Summary
Background Sunitinib is an oral multitargeted tyrosine kinase inhibitor of vascular endothelial growth factor and platelet-derived growth factor receptors, as well as of other receptor types. We have performed a feasibility study to investigate the safety of sunitinib in combination with pemetrexed for treatment of advanced refractory solid tumors. Methods Sunitinib was administered once daily on a continuous daily dosing (CDD) schedule (37.5 mg/day) or a 2-weeks-on, 1-week-off treatment schedule (50 mg/day, Schedule 2/1) in combination with pemetrexed at 500 mg/m2 on day 1 of repeated 21-day cycles. Results Twelve patients were enrolled in the study: six on the CDD schedule and six on Schedule 2/1. None of the treated patients experienced a dose-limiting toxicity. Toxicities were manageable and similar in type to those observed in monotherapy studies of sunitinib and pemetrexed. Pharmacokinetic analysis did not reveal any substantial drug–drug interaction. One patient with squamous cell lung cancer showed a partial response and five patients had stable disease. Conclusions Combination therapy with sunitinib administered on Schedule 2/1 (50 mg/day) or a CDD schedule (37.5 mg/day) together with standard-dose pemetrexed (500 mg/m2) was well tolerated in previously treated patients with advanced solid tumors.
Keywords: Sunitinib, Pemetrexed, Feasibility study, Solid tumors
Introduction
Progress in the molecular biology of solid tumors has established the important role of tumor angiogenesis and the multiple signaling pathways underlying this process in tumor development [1]. Moreover, antiangiogenic therapy that targets signaling by the vascular endothelial growth factor (VEGF) pathway represents a key advance in clinical oncology [2, 3]. Sunitinib (SUTENT®) is an oral multitargeted tyrosine kinase inhibitor of VEGF receptors (VEGFR1 to VEGFR3), platelet-derived growth factor receptors (PDGFRα and PDGFRβ), and other receptor tyrosine kinases [4–6]. It has shown single-agent activity and acceptable tolerability in phase I/II studies of patients with a variety of advanced refractory solid tumors [4]. The clinical benefits observed with sunitinib have resulted in multinational approval for its use in the treatment of patients with advanced renal cell carcinoma or imatinib-resistant or -intolerant gastrointestinal stromal tumors [7, 8].
As targeted agents such as sunitinib enter into clinical practice, there is interest in assessment of the efficacy and safety of these agents administered in combination with chemotherapy in cancer patients, including those with treatment-refractory tumors. Preclinical studies indicate that the combination of sunitinib with chemotherapeutic agents results in increased antitumor activity [9]. One chemotherapeutic agent tested, pemetrexed, is an antimetabolite that suppresses cell replication by inhibiting multiple enzymes in the folate pathway and which shows clinical activity against a broad range of solid tumors, including non-small cell lung cancer (NSCLC) and mesothelioma [10–12]. The adverse effects of sunitinib are largely nonoverlapping with those of pemetrexed, making the latter an appropriate agent, in terms of safety, for combination with sunitinib. We have now performed a feasibility study to assess the safety and tolerability of two dosing schedules of sunitinib (continuous daily dosing [CDD] schedule and 2 weeks on treatment followed by 1 week off treatment [Schedule 2/1]) in combination with fixed-dose (500 mg/m2) pemetrexed.
Patients and methods
Study population
Patients with histologically proven advanced solid tumors and who were 20 years of age or older were enrolled in the study. Other key inclusion criteria included: prior treatment with one or more chemotherapy regimens; an Eastern Cooperative Oncology Group performance status of ≤1; resolution of acute toxicities resulting from prior therapy; adequate organ function; and a life expectancy of ≥3 months. Key exclusion criteria included: prior treatment with pemetrexed or sunitinib or irradiation of ≥ 25% of bone marrow; hemoptysis (≥5 mL per episode or ≥10 mL/day) occurring ≤4 weeks before the onset of study treatment; chemotherapy, surgery, or radiation therapy instituted <4 weeks before the start of the study (with the exception of palliative radiotherapy for nontarget lesions); symptomatic or uncontrolled brain metastases, spinal cord compression, carcinomatous meningitis, or leptomeningeal disease; a history of cardiac disease, cerebrovascular events, or pulmonary embolism within the 12 months prior to the onset of study treatment; ongoing cardiac dysrhythmias of National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) grade ≥ 2, atrial fibrillation (any grade), or a prolonged QTc interval; hemorrhage of CTCAE grade 3 within the 4 weeks before the start of the study treatment; or hypertension that could not be controlled with standard antihypertensive agents.
