Summary
Background Genetic abnormalities in cell cycle control are common in malignant melanoma. UCN-01 (7-hydroxystaurosporine) is an investigational agent that exhibits antitumor activity by perturbing the cancer cell cycle. A patient with advanced melanoma experienced a partial response in a phase I trial of single agent UCN-01. We sought to determine the activity of UCN-01 against refractory metastatic melanoma in a phase II study. Patients and methods Patients with advanced melanoma received UCN-01 at 90 mg/m2 over 3 h on cycle 1, reduced to 45 mg/m2 over 3 h for subsequent cycles, every 21 days. Primary endpoint was tumor response. Secondary endpoints included progression-free survival (PFS) and overall survival (OS). A two-stage (17 + 16), single arm phase II design was employed. A true response rate of ≥20% (i.e., at least one responder in the first stage, or at least four responders overall) was to be considered promising for further development of UCN-01 in this setting. Results Seventeen patients were accrued in the first stage. One patient was inevaluable for response. Four (24%) patients had stable disease, and 12 (71%) had disease progression. As there were no responders in the first stage, the study was closed to further accrual. Median PFS was 1.3 months (95% CI, 1.2–3.0) while median OS was 7.3 months (95% CI, 3.4–18.4). One-year and two year OS rates were 41% and 12%, respectively. A median of two cycles were delivered (range, 1–18). Grade 3 treatment-related toxicities include hyperglycemia (N = 2), fatigue (N = 1), and diarrhea (N = 1). One patient experienced grade 4 creatinine elevation and grade 4 anemia possibly due to UCN-01. No dose modification was required as these patients had disease progression. Conclusion Although well tolerated, UCN-01 as a single agent did not have sufficient clinical activity to warrant further study in refractory melanoma.
Keywords: Metastatic melanoma, UCN-01, 7-hydroxystaurosporine, Cell cycle inhibitor, Phase II, Targeted therapy
Introduction
Melanoma is the sixth most common cancer in the United States, and the number of melanoma cases diagnosed annually is increasing faster than for any other cancer [1]. The management of patients with metastatic melanoma is a challenging clinical problem. Median survival time for patients with metastatic disease is approximately 6 months and less than 10% of patients will survive 5 years [1, 2]. Despite the commercial availability of numerous agents to treat melanoma, standard therapy for this malignancy today is considered experimental (www.nccn.org). Treatment approaches have traditionally included cytotoxic chemotherapy and immunotherapy, used either alone or in combination [3–6]. However, these approaches have not improved outcomes in this disease, with the recent exception of the anti-CTLA4 agent ipilimumab [7]. Thus, there is an unmet need to develop more effective approaches for the treatment of metastatic melanoma.
Genetic abnormalities in cell cycle regulation, particularly G1-S checkpoint control, are common in melanoma [8]. Regulation of G1 phase control consists of inhibition of cyclin-dependent kinases (CDKs) and upreguation of CDK inhibitors, with subsequent hypophosphorylation of the retinoblastoma (Rb) tumor suppressor protein. Particularly, disruption of the CDK inhibitors p21waf1/cip1 (hereafter, p21) and p27kip1 (hereafter, p21), and inactivation of tumor suppressor genes CDKN2A (a single gene that encodes two tumor suppressor proteins p16INK4A and p19ARF) or retinoblastoma (Rb), have been reported in metastatic melanoma [9]. Recent studies demonstrated that p16 protein loss was found in 50% of patients with familial melanomas [10] and was associated with high proliferative activity (as measured by Ki-67 staining) [11]. New agents targeting these cell cycle regulatory mechanisms may be useful in the treatment of melanoma.
