Abstract
Background/Objectives
Vitamin D deficiency is associated with cardiovascular disease, osteoporosis, poor muscle strength, falls, fractures, and mortality. Although older adults are at a high risk of vitamin D deficiency, the relationship of serum 25(OH)D with all-cause and cardiovascular disease mortality has not been well characterized in the elderly. We hypothesized that low serum 25(OH)D predicted mortality in older adults.
Subjects/Methods
Serum 25(OH)D and all-cause and cardiovascular disease mortality were examined in 1006 adults, ≥65 years, who participated in the InCHIANTI study, a population-based, prospective cohort study of aging in Tuscany, Italy. Serum 25(OH)D was measured at enrollment in 1998-1999, and participants were followed for mortality.
Results
During 6.5 years of follow-up, 228 (22.7%) participants died, of whom 107 died from cardiovascular disease. Compared with participants in the highest quartile of serum 25(OH)D (>26.5 ng/mL)(to convert to nmol/L, multiply by 2.496), those in the lowest quartile (<10.5 ng/mL) had increased risk of all-cause mortality (Hazards Ratio [H.R.] 2.11, 95% Confidence Interval [C.I.] 1.22 – 3.64, P = 0.007) and cardiovascular disease mortality (H.R. 2.64, 95% C.I. 1.14 – 4.79, P = 0.02), in multivariate Cox proportional hazards models that adjusted for age, sex, education, season, physical activity, and other potential confounders.
Conclusions
Older community-dwelling adults with low serum 25(OH)D are at higher risk of all-cause and cardiovascular disease mortality.
Keywords: Aging, all-cause mortality, cardiovascular disease mortality, vitamin D
Introduction
Worldwide, the population of persons aged 65 years and older was 420 million in 2000, and it is expected to increase to 973 million by 2030 (Ferrucci et al. 2008). By 2030, it is estimated that persons 65 years and older will comprise over 20% of the population of North America (Ferrucci et al. 2008). Vitamin D deficiency, as assessed by low serum 25-hydroxyvitamin D (25[OH]D) levels, the standard measure of vitamin D status, is highly prevalent among older, community-dwelling adults (Zadshir et al. 2005; Holick, 2006) and has an extremely high prevalence among those who are institutionalized (Gloth et al. 1995; Thomas et al. 1998). Older adults may be at higher risk of vitamin D deficiency because both the level of outdoor activity (Scragg and Camargo, 2008) and cutaneous production of vitamin D (MacLaughlin and Holick, 1985) decrease with age. Vitamin D deficiency will be a growing public health problem because of the global shifts towards an aged population in much of the world.
Vitamin D deficiency is associated with an increased risk of osteopenia, osteoporosis, muscle weakness, poor physical performance (Houston et al. 2007), falls (Bischoff-Ferrari, et al. 2004), and fractures (Bischoff-Ferrari et al. 2005). Low serum 25(OH)D levels are associated with hypertension (Lind et al. 1995; Martins et al. 2007), coronary heart disease (Kim et al. 2008), myocardial infarction (Giovannucci, et al. 2008), heart failure (Zittermann et al. 2007; Kim et al. 2008), stroke (Poole et al. 2006, Pilz et al. 2008), and independently predict cardiovascular disease (Wang et al. 2008). A recent study showed that patients undergoing coronary angiography at a tertiary care center who had low serum 25(OH)D levels were at higher risk of all-cause and cardiovascular disease mortality (Dobnig et al. 2008). Participants, aged 20 years and older, in the Third National Health and Nutrition Examination Survey (NHANES III), who had lower serum 25(OH)D levels were at an increased risk of all-cause but not cardiovascular disease mortality (Melamed et al. 2008).
Although vitamin D deficiency is highly prevalent in the elderly, the relationship between low serum 25(OH)D levels and mortality has not been well characterized. We hypothesized that older, community-dwelling men and women with low serum 25(OH)D levels were at higher risk of all-cause and cardiovascular disease mortality. To address this hypothesis, we examined the relationship between serum 25(OH)D and mortality in a population-based sample of older, community-dwelling men and women in Tuscany, Italy.