Study design and treatment
The study was a randomized, open-label study (NCT00732992) of sunitinib in combination with pemetrexed in patients with advanced solid tumors. The primary objective was assessment of overall safety, including dose-limiting toxicities (DLTs), for two treatment regimens of sunitinib plus pemetrexed. Secondary endpoints included plasma pharmacokinetic evaluations and preliminary antitumor activity.
Sunitinib was administered orally, once daily, according to either the CDD schedule or Schedule 2/1. Pemetrexed (500 mg/m2) was administered as a 10-min infusion on day 1 of a 21-day cycle. Patients were instructed to take 500 μg of folate daily, beginning 1 week before day 1 of cycle 1 until study discontinuation. Vitamin B12 (1 g) was injected intramuscularly 1 week before day 1 of cycle 1 and again every 9 weeks until study discontinuation. A phase I dose-escalation trial of sunitinib in combination with pemetrexed conducted outside of Japan had demonstrated tolerability of the combination of sunitinib at 37.5 mg (CDD schedule) or 50 mg (Schedule 2/1) with pemetrexed at 500 mg/m2 [13]. On the basis of these results, we selected the starting doses of sunitinib in the present study as 37.5 mg for the CDD schedule cohort and 50 mg for the Schedule 2/1 cohort. Doses were interrupted or reduced if adverse events of grade 3 or 4 were observed. Doses were delayed if a patient did not meet the following criteria on the first day of each subsequent cycle: absolute neutrophil count of ≥2,000 cells/μL, platelet count of ≥100,000 cells/μL, and calculated creatinine clearance of ≥45 mL/min. Patients were allowed to undergo a maximum of two dose reductions of either drug; the minimum dose for pemetrexed was 250 mg/m2 and that for sunitinib was 25 mg/day. Treatment was repeated in a 21-day (3-week) cycle until disease progression, unacceptable toxicity, or withdrawal of patient consent occurred. DLTs were assessed during the first treatment cycle and were used to determine whether the dose or schedule was feasible. They were defined as drug-related toxicities of grade 3 or 4, including neutropenia (grade 3 with infection, grade 4 for ≥7 days, or febrile for >24 h), thrombocytopenia (grade ≥ 3 with bleeding or grade 4 for ≥7 days), lymphopenia accompanied by an opportunistic infection, or any nonhematologic toxicity of grade 3 or 4 for ≥7 days. Initially, six patients were randomized to each dosing schedule (three patients each). If no more than one of the three patients experienced a DLT by day 21 of cycle 1, then an additional six patients (three patients each) were randomized for treatment at the same dose. If ≥2/3 or ≥2/6 patients on a schedule experienced a DLT, the dose was reduced and three additional patients were enrolled.
All patients provided written informed consent. The study was approved by the institutional review board of Kinki University Hospital and was performed in accordance with the International Conference on Harmonization of Good Clinical Practice guidelines, as well as with applicable local laws and regulatory requirements.
Study assessments
Safety was assessed according to CTCAE version 3.0. In patients with measurable disease, objective response was determined according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 [14].