UCN-01 (7-hydroxystaurosporine), a derivative of the serine/threonine kinase inhibitor staurosporine, was originally isolated from the culture broth of Streptomyces species as a protein kinase C-selective inhibitor [12]. While UCN-01 is a potent inhibitor of certain PKC isoenzymes [13], the precise mechanism of action for its antitumor activity is still not fully understood. Many clinical studies support the observation that UCN-01 causes arrest of cell cycle progression at G1/S phase and/or abrogation of arrest at G2 phase at concentrations that reduce PKC activity [14–16], although the extent to which PKC inhibition contributes to these effects remains unknown. In addition, UCN-01 exerts its anticancer activity by induction of apoptosis and sensitization to DNA-damaging agents [17, 18]. Several phase I studies of UCN-01 either as monotherapy or in combination with cytotoxic agents have been reported [19–26]. One partial response lasting 8 months was reported in a patient with refractory metastatic melanoma enrolled in a single agent phase I trial of UCN-01 [20]. UCN-01 administered as a 3-h infusion every 3 weeks led to higher dose intensity (mg/m2/h) and less toxicity compared to a 72-h infusion in a phase I study in patients with advanced solid tumors [19]. The primary objective of this single-arm phase II study was to assess the anti-tumor activity of UCN-01 monotherapy as determined by the response rate in metastatic melanoma with intended correlative target validation.
Patients and methods
Eligibility
Patients were required to have histologically or cytologically confirmed diagnosis of melanoma that was incurable by other means such as surgery, radiotherapy or limb perfusion. Patients were required to have none or one prior chemotherapy regimen and/or two or less biological therapies for metastatic disease. At least 4 weeks must have elapsed since prior therapy (6 weeks for nitrosoureas or mitomycin C) and the patient must have recovered from all toxicities attributable to prior therapy. Additional key inclusion criteria included: at least one measurable lesion by Response Evaluation Criteria in Solid Tumors (RECIST) [27], age ≥18 years, life expectancy of greater than 4 months, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; and adequate end-organ function. Patients with known brain metastases were eligible only if disease was controlled and patient was asymptomatic and not receiving corticosteroids.
This trial was reviewed, approved, and sponsored by the Cancer Therapy Evaluation Program of the National Cancer Institute (ClinicalTrials.gov, identifier NCT00072189) under a contract (N01 CM17101) with the California Cancer Consortium. The local institutional review board at each participating institution approved the protocol. All patients gave written, informed consent.
Treatment protocol
UCN-01 was provided to the NCI under a Clinical Trials Agreement (CTA) between Kyowa Hakko Kogyo Company, Ltd. and the NCI Division of Cancer Treatment and Diagnosis. UCN-01 was given at 90 mg/m2 over 3 h on cycle 1, reduced to 45 mg/m2 over 3 h for subsequent cycles. One cycle was defined as 21 days.
Evaluation of response and toxicity
All patients underwent computed tomography (CT) of the chest and abdomen within 4 weeks of registration. Tumor response was assessed every 3 weeks by clinical examination and every two cycles by CT using RECIST. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 2.0. For patients who experienced grade 3–4 toxicity, the UCN-01 was held until resolution to grade 0–1, then resumed in the next cycle with a one-dose level reduction (to 36 mg/m2 for the first reduction and to 27 mg/m2 for the second reduction).
Statistical considerations
A two-stage design was used in this study, with response rate as the primary efficacy endpoint. Secondary endpoints included overall survival (OS), progression-free survival (PFS), and toxicity. Pharmacodynamic correlates were planned to evaluate whether target inhibition (i.e., disruption of G1 phase progression) was achievable. All eligible patients were included in the efficacy analysis, and all treated patients were included in the safety analysis. The study assumed that a true response rate >20% would warrant further study of this regimen, while a true response rate of <5% would not warrant further study. Using this design, the probability of correctly declaring that an agent with a true response rate of 20% warranted further study was 0.91 (power). The probability of declaring that an agent with only a 5% true response rate warranted further study was 0.08 (alpha). In the first stage of accrual, 17 evaluable patients were to be enrolled and assessed. If no response was observed, then accrual would stop, with the conclusion that the regimen was not promising for further study in these patients. If one or more responses were seen in the first 17 patients, an additional 16 patients would be accrued in the second stage. Four or more responses out of 33 would be considered evidence that the regimen warrants further study, provided that other factors such as toxicity and survival also appeared favorable. PFS was defined as the time from registration until disease progression or death without documented progression disease. OS was defined as the time from registration until death. Patients who died without documentation of progression were considered to have progressed on the date of their death. The time to event distributions were estimated using the Kaplan-Meier method. All p-values were two-sided with statistical significance evaluated at the 0.05 alpha level.