Subjects and Methods
The study subjects consisted of men and women, aged 65 and older, who participated in the Invecchiare in Chianti, “Aging in the Chianti Area” (InCHIANTI) study, conducted in two small towns in Tuscany, Italy. The rationale, design, and data collection have been described elsewhere, and the main outcome of this longitudinal study is mobility disability (Ferrucci et al. 2000). Briefly, in August 1998, 1270 people aged 65 years and older were randomly selected from the population registry of Greve in Chianti (pop. 11,709) and Bagno a Ripoli (pop. 4,704), and of 1,256 eligible subjects, 1,155 (90.1%) agreed to participate. Of the 1,155 participants, 1,043 (90.3%) participated in the blood drawing. During 6.5 years of follow-up, 228 participants died, 34 refused to participate in the study and 16 moved away from the area. Those who refused to participate in follow-up or moved away were known to be alive at the time of censoring of this analysis. Participants received an extensive description of the study and participated after written, informed consent. The study protocol complied with the Declaration of Helsinki and was approved by the Italian National Institute of Research and Care on Aging Ethical Committee and by the Institutional Review Board of the Johns Hopkins University School of Medicine.
Demographic information, including total years of formal education, and information on smoking and medication use were collected using standardized questionnaires. All participants were examined by a trained geriatrician, and diseases were ascertained according to standard, pre-established criteria and algorithms based upon those used in the Women’s Health and Aging Study for diabetes mellitus, coronary heart disease, chronic heart failure, stroke, and cancer (Guralnik et al. 1995).
Weight was measured using a high-precision mechanical scale. Standing height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Mini-Mental State Examination (MMSE) was administered at enrollment, and an MMSE score <24 was considered consistent with cognitive impairment (Folstein et al. 1975). Physical activity in the previous year was based upon responses to multiple questions and was rated as sedentary (inactive or light-intensity physical activity such as walking or light housework <1 h/wk), light physical activity (light physical activity 2-4 h/wk), and moderate-high physical activity (light physical activity >4 h/wk and/or moderate physical activity such as brisk walking, playing soccer, gardening ≥1 h/wk) (Ruggiero et al. 2007). Season of the year (winter: December-February; spring: March-May; summer: June-August; autumn: September-November) was included as a categorical variable to account for seasonal effects on vitamin D levels. Renal insufficiency was defined as estimated glomerular filtration rate of <60 mL/min/1.73 m2 using the four-variable Modification of Diet in Renal Disease Study equation of Levey and colleagues (Levey et al. 1999).
Blood samples were collected in the morning after a 12-h fast. Aliquots of serum and plasma were immediately obtained and stored at -80° C. Serum 25(OH)D was measured at enrollment and the 3-year follow-up visit using a radioimmunoassay (DiaSorin, Stillwater, MN) with intra-assay and inter-assay coefficients of variation (CV) of 8.1% and 10.2%, respectively (Maggio et al. 2005). Serum intact PTH levels were measured with a two-site immunoradiometric assay kit (N-tact PTHSP, DiaSorin) with intra-assay and inter-assay CVs of <3.0% and 5.5%, respectively. Serum creatinine was measured by a standard creatinine Jaffe method (Roche Diagnostics, Mannheim, Germany). Commercial enzymatic tests (Roche Diagnostics) were used for measuring serum total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol concentrations. Low-density lipoprotein (LDL) cholesterol was calculated by the Friedewald formula (Friedewald et al. 1972).
Participants were evaluated again for a three-year follow-up visit from 2001-2003 (n = 926) and six-year follow-up visit from 2004-2006 (n = 844). At the end of the field data collection, mortality data of the original InCHIANTI cohort were collected using data from the Mortality General Registry maintained by the Tuscany Region and the death certificates that are deposited immediately after the death at the Registry office of the Municipality of residence. Cardiovascular disease mortality was defined by the death codes 390–459 from the 9th version of the International Classification of Diseases (ICD) (U.S. Department of Health and Human Services, 2006).