Pharmacokinetic evaluations
For patients randomized to the CDD schedule, blood samples were collected on day 1 of cycle 2 (sunitinib, before as well as 2, 4, 6, 8, 10, and 24 h after dosing; pemetrexed, before as well as 10 min and 1, 2, 4, 6, 8, 10, and 24 h after dosing) to evaluate pharmacokinetic parameters. For patients randomized to Schedule 2/1, blood samples were collected on day 14 of cycle 1 to determine the trough level of sunitinib. The plasma concentrations of sunitinib, its active metabolite (SU12662), and pemetrexed were measured by validated high-performance liquid chromatography and tandem mass spectrometry, with the lower limits of detection being 0.1 ng/mL for sunitinib and SU12662 and 0.1 μg/mL for pemetrexed. Standard plasma pharmacokinetic parameters were estimated by noncompartmental methods. They included the maximum plasma concentration (C max), plasma predose concentration (C trough), time to C max (T max), area under the plasma concentration-time profile from time zero to 24 h after dosing (AUC0–24), area under the plasma concentration-time profile from time zero to infinity (AUC0–∞), elimination half-life (t 1/2), oral clearance (CL/F), clearance (CL), and volume of distribution at steady state (V ss).
Statistical analysis
Given the exploratory nature of the study, all analyses were descriptive, with no formal statistical test performed on the data.
Results
Patient characteristics
Twelve patients were enrolled in the study from August to November 2008: six patients for the CDD schedule and six for Schedule 2/1. The most common malignancy in the 12 treated patients was NSCLC (n = 9, 75%). All patients received at least one dose of the study treatment. Patient demographic and baseline characteristics are summarized in Table 1.
Table 1.
Patient characteristics according to dosing schedule
| CDD schedule (n = 6) | Schedule 2/1 (n = 6) | |
|---|---|---|
| Median (range) age (years) | 55.5 (48–69) | 66.0 (57–69) |
| Male/female (n) | 6/0 | 4/2 |
| ECOG performance status 0/1 (n) | 2/4 | 4/2 |
| Primary malignancy (n) | ||
| NSCLC | 6 | 3 |
| Pancreatic cancer | 0 | 1 |
| Pancreatic neuroendocrine tumor | 0 | 1 |
| Uterine sarcoma | 0 | 1 |
| Previous therapy (n) | ||
| Surgery | 2 | 2 |
| Chemotherapy | 6 | 6 |
| Number of prior regimens (n) | ||
| 1 | 3 | 5 |
| 2 | 2 | 1 |
| ≥3 | 1 | 0 |
CDD schedule continuous daily dosing of sunitinib (37.5 mg) plus pemetrexed (500 mg/m2) once every 3 weeks, Schedule 2/1 2-weeks-on and 1-week-off dosing of sunitinib (50 mg) plus pemetrexed (500 mg/m2) once every 3 weeks, ECOG Eastern Cooperative Oncology Group, NSCLC non-small cell lung cancer
Treatment delivery
A total of 66 cycles of treatment with sunitinib plus pemetrexed was completed, with a median number of cycles per patient of four for the CDD schedule and five for Schedule 2/1. All 12 patients were ultimately withdrawn from the study, the most common reason for which was disease progression (three patients on the CDD schedule and five patients on Schedule 2/1). Treatment was withdrawn because of adverse events in one patient on each schedule (hemoglobin decrease for the CDD schedule and febrile neutropenia for Schedule 2/1). Seven dose reductions each for sunitinib and pemetrexed were instituted (three for the CDD schedule and four for Schedule 2/1), mainly as a result of myelosuppression.
Safety
All 12 patients were evaluable for safety analysis. None of the patients treated on the CDD schedule or Schedule 2/1 experienced a DLT, whereas all individuals experienced at least one adverse event during the study. The major adverse events during the entire treatment period are shown in Table 2. The most common nonhematologic toxicities (any grade) across both schedules were fatigue (n = 11), taste alteration (n = 9), skin discoloration (n = 8), anorexia (n = 8), and fever (n = 8). Nonhematologic toxicities of grade 3 included diarrhea (n = 2) as well as fatigue, proteinuria, and dehydration (n = 1 each) on the CDD schedule, and an increase in alanine aminotransferase and hypertension (n = 1 each) on Schedule 2/1. No nonhematologic toxicities of grade 4 were observed for either schedule. The most common hematologic toxicity of grade 3 or 4 was a decrease in neutrophil number, with six patients (CDD schedule, n = 4; Schedule 2/1, n = 2) experiencing this adverse event at grade 3 and two patients (n = 1 for each schedule) at grade 4. Other hematologic toxicities of grade 3 or 4 included a decrease in leukocytes of grade 3 in four patients (CDD schedule, n = 3; Schedule 2/1, n = 1), a decrease in platelets of grade 3 in three patients (CDD schedule, n = 2; Schedule 2/1, n = 1), and a decrease in hemoglobin level of grade 3 in one patient (CDD schedule).