Correlative studies
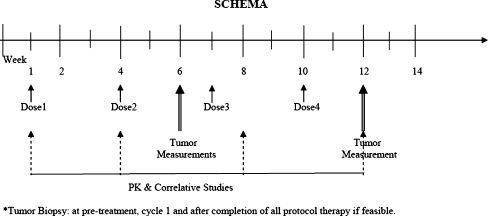
It was hypothesized that the efficacy of UCN-01 in melanoma would be dependent on the status of specific G1-phase cell cycle regulators. Further, it was hypothesized that UCN-01 activity could be seen in surrogate tissues (specifically buccal mucosal cells) and that melanoma response to UCN-01 could be monitored through surrogate markers in patient plasma, specifically secreted IL-6 or shed tumor methylated p16 DNA. It was thought that patients with tumors having low p27 expression, low Ki-67 expression, loss of RB, hypermethylation of the p16 promotor, would be less responsive to UCN-01. Figure 1 illustrates the schema for correlative studies.
Fig. 1.
Schema of the study
Immunohistochemical (IHC) staining
Pretreatment formalin-fixed, paraffin-embedded (FFPE) diagnostic specimens were obtained and analyzed for the expression of the expression of p27, Ki-67, RB and pRB by routine IHC. The commercially available antibodies included: RB (antibody clone mAB245, Chemicon International, Inc.); phosphorylated-RB (sc-7986 goat polyclonal; Santa Cruz Biotechnology); underphosphorylated-Rb (antibody clone mAB549; Chemicon International, Inc.); p27Kip1 (antibody clone DCS72; Oncogene Science); Ki-67 (Mib1 antibody; AMAC, Westbrook, ME). Both percentage of positive cells (from 1% to 100%) and intensity of staining (from 0 to 3+) were scored using the positive control tissues as 3+. Total score was established by multiplying the percentage by intensity, giving scores of 0–300. Final scores of 0, 1, 2, and 3 were given for total scores of 0, 1–50, 50–100, and 100–300, respectively. For p27, tumors staining positive in less than 40% of cells were scored as abnormal for p27.
Buccal mucosal cells
Surrogate epithelial cells from buccal brushings were obtained from selected patients prior to UCN-01 treatment and at 24hs and 48 h following initiation of UCN-01 treatment. Buccal mucosa was scraped with a brush and then transferred to a slide. Slides were fixed with 4% paraformaldehyde solution for 20 min then stored in 70% ethanol. These specimens were analyzed for p27 expression by IHC as described above, using beta-actin expression as internal control.
Tumor DNA and protein in plasma
Blood draws at pre-treatment and at the start of the second and third cycles of UCN-01 treatment were obtained for analyses of plasma proteins and shed tumor DNA. Blood samples were processed within 2 h after drawing. Plasma were assayed for levels of IL-6 using a commercially available ELISA kit (R&D Systems, Minneapolis, MN). The methylation status of p16 promoter were determined using the CpG WIZ™ Amplification Kit assay (Intergen Co. Purchase, NY), which was a two-part assay that detects unmethylated p16 promoter in one tube and any methylated p16 promoter in another reaction.
Results
Patient characteristics
Between 11/04/2003 and 12/13/2004, seventeen eligible patients were accrued to the first stage of the study. Further accrual was terminated after no responses were seen in this initial cohort. Table 1 summarizes the baseline demographic and clinical characteristics of these patients.
Table 1.