Variables are reported as medians (25th, 75th percentiles) or as percentages. Serum 25(OH)D was divided into quartiles: <10.5, 10.5-16.0, 16.1-25.6, >25.6 ng/mL. Characteristics of subjects were compared between groups using Kruskal-Wallis tests. Cox proportional hazards models were used to examine the relationship between serum 25(OH)D and all-cause and cardiovascular disease mortality. We adjusted for possible confounders in multivariate Cox proportional hazards models for mortality using conventional cardiovascular risk factors. The models were tested for possible interactions and collinearity, and no significant interactions or collinearity were found. Pearson correlation was used to examine the correlation between serum 25(OH)D measurements at enrollment and 3 year follow-up. Kaplan-Meier curves were used for survival by quartile of serum 25(OH)D, and survival curves were compared using log-rank tests. The statistical program used was SAS (SAS Institute, Cary, NC). The level of significance used in this study was P <0.05.
Results
The characteristics of participants by quartile of serum 25(OH)D at enrollment are shown in Table 1. Participants in the lower quartiles of serum 25(OH)D were older, more likely to be female, current non-smokers, physically inactive, and more likely to have low education and MMSE scores, heart failure, stroke, or renal insufficiency. Participants in the lower quartiles of serum 25(OH)D were more likely to have had their serum vitamin D measured in the autumn or winter. The participants did not have any significant differences across quartile of serum 25(OH)D by total cholesterol, HDL cholesterol, LDL cholesterol, or triglycerides, or by prevalence of hypertension, angina, peripheral artery disease, diabetes mellitus, or cancer.
Table 1.
Baseline characteristics of adults, aged ≥65 years, in the InCHIANTI study, by quartiles of serum 25(OH)D
| Characteristic1 | 25(OH)D Quartiles ng/mL2 | P | ||||
|---|---|---|---|---|---|---|
| <10.5 (n = 252) | 10.5 – 16.0 (n = 254) | 16.1 – 25.6 (n = 247) | >25.6 (n = 253) | |||
| Age, y | 78.0 (72.0, 85.0) | 75.0 (70.0, 81.0) | 72.0 (69.0, 77.0) | 71.0 (68.0, 74.0) | <0.0001 | |
| Sex, female (%) | 32.7 | 29.5 | 21.7 | 16.0 | <0.0001 | |
| Education, years | 5.0 (3.0, 5.0) | 5.0 (3.0, 5.0) | 5.0 (4.0, 6.0) | 5.0 (5.0, 7.0) | <0.0001 | |
| Current smoker (%) | 9.9 | 11.0 | 15.8 | 19.0 | 0.01 | |
| Aspirin use (%) | 36.1 | 40.2 | 29.6 | 25.7 | 0.002 | |
| Body mass index (kg/m2) | 27.1 (23.9, 30.0) | 27.3 (24.5, 30.1) | 27.4 (25.0, 30.1) | 26.7 (24.5, 29.1) | 0.25 | |
| Physical activity (%) | Inactive | 45.5 | 29.5 | 18.0 | 6.0 | <0.0001 |
| Low | 23.3 | 27.8 | 22.8 | 26.1 | ||
| Moderate-High | 13.9 | 20.0 | 30.5 | 35.6 | ||
| Parathyroid hormone (ng/L) | 30.9 (21.2, 42.8) | 24.7 (17.9, 33.4) | 20.3 (16.1, 29.0) | 16.6 (13.2, 23.5) | <0.0001 | |
| Serum calcium (mg/dL) | 9.4 (9.1, 9.7) | 9.4 (9.2, 9.7) | 9.5 (9.2, 9.7) | 9.4 (9.2, 9.6) | 0.10 | |
| Total cholesterol (mg/dL) | 212 (186, 240) | 217 (186, 242) | 212 (187, 245) | 217 (191, 244) | 0.24 | |
| HDL cholesterol (mg/dL) | 54 (45, 67) | 56 (47, 65) | 51 (44, 62) | 53 (45, 64) | 0.06 | |
| LDL cholesterol (mg/dL) | 132 (107, 154) | 135 (116, 155) | 133 (133, 157) | 137 (113, 163) | 0.19 | |
| Triglycerides (mg/dL) | 109 (82, 141) | 113 (84, 169) | 114 (84, 158) | 108 (82, 144) | 0.52 | |
| Mini-Mental Exam Score <24 (%) | 42.9 | 33.4 | 22.7 | 18.6 | <0.0001 | |
| Hypertension (%) | 47.6 | 50.8 | 44.5 | 44.7 | 0.45 | |
| Angina (%) | 4.8 | 3.9 | 5.3 | 3.9 | 0.86 | |
| Heart failure (%) | 9.5 | 6.7 | 3.2 | 2.4 | 0.001 | |
| Peripheral artery disease (%) | 5.9 | 6.7 | 5.3 | 6.7 | 0.89 | |
| Stroke (%) | 9.1 | 5.9 | 3.2 | 2.4 | 0.003 | |
| Diabetes mellitus (%) | 16.7 | 11.4 | 11.7 | 13.4 | 0.29 | |
| Cancer (%) | 7.5 | 7.8 | 5.7 | 4.7 | 0.42 | |
| Renal insufficiency (%) | 17.7 | 20.5 | 14.1 | 9.5 | 0.004 | |
| Season (%) | Winter | 32.7 | 35.2 | 21.4 | 10.7 | <0.0001 |
| Spring | 18.3 | 20.9 | 24.3 | 36.5 | ||
| Summer | 17.6 | 18.5 | 26.1 | 37.8 | ||
| Autumn | 30.2 | 27.7 | 24.8 | 17.2 | ||
Data are given as median and interquartile range or percentage, as indicated.
