM ZnCl2 for 2h at 37°C. At selected time points (0 - 250min), fluorescence emission spectra were collected using Cary Eclipse spectrofluorometer (Varian Inc., Australia). Measurements were performed in 0.5×0.5 cm quartz cells with the excitation at 470 nm.
Lifetime Measurements
Fluorescence lifetime measurements were done using FluoTime 200 fluorometer (PicoQuant, GmbH, Berlin, Germany). This time-resolved instrument is equipped with an ultrafast detector, a Hamamatsu R3809U-50 microchannel plate photomultiplier (MCP). For the excitation we used a 470 nm picosecond pulsed laser diode. The detection was through a monochromator supported by 495 nm long wave pass filter in order to eliminate a scattering excitation light. The decay data were analyzed with FluoFit, version 5.0 software (PicoQuant, GmbH). The amplitude average life-time was calculated as 〈 τ 〉 = Σi αiτi.
RESULTS AND DISCUSSION
Spectral Properties of 5-FAM/Cy5 Peptide
An absorption spectrum of a synthesized peptide with fluorophores on both ends is presented in Fig. (2). This spectrum consists of two bands, one characteristic for carboxy-fluorescein (5-FAM) at 480nm and second long wavelength band which originates from a Cy5 chromophore. The long-wavelength (Cy5) absorption is about 2.5 fold stronger than short wavelength. This ratio corresponds well to the ratio of Cy5 and fluorescein extinction coefficients. We concluded that the fluorophores attached to the peptide are not interacting in the ground state.
Fig. (2).
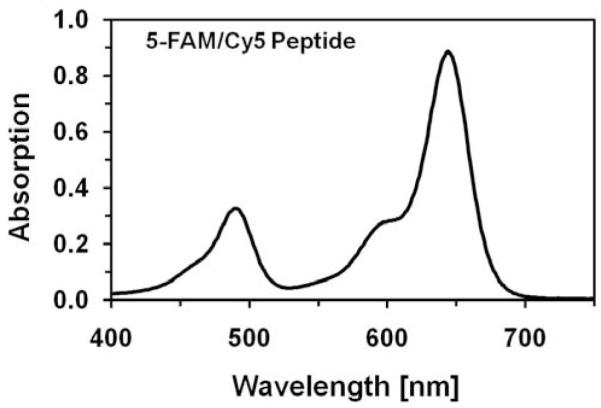
Absorption spectrum of 5-FAM/Cy5 peptide. Characteristic absorption bands of 5-FAM and Cy5 are clearly separated. The concentration of the peptide was 10μM.
Next, we measured fluorescence spectrum of the labeled peptide at 470nm excitation Fig. (3), solid line. This spectrum also contains two bands related to fluorescein (green, 520nm) and Cy5 (red, 670nm) fluorophores. The Cy5 fluorescence band is about two times more intense than fluorescein. However, the Cy5 fluorophore is very weekly excited at 470nm Fig. (3), dotted line. Such a great gain of excitation energy by the Cy5 molecule is due to Förster Resonance Energy Transfer (FRET), a phenomenon that occurs through the space. The fluorescein serves as an energy donor (D) and Cy5 is an acceptor (A). The characteristic R0 distance, where a probability of energy transfer is 50%, has been calculated for this D-A pair and is equal 49Å. The fluorophores attached to the peptide are at much shorter distance than R0 and we observe a very efficient FRET. Of course, the peptide exists in the solution at different conformations and the distance as well as orientation of the attached fluorophores may be different. The measured fluorescence spectrum reflects averaged conformations.
Fig. (3).
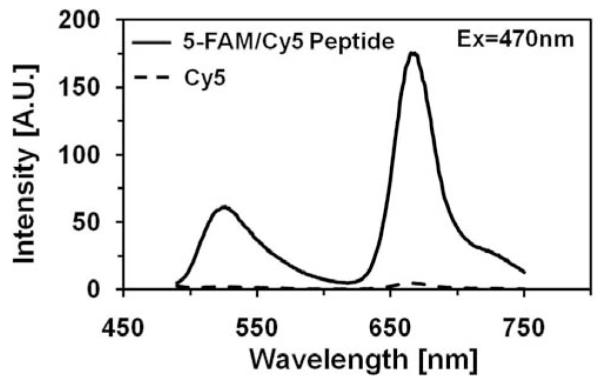
Fluorescence emission spectrum of 5-FAM/Cy5 peptide (1μM) at the excitation at 470nm. The characteristic emission bands of 5-FAM and Cy5 are easily distinguishable. The Cy5 fluorescence band is about 2.5 fold stronger than 5-FAM. The observed fluorescence is weak and red.