Baseline demographics and clinical characteristics of study patients
| Baseline characteristics | No. | % |
|---|---|---|
| Patients evaluable/enrolled | 16/17 | 94 |
| Gender: Female % | 11 | 65 |
| Age at enrollment, years | ||
| Median | 53 | |
| Range | 34–85 | |
| Race/Ethnicity | ||
| White | 13 | 76 |
| Hispanic | 3 | 18 |
| Asian | 1 | 6 |
| ECOG performance status | ||
| 0 | 10 | 59 |
| 1 | 6 | 35 |
| 2 | 1 | 6 |
| Primary malignant melanoma | ||
| Skin | 14 | 82 |
| Esophagus | 1 | 6 |
| Peritonium | 1 | 6 |
| Unknown | 1 | 6 |
| Primary histology | ||
| Malignant melanoma, NOS | 13 | 76 |
| Nodular melanoma | 3 | 18 |
| Malignant melanoma in junction nevus | 1 | 6 |
| Sites of metastatic disease | ||
| M1a: skin, subcutaneous or lymph nodes | 4 | 24 |
| M1b: lung | 13 | 76 |
| M1c: distant organs | 14 | 82 |
| No. of metastatic sites | ||
| 1 | 3 | 18 |
| 2 | 4 | 24 |
| 3 | 5 | 29 |
| >3 | 5 | 29 |
| Prior adjuvant/Neoadjuvant therapy | ||
| Surgery | 17 | 100 |
| Radiation | 3 | 18 |
| Immunotherapy (alpha interferon) | 5 | 29 |
| Prior metastatic therapy | ||
| Surgery | 0 | 0 |
| Radiation | 0 | 0 |
| Chemotherapy/Molecularly targeted agents | 15 | 88 |
| Chemotherapy | ||
| 0 | 7 | 41 |
| 1 | 6 | 36 |
| 2 | 4 | 24 |
| Molecularly targeted agents | ||
| 0 | 8 | 48 |
| 1 | 9 | 54 |
| Immunotherapy | 9 | 53 |
| Vaccine | 1 | 6 |
Efficacy
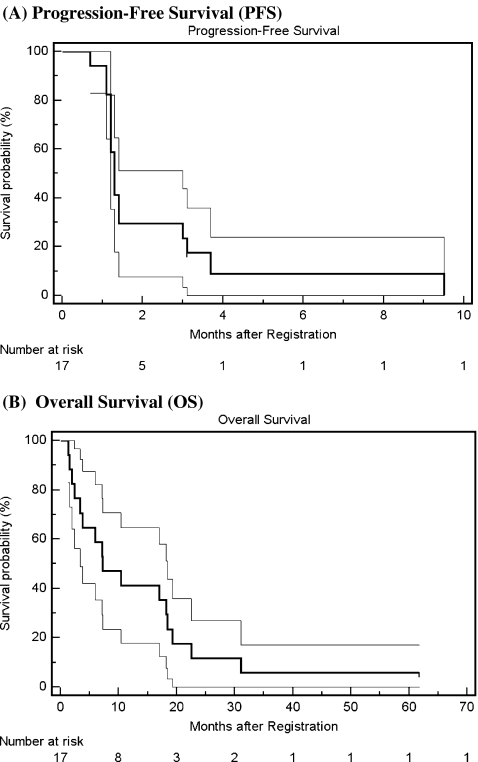
Of the 17 patients, one patient was inevaluable for response due to early withdrawal after acute abdominal pain deemed unlikely related to treatment. No objective response was observed. Four (24%) patients had stable disease after more than two cycles of treatment, and 12 (71%) patients had disease progression. All 16 evaluable patients had disease progression before death. Median PFS was 1.3 months (95% CI, 1.2–3.0; range 0.7–9.5 months). Sixteen of the 17 eligible patients had died at the time of this report. The follow-up time for the early withdrawal patient, who had been subsequently treated with temozolomide, was over 5 years at the time of data cut-off on March 25, 2010. The median OS was 7.3 months (95% CI, 3.4–18.4; range 2.4–61.8 months). One-year survival rate was 41% and two-year survival rate was 12%. Figure 2 shows the Kaplan-Meier curves of PFS and OS for all 17 patients.
Fig. 2.