To convert to nmol/L, multiply by 2.496.
During 6.5 years of follow-up, 228 (22.7%) of 1,006 participants died. The main causes of death were cardiovascular disease (47.3%), cancer (24.1%), respiratory disease (9.0%), and other (19.2%), and unknown (0.4%). The survival of participants by quartile of serum 25(OH)D for all-cause mortality is shown in Figure 1. Participants in the lowest quartile of serum 25(OH)D had significantly shorter survival than participants in the highest quartile of serum 25(OH)D for all-cause mortality. In multivariate Cox proportional hazards models adjusting for age, sex, education, and season, and additionally for BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, MMSE score, and chronic diseases, participants in the lowest quartile of serum 25(OH)D had a significantly increased risk of all-cause mortality compared with participants in the highest quartile of serum 25(OH)D (Table 2). Participants in the second and third quartile of serum 25(OH)D also had a higher risk of all-cause mortality compared with those in the highest quartile of serum 25(OH)D, but the results did not reach statistical significance for those in the second quartile of serum 25(OH)D.
Figure 1.
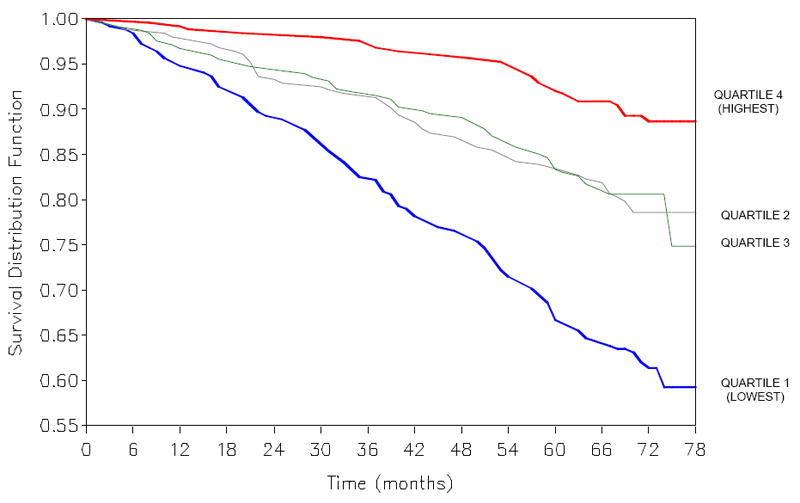
Kaplan-Meier plots of all-cause mortality by quartiles of serum 25-hydroxyvitamin D. P <0.0001 by log-rank test.
Table 2.
Relationship between serum 25(OH)D and all-cause mortality in separate multivariate Cox proportional hazards models
| Covariates in models | 25(OH)D Quartiles ng/mL1,2 | |||
|---|---|---|---|---|
| <10.5 | 10.5 – 16.0 | 16.1 – 25.6 | >25.6 | |
| Age, sex, education, season | 3.53 (2.19, 5.68) | 2.15 (1.33, 3.50) | 2.22 (1.39, 3.55) | 1.00 |
| Age, sex, education, season, BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, MMSE score. | 2.20 (1.28, 3.77) | 1.57 (0.93, 2.66) | 1.96 (1.20, 3.20) | 1.00 |
| Age, sex, education, season, BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, MMSE score, and chronic diseases.3 | 2.11 (1.22, 3.64) | 1.41 (0.83, 2.40) | 1.12 (1.09, 1.15) | 1.00 |
Hazards ratios shown for each quartile of 25(OH) relative to the highest quartile (reference).