Table 2.
Treatment-emergent (all-causality) adverse events (NCI CTCAE version 3.0) occurring with an incidence of ≥ 2 cases (or of special interest) in patients on either the CDD schedule or Schedule 2/1
| Adverse event | CDD schedule (n = 6) | Schedule 2/1 (n = 6) | Total (n = 12) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | All gradesa | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| Nonhematologic | |||||||||
| Fatigue | 3 | 1 | 1 | 0 | 6 | 0 | 0 | 0 | 11 |
| Taste alteration | 1 | 2 | 0 | 0 | 6 | 0 | 0 | 0 | 9 |
| Skin discoloration | 5 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 8 |
| Anorexia | 3 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 8 |
| Fever | 3 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 8 |
| AST increased | 4 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 6 |
| Diarrhea | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 5 |
| Stomatitis | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 5 |
| ALT increased | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 5 |
| Hypoalbuminemia | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 5 |
| Hand–foot syndrome | 2 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 5 |
| Vomiting | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 4 |
| Cough | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 4 |
| Rash | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 |
| Eyelid edema | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 |
| Cheilitis | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 |
| Nausea | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 3 |
| Nasopharyngitis | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 |
| Proteinuria | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 3 |
| Constipation | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Edema | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Infection | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| Dehydration | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Pain-joint | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Headache | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Neuropathy | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Hypertension | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Hypothyroidismb | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| TSH increasedb | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Epistaxisb | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hemorrhageb | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hematologic | |||||||||
| Platelets decreased | 3 | 1 | 2 | 0 | 3 | 0 | 1 | 0 | 10 |
| Leukocytes decreased | 0 | 2 | 3 | 0 | 0 | 4 | 1 | 0 | 10 |
| Neutrophils decreased | 0 | 0 | 4 | 1 | 0 | 1 | 2 | 1 | 9 |
| Hemoglobin decreased | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 5 |
| Lymphopenia | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Febrile neutropeniab | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
NCI CTCAE National Cancer Institute Common Terminology Criteria for Adverse Events, CDD continuous daily dosing, AST aspartate aminotransferase, ALT alanine aminotransferase, TSH thyroid-stimulating hormone
aNo adverse events of grade 5 occurred
bAdverse events of special interest occurring with an incidence of <2 on either the CDD schedule or Schedule 2/1
Adverse events considered to be serious occurred in three patients (CDD schedule, n = 2; Schedule 2/1, n = 1): one patient on the CDD schedule had dehydration (grade 2), one patient on the CDD schedule had infectious enteritis and dehydration (both of grade 3), and one patient on Schedule 2/1 had pyrexia (grade 2), pneumothorax (grade 1), pleural effusion (grade 1), and febrile neutropenia (grade 3). There were no deaths during the study.