Kaplan-Meier curves of progression-free survival (A) and overall survival (B) in all 17 patients
Treatment administered and adverse effects
A total of 54 cycles of treatment was given to the 17 eligible patients, with a median number of two cycles (rang, 1–18). Table 2 summarizes the possibly, probably, or definitely treatment-related adverse events in all 17 patients who received at least one dose of treatment. The most common toxicities included hyperglycemia (N = 11, 65%), nausea (N = 9, 53%), anemia (N = 7, 41%), fatigue (N = 6, 35%), vomiting (N = 5, 29%), and pain (N = 4, 24%). Grade 3 treatment-related toxicities include hyperglycemia (N = 2), fatigue (N = 1), and diarrhea (N = 1) and one patient experienced grade 4 creatinine elevation and grade 4 anemia possibly due to UCN-01. No dose modifications were required as these patients had disease progression.
Table 2.
Treatment-related adverse events
| Adverse event | Grades 1 or 2 | Grade 3 | Grade 4 | Any grade | ||||
|---|---|---|---|---|---|---|---|---|
| (N = 17) | No. of patients | % | No. of patients | % | No. of patients | % | No. of patients | % |
| Hematologic | ||||||||
| Anemia | 6 | 35 | 1 | 6 | 7 | 41 | ||
| Neutropenia | 3 | 18 | 3 | 18 | ||||
| Lymphopenia | 1 | 6 | 1 | 6 | ||||
| Thrombocytopenia | 1 | 6 | 1 | 6 | ||||
| Gastrointestinal | ||||||||
| Nausea | 9 | 53 | 9 | 53 | ||||
| Vomiting | 5 | 29 | 5 | 29 | ||||
| Diarrhea | 2 | 12 | 1 | 6 | 3 | 18 | ||
| Dehydration | 1 | 6 | 1 | 6 | ||||
| Constipation | 1 | 6 | 1 | 6 | ||||
| Abdominal pain* | 1 | 6 | 1 | 6 | ||||
| Constitutional | ||||||||
| Fatigue | 5 | 29 | 1 | 6 | 6 | 35 | ||
| Anorexia | 2 | 12 | 2 | 12 | ||||
| Fever | 1 | 6 | 1 | 6 | ||||
| Metabolic | ||||||||
| Hyperglycemia | 9 | 53 | 2 | 12 | 11 | 65 | ||
| Hypomagnesemia | 2 | 12 | 2 | 12 | ||||
| Hypermagnesemia | 1 | 6 | 1 | 6 | ||||
| Hyponatremia | 3 | 18 | 3 | 18 | ||||
| Hypocalcemia | 3 | 18 | 3 | 18 | ||||
| Hypokalemia | 2 | 12 | 2 | 12 | ||||
| Hypophosphatemia | 1 | 6 | 1 | 6 | ||||
| Hypoalbuminemia | 3 | 18 | 3 | 18 | ||||
| ALT, SGPT | 2 | 12 | 2 | 12 | ||||
| AST, SGOT | 2 | 12 | 2 | 12 | ||||
| Hyperbilirubinemia | 1 | 6 | 1 | 6 | ||||
| Hypercholesteremia | 2 | 12 | 2 | 12 | ||||
| Hypertriglyceridemia | 2 | 12 | 2 | 12 | ||||
| Hyperuricemia | 1 | 6 | 1 | 6 | ||||
| Neurologic | ||||||||
| Pain | 4 | 24 | 4 | 24 | ||||
| Neuropathy, sensory | 2 | 12 | 2 | 12 | ||||
| Dizziness | 1 | 6 | 1 | 6 | ||||
| Somnolence | 1 | 6 | 1 | 6 | ||||
| Renal | ||||||||
| Creatinine | 1 | 6 | 1 | 6 | 2 | 12 | ||
| Musculoskeletal | ||||||||
| Musculoskeletal disorder | 1 | 6 | 1 | 6 | ||||
| Cardiovascular | ||||||||
| Supraventricular and nodal arrhythmia | 1 | 6 | 1 | 6 | ||||
| Skin | ||||||||
| Rash/desquamation | 2 | 12 | 2 | 12 | ||||
Patient #11 had severe abdominal pain shortly after starting the treatment, and was off the study as “unlikely” related to treatment.