To convert to nmol/L, multiply by 2.496.
Chronic diseases include hypertension, diabetes, heart failure, stroke, and renal insufficiency.
During follow-up, 107 participants died from cardiovascular diseases. The survival of participants by quartile of serum 25(OH)D for cardiovascular disease mortality is shown in Figure 2. Participants in the lowest quartile of serum 25(OH)D had significantly shorter survival than participants in the highest quartile of serum 25(OH)D for cardiovascular disease mortality. In multivariate Cox proportional hazards models adjusting for age, sex, education, and season, and additionally for BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, MMSE score, and renal insufficiency, participants in the lowest quartile of serum 25(OH)D had a significantly increased risk of cardiovascular disease mortality compared with participants in the highest quartile of serum 25(OH)D (Table 3). Participants in the second and third quartile of serum 25(OH)D also had a higher risk of cardiovascular mortality compared with those in the highest quartile of serum 25(OH)D, but the results did not reach statistical significance for those in the second quartile of serum 25(OH)D. There were 52 deaths from cancer, and quartiles of 25(OH)D were not significantly related with cancer mortality.
Figure 2.
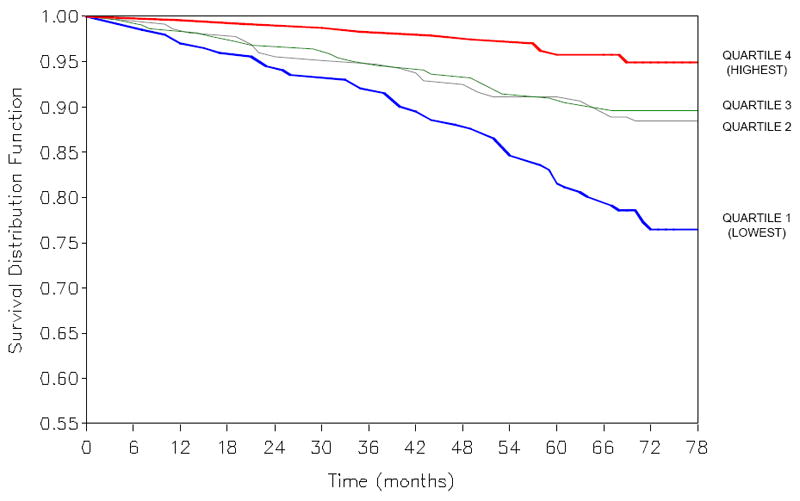
Kaplan-Meier plots of cardiovascular disease mortality by quartiles of serum 25-hydroxyvitamin D. P <0.0001 by log-rank test.
Table 3.
Relationship between serum 25(OH)D and cardiovascular disease mortality in separate multivariate Cox proportional hazards models
| Covariates in models | 25(OH)D Quartiles ng/mL1,2 | |||
|---|---|---|---|---|
| <10.5 | 10.5 – 16.0 | 16.1-25.6 | >25.6 | |
| Age, sex, education, season | 4.15 (2.01, 8.57) | 2.63 (1.27, 5.46) | 2.65 (1.31, 5.39) | 1.00 |
| Age, sex, education, season, BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, MMSE score. | 2.57 (1.12, 5.91) | 1.76 (0.80, 3.89) | 2.28 (1.09, 4.79) | 1.00 |
| Age, sex, education, season, BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, MMSE score, and renal insufficiency. | 2.64 (1.68, 2.19) | 1.68 (0.76, 3.72) | 2.19 (1.05, 4.60) | 1.00 |
Hazards ratios shown for each quartile of 25(OH) relative to the highest quartile (reference).
To convert to nmol/L, divide by 2.496.