We also measured lifetimes of the labeled peptide at 470nm excitation and 520nm or 670nm observation Fig. (4). The lifetime of 5-FAM in the peptide is about 10 fold shorter than the lifetime of free 5-FAM whereas the lifetime of Cy5 is longer in the peptide (not shown). We needed four components in order to fit the quenched donor data with dominant amplitudes associated with short lifetimes (72% with 29ps and 18% with 237ps). It is clear that time-resolved measurements confirmed the efficient FRET occurring between 5-FAM and Cy5 molecules.
Fig. (4).
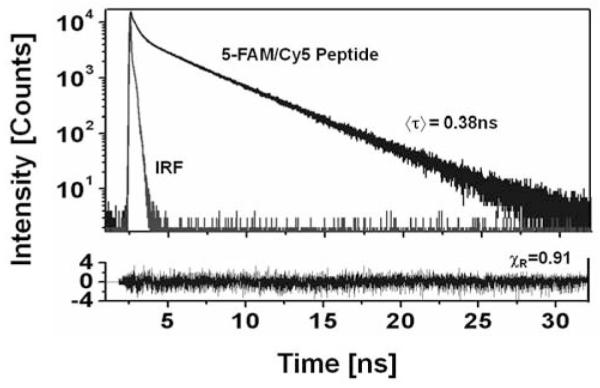
Fluorescence intensity decay of the 5-FAM/Cy5 peptide (1μM) measured with 470nm excitation and 520nm (5-FAM band) observation. The fluorescence of 5-FAM is strongly quenched and the decay is very heterogeneous. After the cleavage reaction the lifetime is long (3.4 ns) and homogeneous.
Spectral Changes of 5-FAM/Cy5 Peptide Upon Addition of MMP-9 Enzyme
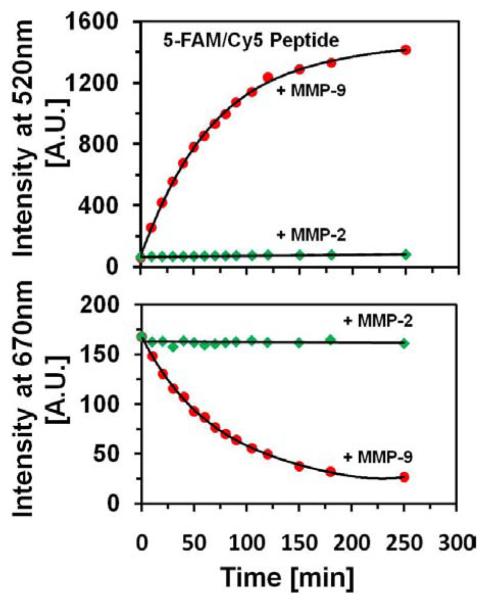
To the 250 μl solution of 1μM 5-FAM/Cy5 peptide, we added 2.5 nM MMP-9 enzyme; then recorded fluorescence spectrum every 10 min. The time dependent spectra of the peptide are presented in Fig. (5). With the time the spectrum of 5-FAM/Cy5 peptide dramatically changes. The fluorescence emission at 520 nm strongly increases whereas the Cy5 emission progressively decreases. This is illustrated on the photographs taken before addition of MMP-9 enzyme and after 4 hours Fig. (5), top. These changes are possible only if the FRET is being reduced.
Fig. (5).