Correlative studies
Only pretreatment tumor specimens were collected, including 20 blood specimens and ten tumor biopsies that were performed 72 h after initiation of UCN-01 treatment (seven patients) and 16 buccal mucosa samples (six patients). Ten pre-treatment tumor specimens were analyzed for p27, ki-67, RB and p-RB expression by IHC. As shown in Table 3, three patients had low p27 expression and two had high Ki-67 expression. Of four patients who had sufficient tumor specimens for the analyses of RB and pRB expression, two had no RB expression and none had pRB expression. Of note, except for one patient who did not have sufficient tumor specimen for the assessment of RB and pRB expression, all other six patients had at least one abnormal expression of these cell cycle markers.
Table 3.
Summary of correlative studies
| Patient no. | IHC | IL-6 level (pg/mL) by ELISA | |||||
|---|---|---|---|---|---|---|---|
| (N = 7) | p27 | Ki-67 | RB | p-RB | Pre-treatment | Pre-second treatment | At disease progression |
| UCD-001 | 30% | 30% | 30% | 0 | 1.8 | – | 12.5 |
| UCD-002 | 0 | 50% | 60% | 0 | 110.5 | 328.7 | 213.2 |
| UCD-003 | 90% | 60% | – | – | 0 | 0.3 | 10.9 |
| UCD-004 | 80% | 35% | 0 | 0 | 6.3 | 7.8 | 16.7 |
| UCD-005 | 90% | 40% | – | – | – | – | – |
| UCD-006 | 100% | 90% | – | – | 2.6 | 5.0 | 6.0 |
| UCD-007 | 0 | 10% | 0 | 0 | 3.7 | 13.5 | 7.8 |
IHC immunohistochemistry stain. ELISA Enzyme-Linked Immunosorbent Assay
The reported normal value for IL-6 was 4.3 pg/mL
Buccal mucosa samples were assayed for p27 expression by Western blot. No p27 expression was detected in these samples. Only two of the seven serum samples had sufficient tumor-shed serum DNA for assessing the methylation status of p16 promoter: patient 002 had methylated p16 (RB IHC 60% positive) and patient 004 had unmethylated p16 expression (RB IHC zero; data not shown). These data are consistent with the previous report that abnormalities of p16 and RB genes, both constituents of the G1 checkpoint pathway, are mutually exclusive in clinical samples [28]. Interleukin-6 (IL-6) is a pleiotropic cytokine that modulates gene expression in a number of target cell types, including melanoma cells. We observed that plasma IL-6 levels by ELISA were elevated by at least two folds in post-treatment samples compared to the pre-treatment samples, reflecting the progression of disease. Since no objective response was observed in this study, no statistical correlation could be performed.
Discussion
Identification of genetic abnormalities in various components of signaling pathways involved in the initiation and progression of melanoma has provided potential new therapeutic targets. It is well known that cell cycle dysregulation, such as the control of G1 phase progression, contributes to the progression and therapeutic resistance of malignant tumors, including metastatic melanoma. Thus, cell cycle inhibitors have since been developed as possible anti-cancer agents. UCN-01 was one of the first cell cycle inhibitors evaluated in the clinical trials [23, 25, 26]. It was thought to exert its antitumor effect by through several mechanisms, such as inhibition of PKC and CDKs and up-regulation of endogenous CDK inhibitors p21 and p27. These effects have been associated with loss in G1 CDK activity and RB dephosphorylation [8]. In this study, we evaluated the clinical efficacy of UCN-01 in refractory, metastatic melanoma and found insufficient activity to warrant further study.
Since no clinical activity was observed, appropriate correlative studies could not be pursued. However, analyses on limited pre-treatment samples confirmed that genetic abnormalities in key cell cycle components, such as low level of p27, RB or pRB expression, high Ki-67 expression, methylation of p16 promoter, are common in metastatic melanoma despite expected variation in individual patients. Consistent with a previous report that plasma IL-6 level is significantly higher in melanoma patients compared to those of healthy controls [29, 30], we found that elevation of plasma IL-6 level is associated with disease progression, supporting that IL-6 might be used as a surrogate biomarker for disease progression and a valid target for the treatment of advanced melanoma.