Median (25th, 75th percentile) serum PTH concentrations were 27.2 (19.7, 38.3) ng/L among participants who died from all causes compared to 21.6 (15.4, 30.3) ng/L among participants who survived (P <0.0001). Addition of PTH and calcium to the multivariate Cox proportional hazards model that also adjusted for age, sex, season, BMI, smoking, aspirin use, physical activity, total and HDL cholesterol, MMSE score, and chronic diseases did not substantially change the relationship between the highest and lowest quartile of serum 25(OH)D and mortality (H.R. 1.97, 95% C.I. 1.11-3.47, P = 0.02), or between the highest and second and highest and third quartiles, respectively (H.R. 1.51, 95% C.I. 0.89-2.57, P = 0.13; H.R. 1.88, 95% C.I. 1.15-3.09, P = 0.01).
Median (25th, 75th percentile) serum PTH concentrations were 28.2 (20.3, 39.8) ng/L among participants who died from cardiovascular diseases compared to 21.6 (15.4, 30.3) ng/L among participants who survived (P <0.0001). Addition of PTH and calcium to the multivariate Cox proportional hazards model that also adjusted for age, sex, education, season, BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, MMSE score, and renal insufficiency, did not substantially change the relationship between the highest and lowest quartile of serum 25(OH)D and mortality (H.R. 2.23, 95% C.I. 0.95-5.25, P = 0.06), or between the highest and second and highest and third quartiles, respectively (H.R. 1.58, 95% C.I. 0.71-3.53, P = 0.26; H.R. 2.11, 95% C.I. 1.01-4.43, P = 0.049).
In an alternative analysis, we excluded participants who died within one year of enrollment in the study in order to have a time lag. There were 160 participants who died from all causes after the one year time lag. In a multivariate Cox proportional hazards model for all-cause mortality that adjusted for age, sex, education, season, BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, MMSE score, and chronic diseases as in the final model in Table 2, the H.R. (95% C.I.) for 25(OH)D quartiles of <10.5, 10.5-16.0, 16.1-25.6 ng/mL were 1.82 (1.03, 3.21), 1.38 (0.80, 2.38), and 1.67 (1.01, 2.79), respectively. There were 79 participants who died from cardiovascular disease after the one year time lag. In a multivariate Cox proportional hazards model for cardiovascular mortality that adjusted for age, sex, education, season, BMI, smoking, aspirin use, physical activity, total cholesterol, HDL cholesterol, MMSE score, and renal insufficiency as in the final model in Table 4, the H.R. (95% C.I.) for 25(OH)D quartiles of <10.5, 10.5-16.0, 16.1-25.6 ng/mL were 2.15 (0.92, 5.04), 1.49 (0.67, 3.34), and 1.78 (0.84, 3.77), respectively.
There were 729 participants who had serum 25(OH)D measurements at both enrollment and the 3 year follow-up visit, of whom 92 (12.6%) died from all causes during the follow-up period from 3 to 6.5 years after enrollment. The Pearson correlation between serum 25(OH)D at enrollment and the 3 year follow-up visit was 0.45 (P <0.0001). Participants were divided into four groups based upon serum 25(OH)D measurements at enrollment and the 3 year follow-up visit: (1) above the median at both enrollment and 3-year follow-up, (2) below the median at enrollment and above the median at the 3-year follow-up, (3) above the median at enrollment and below the median at the 3-year follow-up, and (4) below the median at both enrollment and 3-year follow-up. The mortality rates in the four groups, respectively, were 8.3%, 10.8%, 14.5%, and 17.3% (P = 0.02).
Discussion
This study shows that older, community-dwelling men and women with low serum 25(OH)D levels (lowest quartile, <10.5 ng/mL; <26.2 nmol/L) had a significantly higher risk of both all-cause and cardiovascular disease mortality compared with those with higher serum 25(OH)D concentrations (highest quartile, >25.6 ng/mL; >63.9 nmol/L), after adjusting for demographic and other risk factors. The study corroborates the findings of the Hoorn study (Pilz et al. 2009) and extends the association between vitamin D and cardiovascular mortality in a larger study cohort with lower levels of 25(OH)D. Another previous study showed that in a selected group of patients undergoing coronary angiography, low serum 25(OH)D was associated with increased all-cause and cardiovascular disease mortality (Dobnig et al. 2008). In the general population of adults, aged 20 years and older in NHANES III, low serum 25(OH)D was an independent predictor of all-cause mortality but the relationship of low serum 25(OH)D and cardiovascular disease mortality did not reach statistical significance (Melamed et al. 2008). The results from NHANES III and the present study are not directly comparable due to the differences in age ranges between the two studies.