Pharmacokinetics
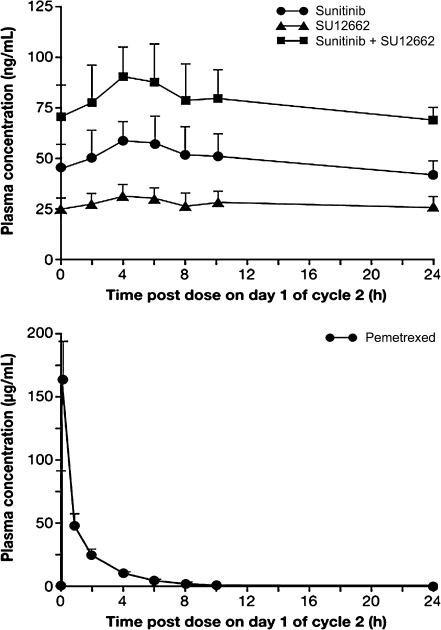
The mean plasma concentration-time profiles and pharmacokinetic parameters for sunitinib, its active metabolite (SU12662), total drug (sunitinib + SU12662), and pemetrexed for three patients who received the planned treatment on the CDD schedule are shown in Fig. 1 and Tables 3 and 4. The mean C trough for day 1 of cycle 2 was 45.6 ng/mL for sunitinib, 25.1 ng/mL for SU12662, and 70.6 ng/mL for total drug, and each of the corresponding mean plasma concentration-time profiles showed relatively slow absorption and elimination, consistent with previous observations [15]. The pharmacokinetic parameters obtained for sunitinib (37.5 mg) on the CDD schedule with pemetrexed (500 mg/m2) in the present study did not appear to differ substantially from the dose-normalized parameters previously obtained for single dosing of sunitinib at 25 or 50 mg [15, 16]. The plasma concentration of pemetrexed during sunitinib continuous dosing declined with a fast elimination rate (mean t 1/2 was 2.75 h), and the t 1/2, CL, and V ss values were similar to those previously obtained for single dosing of pemetrexed at 500 mg/m2 [17]. For Schedule 2/1, the mean C trough for day 14 of cycle 1 in six patients who received the planned treatment was 78.5 ng/mL for sunitinib, 38.2 ng/mL for SU12662, and 117.0 ng/mL for total drug. The plasma concentration of sunitinib observed for both schedules was considered to have achieved a steady state on the basis of previous results [15]. The C trough values of sunitinib, SU12662, and total drug observed for both the CDD schedule (sunitinib, 37.5 mg/day) and Schedule 2/1 (sunitinib, 50 mg/day) suggested that the plasma concentrations increased in a dose-dependent manner.
Fig. 1.
Plasma concentration-time profiles for sunitinib, SU12662, total drug (sunitinib + SU12662), and pemetrexed on day 1 of cycle 2 for the CDD schedule. Data are means ± standard deviation (SD) for three patients
Table 3.
Pharmacokinetic parameters of sunitinib, SU12662, and total drug (sunitinib + SU12662) for the CDD schedule
| Parameter | Sunitinib | SU12662 | Total drug |
|---|---|---|---|
| C trough (ng/mL) | 45.6 ± 11.7 (26) | 25.1 ± 5.08 (20) | 70.6 ± 13.9 (20) |
| [41.3] | [28.0] | [69.3] | |
| T max (h) | 4 (4–6) | 4 (4–4) | 4 (4–6) |
| C max (ng/mL) | 59.9 ± 10.9 (18) | 31.6 ± 5.49 (17) | 91.56 ± 14.2 (15) |
| [59.6] | [34.7] | [94.3] | |
| AUC0–24 (ng·h/mL) | 1,190 ± 247 (21) | 675 ± 107 (16) | 1,866 ± 269 (14) |
| [1,161] | [665] | [1,951] | |
| CL/F (L/h) | 32.4 ± 6.65 (20) | ND | ND |
| [32.3] |
CDD continuous daily dosing, ND no data, SD standard deviation
Data are arithmetic means ± SD (coefficient of variation, (%) [median], with the exception of those for T max, which are medians (range). Sampling was performed on day 1 of cycle 2
Table 4.