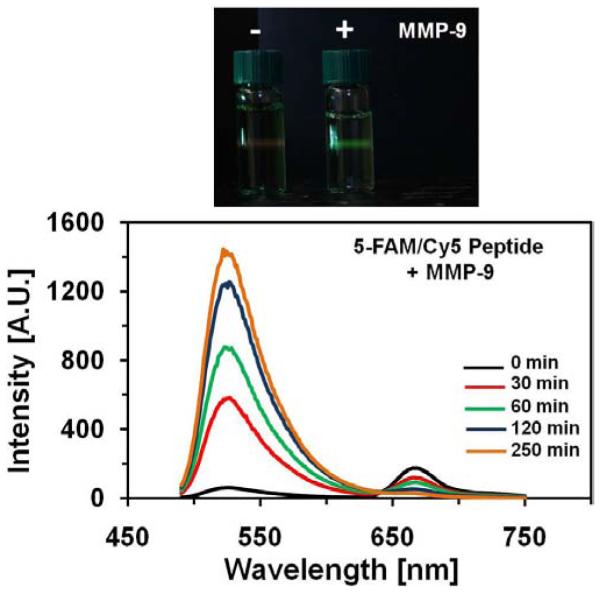
Upper Panel - Selected Emission Spectra Of 1 μM Solution Of 5-Fam/Cy5 Peptide With The Addition Of 2.5Nm Of Mmp-9 En-Zyme. Before The Cleavage Reaction The Fluorescence Was Weak And Red; After The Reaction The Fluorescence Is Strong And Green. The Exposure Time In The Photograph Before The Reaction Was Few Times Longer Than For The Solution After The Reaction. Bottom Panel - Selected Emission Spectra Of 1 μM Solution Of 5-Fam/Cy5 Peptide With The Addition Of 2.5Nm Of Mmp-9 Enzyme. After The Cleavage Reaction The Fluorescence Is Strong.
The time-dependent changes of the green emission are shown in Fig. (6), top and the changes of red Cy5 emission are shown in the Fig. (6), bottom. In the control experiment, we added MMP-2 instead of MMP-9, enzyme. The results are shown in Fig. (6). MMP-2 enzyme does not cause any significant changes in 5-Fam/Cy5 peptide fluorescence. We also checked that peptide without MMP-9 enzyme does not change its spectral properties during the time of the experiment. However, we noticed that after several days at 4°C in a refrigerator, the ratio of 520/670 emission slightly decreased.
Fig. (6).
Kinetics of the cleavage reaction. Top panel, red dots: Fluorescence intensity changes observed at 520nm emission (5-FAM) upon addition of MMP-9 enzyme; green diamonds: Control experiment with addition of MMP-2 enzyme. Bottom panel, red dots: Fluorescence intensity changes observed at 670nm emission (Cy5) upon addition of MMP-9 enzyme; green diamonds: Control experiment with addition of MMP-2 enzyme. The concentration of peptide was 1μM, and concentration of enzyme used was 2.5nM. The excitation was at 470nm.
Finally, we measured lifetime of 5-FAM/Cy5 peptide after the experiment with MMP-9 enzyme. The lifetime measured with 470 nm excitation and at 520 nm observation becomes almost homogeneous with the average value of about 3.4 ns. After the reaction with the enzyme there is no longer any evidence for the FRET.
CONCLUSIONS
The cleavage of 5-FAM/Cy5 peptide by MMP-9 enzyme results in a separation of both fluorophores knocking down the FRET. The fluorescence of 5-FAM significantly increases with a simultaneous decrease of Cy5 emission. The presence of MMP-9 monitors itself in a color change from red to green as shown on the photographs in Fig. (5). Interestingly, another metalloproteinase, MMP-2, does not cleavage the 5-FAM/Cy5 peptide. We believe that this is an important finding which will help researchers from the biosensing field to detect MMP-9 enzyme.
ACKNOWLEDGEMENTS
This work was supported by NIHR01 EB012003 and DoD W81XWH-09-1-0506 grants (Z.G.), R01 HL090786 (J.B.) and Susan G. Komen KG101213 grant (AM).