In the present study, participants who maintained a serum 25(OH)D level above the median at both enrollment and three years later or those who had a serum 25(OH)D level below the median at enrollment but above the median three years later were at a lower risk of mortality, compared to participants who remained below the median at both enrollment and three year follow-up, or who dropped from above the median at enrollment to below the median at the three year follow-up. These findings suggest that those who maintain a high serum 25(OH)D level over time are at a lower risk of all-cause mortality.
Several biological mechanisms have been proposed that could explain a possible causal relationship between vitamin D deficiency and mortality. The active form of vitamin D, 1,25-dihydroxyvitamin D, has pleiotropic effects, and the vitamin D receptor is widely distributed in tissues (Holick, 2007). Vitamin D plays a role in regulation of the renin-angiotensin axis (Li et al. 2002), modulation of cellular proliferation and differentiation (Thorne and Campbell, 2008), cytokine production (Schleithoff et al. 2006), and atherosclerosis (Richart et al. 2007; Cardus et al. 2008). Low serum 25(OH)D levels have been shown to predict the risk of coronary artery calcification (de Boer et al. 2009), and vitamin D may also play a role in arterial stiffness (Andrade et al. 2008). The wide role that vitamin D plays in different tissues may account for the associations between vitamin D deficiency and cardiovascular disease (Martins et al. 2007; Wang et al. 2008), cancer (Davis, 2008), and mortality (Dobnig et al. 2008; Melamed et al. 2008). The effect of vitamin D on mortality may be indirect via hyperparathyroidism secondary to vitamin D insufficiency (Lips, 2001). PTH levels were significantly higher among those who died, however, the addition of PTH to models of 25(OH)D and all-cause and cardiovascular disease mortality did not substantially change the relationship between serum 25(OH)D and mortality.
Vitamin D deficiency is often defined as a serum 25(OH)D level less than 20 ng/mL (50 nmol/L) (Holick, 2007). Using this criterion, the majority of participants in the present study were deficient in vitamin D. The median serum 25(OH)D concentration of 16 ng/mL (40 nmol/L) among participants in the present study is lower than the mean 25(OH)D concentrations of about 28 ng/mL described among participants, aged 60 years and older, in NHANES III (Zadshir et al. 2005), but serum 25(OH)D was measured in NHANES III from 89 survey locations across the US and during the warmer months in the northern locations, when sunlight is more abundant (Martins et al. 2007).
Vitamin D is found in a limited number of dietary sources such as oily fish, eggs, and vitamin D-fortified milk, cereals, margarine, and yogurts. The current dietary recommendations for adequate daily intake of vitamin D are 400 IU for adults 51 to 70 years of age and 600 IU for adults 71 years of age and older (Food and Nutrition Board, 1997), but recent evidence suggests that the recommended adequate daily intake may need to be increased to 800 IU per day (Holick, 2007). Among the participants 60 years and older in NHANES III, those who participated in daily outdoor activities had mean serum 25(OH)D concentrations that were 30.8 ng/mL (77 nmol/L) (Scragg, et al. 2008), well above the cut-off of 50 nmol/L for vitamin D sufficiency. Older adults may require both greater daily outdoor activities and higher dietary intake of vitamin D to reduce the risk of vitamin D deficiency.
This study is limited in that phosphate levels were not collected and could be a potential confounder in the relationship between vitamin D and mortality. There may be other unmeasured confounding factors in the study, as it is generally not possible to account for all confounders in longitudinal studies. As the study is observational, causality cannot be established. Further studies are also needed to examine the relationship between serum 25(OH) D over time and mortality. In an alternative analysis, PTH was added as a covariate to the model, and it may not be completely accurate to adjust for PTH since PTH might mediate the relationship between 25(OH)D and mortality. The present study may possibly underestimate the relationship between low 25(OH)D and mortality since the reference quartile of 25(OH)D consisted of many participants who were still in the insufficient range of 25(OH)D.