Pharmacokinetic parameters of pemetrexed for the CDD schedule
| Parameter | Value |
|---|---|
| T max (h) | 0.167 (0.167–0.167) |
| C max (μg/mL) | 1636 ± 30.7 (19) [167] |
| AUC0–∞ (μg·h/mL) | 1916 ± 36.3 (19) [202] |
| t 1/2 (h) | 2.7546 ± 0.531 (19) [2.558] |
| CL (L/h) | 4.976 ± 1.38 (28) [4.30] |
| V ss (L) | 10.46 ± 3.13 (30) [10.9] |
CDD continuous daily dosing, SD standard deviation
Data are arithmetic means ± SD (coefficient of variation, (%) [median], with the exception of those for T max, which are medians (range). Sampling was performed on day 1 of cycle 2
Tumor response
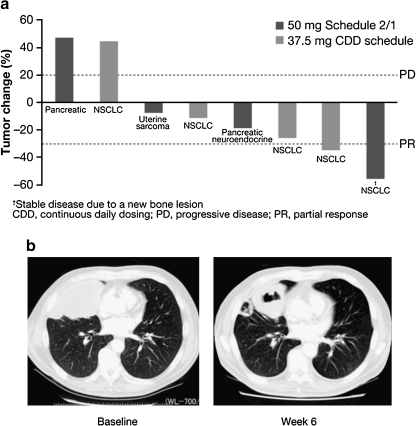
Eight of the 12 patients were evaluable for response by RECIST. A partial response was observed in one patient with NSCLC on the CDD schedule, whereas five patients (two on the CDD schedule and three on Schedule 2/1) had stable disease (Fig. 2a). Most patients showed a decrease in the size of the target lesion while on the study treatment.
Fig. 2.
Tumor response. a maximum percentage change in the size of the target lesion in the eight evaluable patients. PD progressive disease, PR partial response, †stable disease due to a new bone lesion. b computed tomography of a solid tumor in the right lung of a patient indicated by † in part a at baseline (left panel) and on day 14 of cycle 2 for the CDD schedule. The tumor showed marked central cavitation after treatment
Discussion
Our feasibility study investigated the overall safety of sunitinib administered on the CDD schedule or Schedule 2/1 in combination with pemetrexed for the treatment of subjects with advanced refractory solid tumors. Phase I studies of sunitinib monotherapy have been performed according to various schedules, including a 3-week cycle consisting of treatment for 2 weeks followed by a 1-week rest period (Schedule 2/1), a 4-week cycle comprising treatment for 2 weeks followed by 2 weeks off treatment (Schedule 2/2), or a 6-week cycle of treatment for 4 weeks followed by 2 weeks off treatment (Schedule 4/2) [18, 19]. Daily dosing with sunitinib at 50 mg resulted in a target plasma concentration greater than the 50 ng/mL required to inhibit PDGFR and VEGFR, and DLTs of fatigue, asthenia, and thrombocytopenia occurred at a dose of 75 mg on all schedules; a recommended dose of 50 mg was thus established for Schedules 2/1, 2/2, and 4/2 [4]. Preclinical and clinical studies showing tumor regrowth during the off-dosing period suggested that better tumor control might be achieved with sunitinib on a CDD schedule [20, 21]. Subsequent clinical trials demonstrated that CDD of sunitinib at 37.5 mg was well tolerated and showed clinical activity largely similar to that observed for administration on intermittent schedules, providing flexibility in dosing schedule [22–24].
Phase I studies have shown that myelosuppression is the predominant DLT of pemetrexed [25]. We previously found that the maximum tolerated dose of pemetrexed supplemented with folic acid and vitamin B12 was 1,200 mg/m2, which was twice the previously determined such dose (600 mg/m2) for administration without vitamin supplementation [17, 26]. The results of randomized trials comparing pemetrexed at 500 mg/m2 versus 900 mg/m2 or 1,000 mg/m2 in patients with recurrent NSCLC showed that the higher doses did not exhibit a greater clinical efficacy than the lower dose, thereby establishing the clinically recommended dose of 500 mg/m2 for pemetrexed supplemented with folic acid and vitamin B12 [27, 28].
Given the differences in metabolism and elimination between sunitinib and pemetrexed, we assessed the safety of the combination of recommended doses of these drugs. We initiated treatment with sunitinib at 50 mg/day on Schedule 2/1 or at 37.5 mg on the CDD schedule together with pemetrexed at 500 mg/m2. There were no DLTs in the 12 patients of the present study who received both drugs at the recommended single-agent doses. Most toxicities were mild or moderate in extent, and similar in type to those observed in the monotherapy studies of sunitinib and pemetrexed. All toxicities of grade 3 or 4 were reversible and manageable with symptomatic treatment and dose reduction or interruption. Hypertension is often associated with treatment with angiogenesis inhibitors, including sunitinib, but this condition developed in only two patients in the present study and, in both cases, blood pressure was controlled with standard antihypertensive therapy. No patients experienced cardiac abnormalities, including electrocardiogram (ECG) changes or a decline in left ventricular ejection fraction to below the lower limit (50%).