REFERENCES
- [1].Didenko VV. DNA probes using fluorescence resonance energy transfer (FRET): designs and applications. Biotechniques. 2001;31:1106–1116. doi: 10.2144/01315rv02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Orpana AK. Fluorescence resonance energy transfer (FRET) using ssDNA binding fluorescent dye. Biomol. Eng. 2004;21:45–50. doi: 10.1016/j.bioeng.2003.09.001. [DOI] [PubMed] [Google Scholar]
- [3].Tan W, Wang K, Drake TJ. Molecular beacons. Curr. Opin. Chem. Biol. 2004;8:547–553. doi: 10.1016/j.cbpa.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [4].Schäferling M, Nagl S. Optical technologies for the read out and quality control of DNA and protein microarrays. Anal. Bioanal. Chem. 2006;385:500–517. doi: 10.1007/s00216-006-0317-5. [DOI] [PubMed] [Google Scholar]
- [5].Kridel SJ, Chen E, Kotra LP, Howard EW, Mobasher S, Smith JW. Substrate hydrolysis by matrix metalloproteinase-9. J Biol Chem. 2001;276:20572–20578. doi: 10.1074/jbc.M100900200. [DOI] [PubMed] [Google Scholar]
- [6].DeClerck YA, Yean TD, Ratzkin BJ. Purification and characterization of two related but distinct metalloproteinase inhibitors secreted by bovine endothelial cells. J. Biol. Chem. 1989;264:17445–17453. [PubMed] [Google Scholar]
- [7].Stetler-Stevenson WG, Krutzsch HC, Liotta LA. A new member of the metalloproteinase inhibitor family. J. Biol. Chem. 1989;264:17374–17378. [PubMed] [Google Scholar]
- [8].Williamsen RA, Marston F, Angel S, Koklitis P, Panico M, Morris HR, Carne AF, Smith BJ, Harris T, Freedman RB. Disulphide bond assignment in human tissue inhibitor of metalloproteinases (TIMP) Biochem. J. 1990;268:267–274. doi: 10.1042/bj2680267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nagase H, Woessner JF. Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- [10].Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr. Opin. Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- [11].Westermarck J, Kähäri V-M. Regulation of matrix metalloproteinase expression in tumour invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- [12].Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell. 2000;102(5):647–655. doi: 10.1016/s0092-8674(00)00087-8. [DOI] [PubMed] [Google Scholar]
- [13].Lochter A, Sternligcht MD, Werb Z, Bissel MJ. The significance of matrix metalloproteinases during early stages of tumor progression. Ann. N. Y. Acad. Sci. 1998;857:180–193. doi: 10.1111/j.1749-6632.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- [14].Stöcker W, Grams F, Baumann U, Reinemer P, Gomis-Rüth FX, McKay DB, Bode W. The metzincins--topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4(5):823–840. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bode W, Fernandez-Catalan C, Tschesche H, Grams F, Nagase H, Maskos K. Structural properties of matrix metalloproteinases. Cell. Mol. Life. Sci. 1999;55(4):639–652. doi: 10.1007/s000180050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sternligcht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Ann. Rev. Cell Dev., Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Garbisa S, Scagliotti G, Masiero L, Di Francesco C, Caenazzo C, Onisto M, Micela M, Stetler-Stevenson WG, Liotta LA. Correlation of serum metalloproteinase levels with lung cancer metastasis and response to therapy. Cancer Res. 1992;52:4548–4549. [PubMed] [Google Scholar]
- [18].Nakajima M, Welch DR, Wynn DM, Tsuruo T, Nicolson GL. Serum and plasma M(r) 92,000 progelatinase levels correlate with spontaneous metastasis of rat 13762NF mammary adenocarcinoma. Cancer Res. 1993;53:5802–5807. [PubMed] [Google Scholar]
- [19].Gohji K, Fujimoto N, Komiyama T, Fujii A, Ohkawa J, Kamidono S, Nakajima M. Elevation of serum levels of matrix metalloproteinase-2 and -3 as new predictors of recurrence in patients with urothelial carcinoma. Cancer. 1996;78:2379–2387. [PubMed] [Google Scholar]
- [20].Guan KP, Ye HY, Yan Z, Wang Y, Hou SK. Serum levels of endostatin and matrix metalloproteinase-9 associated with high stage and grade primary transitional cell carcinoma of the bladder. Urology. 2003;61:719–723. doi: 10.1016/s0090-4295(02)02429-9. [DOI] [PubMed] [Google Scholar]
- [21].Vasala K, Turpeenniemi-Hujanen T. Serum tissue inhibitor of metalloproteinase-2 (TIMP-2) and matrix metalloproteinase-2 in complex with the inhibitor (MMP-2:TIMP-2) as prognostic markers in bladder cancer. Clin. Biochem. 2007;40:640–644. doi: 10.1016/j.clinbiochem.2007.01.021. [DOI] [PubMed] [Google Scholar]
- [22].Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, Xu XC. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int. J. Cancer. 2008;122:2050–2056. doi: 10.1002/ijc.23337. [DOI] [PubMed] [Google Scholar]