Although our trial failed to demonstrate single agent activity of UCN-01 in metastatic melanoma, it does not prohibit further clinical evaluation of agents targeting cell cycle dysregulation [31, 32], either as a single agent or in combination with other therapeutics. Recent breakthroughs in targeting BRAF mutation and breaking down the barriers to tumor tolerance/immunity by an antibody against cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) hold great promise to improve the treatment for this deadly disease [7, 33].
Acknowledgement
Supported by: NIH N01 CM62209 (Gandara), UL1 RR024146 from the National Center for Research Resources (Li), and the American Cancer Society Institutional Research Grant 95-125-07 (Li). We thank Dr. Paul H. Gumerlock for performing the correlative studies, supported by 22XS070 TO 02.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Jemal A et al (2010) Cancer Statistics, 2010. CA Cancer J Clin
- 2.Barth A, Wanek LA, Morton DL. Prognostic factors in 1, 521 melanoma patients with distant metastases. J Am Coll Surg. 1995;181(3):193–201. [PubMed] [Google Scholar]
- 3.Tawbi HA, Buch SC. Chemotherapy resistance abrogation in metastatic melanoma. Clin Adv Hematol Oncol. 2010;8(4):259–266. [PubMed] [Google Scholar]
- 4.Mouawad R, et al. Treatment for metastatic malignant melanoma: old drugs and new strategies. Crit Rev Oncol Hematol. 2010;74(1):27–39. doi: 10.1016/j.critrevonc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Yang AS, Chapman PB. The history and future of chemotherapy for melanoma. Hematol Oncol Clin North Am. 2009;23(3):583–597. doi: 10.1016/j.hoc.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middleton MR, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauroja I, et al. Analysis of G(1)/S checkpoint regulators in metastatic melanoma. Genes Chromosom Cancer. 2000;28(4):404–414. doi: 10.1002/1098-2264(200008)28:4<404::AID-GCC6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 9.High WA, Robinson WA. Genetic mutations involved in melanoma: a summary of our current understanding. Adv Dermatol. 2007;23:61–79. doi: 10.1016/j.yadr.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri G, et al. Definition of the role of chromosome 9p21 in sporadic melanoma through genetic analysis of primary tumours and their metastases. The Melanoma Cooperative Group. Br J Cancer. 2000;83(12):1707–1714. doi: 10.1054/bjoc.2000.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuhahula E, Straume O, Akslen LA. Frequent loss of p16 protein expression and high proliferative activity (Ki-67) in malignant melanoma from black Africans. Anticancer Res. 2000;20(6C):4857–4862. [PubMed] [Google Scholar]
- 12.Takahashi I, et al. UCN-01, a selective inhibitor of protein kinase C from Streptomyces. J Antibiot (Tokyo) 1987;40(12):1782–1784. doi: 10.7164/antibiotics.40.1782. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi I, et al. Potent selective inhibition of 7-O-methyl UCN-01 against protein kinase C. J Pharmacol Exp Ther. 1990;255(3):1218–1221. [PubMed] [Google Scholar]
- 14.Akiyama T, et al. G1-checkpoint function including a cyclin-dependent kinase 2 regulatory pathway as potential determinant of 7-hydroxystaurosporine (UCN-01)-induced apoptosis and G1-phase accumulation. Jpn J Cancer Res. 1999;90(12):1364–1372. doi: 10.1111/j.1349-7006.1999.tb00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akiyama T, et al. G1 phase accumulation induced by UCN-01 is associated with dephosphorylation of Rb and CDK2 proteins as well as induction of CDK inhibitor p21/Cip1/WAF1/Sdi1 in p53-mutated human epidermoid carcinoma A431 cells. Cancer Res. 1997;57(8):1495–1501. [PubMed] [Google Scholar]