In the present study, the full pharmacokinetic profile was evaluated at steady state only for the CDD schedule, given that pharmacokinetic interaction is generally assessed with high drug exposure. The concomitant administration of pemetrexed and sunitinib showed no marked effect on the pharmacokinetics of either drug, compared with previous single-dosing results. These findings suggest that there was no substantial pharmacokinetic interaction between sunitinib and pemetrexed, consistent with the differences in the pathways of metabolism and elimination for these drugs. Sunitinib is primarily metabolized by cytochrome P450-3A4 (CYP3A4) in hepatic microsomes, whereas pemetrexed is not metabolized to an appreciable extent, but is primarily eliminated renally [4, 29]. It is not likely that sunitinib or its metabolites inhibit the renal elimination of pemetrexed. In addition, in vitro studies with human liver microsomes suggested that pemetrexed administration is not likely to result in clinically relevant inhibition of the metabolic clearance of drugs metabolized by CYP3A [30]. The trough plasma concentrations for total drug (sunitinib + SU12662) in both treatment arms of the present study suggest that sufficient exposure was achieved with regard to target inhibition, according to the required inhibitory concentration values.
Although tumor evaluation was not the primary objective of the present study, and the small sample size precludes any definitive conclusions regarding treatment efficacy, antitumor activity data were suggestive of a potential clinical benefit. It is possible that further pemetrexed studies might be restricted to patients with nonsquamous NSCLC because of the pemetrexed label indications [31]. However, the one partial response in the present study was observed in a patient with squamous NSCLC; the tumor cavitation apparent in this patient after study treatment (Fig. 2b) is characteristic of the antitumor effect of antiangiogenic therapy. Given that sunitinib has shown promising single-agent activity in patients with recurrent NSCLC [22, 32], further research is warranted to determine whether sunitinib might improve the effect of pemetrexed, not only in nonsquamous NSCLC, but also in squamous NSCLC.
In conclusion, combination therapy with sunitinib administered according to Schedule 2/1 (50 mg/day), or a CDD schedule (37.5 mg/day) together with standard-dose pemetrexed (500 mg/m2), was well tolerated in previously treated patients with advanced solid tumors. In both dosing schedules, sunitinib exposure remained above the target plasma concentration in the presence of pemetrexed. Given that both sunitinib and pemetrexed have shown antitumor activity as single agents for various types of solid tumors including NSCLC, sunitinib in combination with pemetrexed is a viable therapeutic regimen that warrants future investigation.
Acknowledgments
This work was sponsored by Pfizer Inc. Medical writing support was provided by Lisa Cheyne at ACUMED® (Tytherington, UK) and was funded by Pfizer Inc. We would like to thank all of the participating patients and their families, as well as the global network of investigators, research nurses, study coordinators, and operations staff.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
ClinicalTrials.gov identifier = NCT00732992.
References
- 1.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 2.Morabito A, De Maio E, Di Maio M, et al. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11:753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 3.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6:569–579. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 4.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 5.Papaetis GS, Syrigos KN. Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs. 2009;23:377–389. doi: 10.2165/11318860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Heng DY, Kollmannsberger C. Sunitinib. Recent Results Cancer Res. 2010;184:71–82. doi: 10.1007/978-3-642-01222-8_6. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 9.Christensen JG, Hall C, Hollister BA (2008) Antitumor efficacy of sunitinib malate in concurrent and sequential combinations with standard chemotherapeutic agents in non-small cell lung cancer (NSCLC) nonclinical models. Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; San Diego, CA. Abstract nr 1433.