- 16.Abe S, et al. UCN-01 (7-hydoxystaurosporine) inhibits in vivo growth of human cancer cells through selective perturbation of G1 phase checkpoint machinery. Jpn J Cancer Res. 2001;92(5):537–545. doi: 10.1111/j.1349-7006.2001.tb01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuse E, et al. Review of UCN-01 development: a lesson in the importance of clinical pharmacology. J Clin Pharmacol. 2005;45(4):394–403. doi: 10.1177/0091270005274549. [DOI] [PubMed] [Google Scholar]
- 18.Kurata N, et al. Pharmacokinetics and pharmacodynamics of a novel protein kinase inhibitor, UCN-01. Cancer Chemother Pharmacol. 1999;44(1):12–18. doi: 10.1007/s002800050939. [DOI] [PubMed] [Google Scholar]
- 19.Dees EC, et al. A phase I and pharmacokinetic study of short infusions of UCN-01 in patients with refractory solid tumors. Clin Cancer Res. 2005;11(2 Pt 1):664–671. [PubMed] [Google Scholar]
- 20.Sausville EA, et al. Phase I trial of 72-hour continuous infusion UCN-01 in patients with refractory neoplasms. J Clin Oncol. 2001;19(8):2319–2333. doi: 10.1200/JCO.2001.19.8.2319. [DOI] [PubMed] [Google Scholar]
- 21.Lara PN, Jr, et al. The cyclin-dependent kinase inhibitor UCN-01 plus cisplatin in advanced solid tumors: a California cancer consortium phase I pharmacokinetic and molecular correlative trial. Clin Cancer Res. 2005;11(12):4444–4450. doi: 10.1158/1078-0432.CCR-04-2602. [DOI] [PubMed] [Google Scholar]
- 22.Hotte SJ, et al. Phase I trial of UCN-01 in combination with topotecan in patients with advanced solid cancers: a Princess Margaret Hospital Phase II Consortium study. Ann Oncol. 2006;17(2):334–340. doi: 10.1093/annonc/mdj076. [DOI] [PubMed] [Google Scholar]
- 23.Jimeno A, et al. Phase I and pharmacokinetic study of UCN-01 in combination with irinotecan in patients with solid tumors. Cancer Chemother Pharmacol. 2008;61(3):423–433. doi: 10.1007/s00280-007-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welch S, et al. UCN-01 in combination with topotecan in patients with advanced recurrent ovarian cancer: a study of the Princess Margaret Hospital Phase II consortium. Gynecol Oncol. 2007;106(2):305–310. doi: 10.1016/j.ygyno.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Fracasso PM et al (2010) A phase 1 study of UCN-01 in combination with irinotecan in patients with resistant solid tumor malignancies. Cancer Chemother Pharmacol [DOI] [PMC free article] [PubMed]
- 26.Kummar S, et al. A phase I trial of UCN-01 and prednisone in patients with refractory solid tumors and lymphomas. Cancer Chemother Pharmacol. 2010;65(2):383–389. doi: 10.1007/s00280-009-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Rodolfo M, Pierotti MA, Parmiani G. A merging duo in melanoma formation. J Invest Dermatol. 2005;125(6):xii–xiii. doi: 10.1111/j.0022-202X.2005.23965.x. [DOI] [PubMed] [Google Scholar]
- 29.Lazar-Molnar E, et al. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12(6):547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]
- 30.Porter GA, et al. Significance of plasma cytokine levels in melanoma patients with histologically negative sentinel lymph nodes. Ann Surg Oncol. 2001;8(2):116–122. doi: 10.1007/s10434-001-0116-3. [DOI] [PubMed] [Google Scholar]
- 31.Sausville EA. Cyclin-dependent kinase modulators studied at the NCI: pre-clinical and clinical studies. Curr Med Chem Anticancer Agents. 2003;3(1):47–56. doi: 10.2174/1568011033353560. [DOI] [PubMed] [Google Scholar]
- 32.Senderowicz AM. Inhibitors of cyclin-dependent kinase modulators for cancer therapy. Prog Drug Res. 2005;63:183–206. doi: 10.1007/3-7643-7414-4_8. [DOI] [PubMed] [Google Scholar]
- 33.Flaherty KT, McArthur G (2010) BRAF, a target in melanoma: implications for solid tumor drug development. Cancer [DOI] [PubMed]