- 10.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 11.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 12.Walling J. From methotrexate to pemetrexed and beyond. A review of the pharmacodynamic and clinical properties of antifolates. Invest New Drugs. 2006;24:37–77. doi: 10.1007/s10637-005-4541-1. [DOI] [PubMed] [Google Scholar]
- 13.Chow LQM, Jonker DJ, Call JA, et al. A phase I dose-escalation study of sunitinib in combination with pemetrexed in patients with advanced solid malignancies. Ann Oncol. 2008;19(Suppl 8):480P. [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Shirao K, Nishida T, Doi T et al. (2009) Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. Invest New Drugs Sep 3 (Epub ahead of print) [DOI] [PubMed]
- 16.Uemura H, Shinohara N, Yuasa T, et al. A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol. 2010;40:194–202. doi: 10.1093/jjco/hyp146. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa K, Kudoh S, Matsui K, et al. A phase I study of pemetrexed (LY231514) supplemented with folate and vitamin B12 in Japanese patients with solid tumours. Br J Cancer. 2006;95:677–682. doi: 10.1038/sj.bjc.6603321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 19.Britten CD, Kabbinavar F, Hecht JR, et al. A phase I and pharmacokinetic study of sunitinib administered daily for 2 weeks, followed by a 1-week off period. Cancer Chemother Pharmacol. 2008;61:515–524. doi: 10.1007/s00280-007-0498-4. [DOI] [PubMed] [Google Scholar]
- 20.Abrams TJ, Murray LJ, Pesenti E, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with “standard of care” therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2:1011–1021. [PubMed] [Google Scholar]
- 21.Burstein HJ, Elias AD, Rugo HS, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 22.Novello S, Scagliotti GV, Rosell R, et al. Phase II study of continuous daily sunitinib dosing in patients with previously treated advanced non-small cell lung cancer. Br J Cancer. 2009;101:1543–1548. doi: 10.1038/sj.bjc.6605346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Escudier B, Roigas J, Gillessen S, et al. Phase II study of sunitinib administered in a continuous once-daily dosing regimen in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27:4068–4075. doi: 10.1200/JCO.2008.20.5476. [DOI] [PubMed] [Google Scholar]
- 24.George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959–1968. doi: 10.1016/j.ejca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Rinaldi DA. Overview of phase I trials of multitargeted antifolate (MTA, LY231514) Semin Oncol. 1999;26:82–88. [PubMed] [Google Scholar]
- 26.Rinaldi DA, Kuhn JG, Burris HA, et al. A phase I evaluation of multitargeted antifolate (MTA, LY231514), administered every 21 days, utilizing the modified continual reassessment method for dose escalation. Cancer Chemother Pharmacol. 1999;44:372–380. doi: 10.1007/s002800050992. [DOI] [PubMed] [Google Scholar]
- 27.Cullen MH, Zatloukal P, Sorenson S, et al. A randomized phase III trial comparing standard and high-dose pemetrexed as second-line treatment in patients with locally advanced or metastatic non-small-cell lung cancer. Ann Oncol. 2008;19:939–945. doi: 10.1093/annonc/mdm592. [DOI] [PubMed] [Google Scholar]
- 28.Ohe Y, Ichinose Y, Nakagawa K, et al. Efficacy and safety of two doses of pemetrexed supplemented with folic acid and vitamin B12 in previously treated patients with non-small cell lung cancer. Clin Cancer Res. 2008;14:4206–4212. doi: 10.1158/1078-0432.CCR-07-5143. [DOI] [PubMed] [Google Scholar]
- 29.Villela LR, Stanford BL, Shah SR. Pemetrexed, a novel antifolate therapeutic alternative for cancer chemotherapy. Pharmacotherapy. 2006;26:641–654. doi: 10.1592/phco.26.5.641. [DOI] [PubMed] [Google Scholar]
- 30.Tanai C, Yamamoto N, Ohe Y, et al. A phase I study of enzastaurin combined with pemetrexed in advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:1068–1074. doi: 10.1097/JTO.0b013e3181da3899. [DOI] [PubMed] [Google Scholar]
- 31.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 32.Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:650–656. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]