A recent meta-analysis of randomized controlled trials of vitamin D supplementation suggests that vitamin D supplementation may reduce mortality among older adults (Autier et al. 2007). Most of the trials in the meta-analysis were conducted among older adults who were at high risk of falls or fractures, and the main outcomes were usually bone density, falls, and clinical fractures, rather than mortality (Autier et al 2007). The authors recommended that randomized, placebo controlled clinical trials were needed among people 50 years and older for at least six years with mortality as the primary outcome in order to definitively address the issue of vitamin D supplementation and mortality (Autier et al.2007).
In conclusion, older community-dwelling men and women with low serum 25(OH)D levels are at an increased risk of all-cause and cardiovascular disease mortality.
Acknowledgments
This work was supported by National Institute on Aging (NIA) Grant R01 AG027012, the Italian Ministry of Health (ICS110.1/RF97.71), NIA contracts 263 MD 9164, 263 MD 821336, N.1-AG-1-1, N.1-AG-1-2111, and N01-AG-5-0002, the Intramural Research Program of NIA, National Institutes of Health, Baltimore, Maryland.
Footnotes
Conflict of interest None of the authors had a conflict of interest.
References
- Andrade J, Er L, Ignaszewski A, Levin A. Exploration of association of 1,25-OH2D3 with augmentation index, a composite measure of arterial stiffness. Clin J Am Soc Nephrol. 2008;3:1800–1806. doi: 10.2215/CJN.00900208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–1812. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Dawson-Hughes B, Willett SC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- Cardus A, Panizo S, Encinas M, Dolcet X, Gallego C, Aldea M, et al. 1,25-dihydroxyvitamin D(3) regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis. 2008 Aug 29; doi: 10.1016/j.atherosclerosis.2008.08.020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Davis CD. Vitamin D and cancer: current dilemmas and future research needs. Am J Clin Nutr. 2008;88(suppl):565S–569S. doi: 10.1093/ajcn/88.2.565S. [DOI] [PubMed] [Google Scholar]
- Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Giallauria F, Guralnik JM. Epidemiology of aging. Radiol Clin North Am. 2008;46:643–52. doi: 10.1016/j.rcl.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board, National Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, D.C.: National Academy Press; 1997. [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Frederickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparation ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloth FM, III, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–1686. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Publication No. 95-4009. [Google Scholar]
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Houston DK, Cesari M, Ferrucci L, Cherubini A, Maggio D, Bartali B, et al. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:440–446. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004) Am J Cardiol. 2008;102:1540–1544. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Li YC, Kong J, Wei M, Chen ZF, Lui SQ, Cao LP. 1,25-dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind L, Hanni A, Lithell H, Hvarfner A, Sorensen OH, Ljunghall S. Vitamin D is related to blood pressure and other cardiovascular disease factors in middle-aged men. Am J Hypertens. 1995;8:894–901. doi: 10.1016/0895-7061(95)00154-H. [DOI] [PubMed] [Google Scholar]
- Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio D, Cherubini A, Lauretani F, Russo RC, Bartali B, Pierandrei M, et al. 25(OH)D serum levels decline with age earlier in women than in men and less efficiently prevent compensatory hyperparathyroidism in older adults. J Gerontol Biol Sci Med Sci. 2005;60:1414–1419. doi: 10.1093/gerona/60.11.1414. [DOI] [PubMed] [Google Scholar]
- Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, et al. Low vitamin D levels predict stroke in patients referred to coronary angiography. Stroke. 2008;39:2611–2613. doi: 10.1161/STROKEAHA.107.513655. [DOI] [PubMed] [Google Scholar]
- Pilz S, Dobnig N, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, et al. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 2009 Fed; doi: 10.1111/j.1365-2265.2009.03548.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Poole KES, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- Richart T, Li Y, Staessen JA. Renal versus extrarenal activation of vitamin D in relation to atherosclerosis, arterial stiffening, and hypertension. Am J Hypertens. 2007;20:1007–1015. doi: 10.1016/j.amjhyper.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Ruggiero C, Cherubini A, Guralnik J, Semba RD, Maggio M, Ling SM, et al. The interplay between uric acid and antioxidants in relation to physical function in older persons. J Am Geriatr Soc. 2007;55:1206–1215. doi: 10.1111/j.1532-5415.2007.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168:577–586. doi: 10.1093/aje/kwn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc. 2008;67:115–127. doi: 10.1017/S0029665108006964. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. International Classification of Diseases Ninth revision, clinical modification. Washington, D.C.: U S Health and Human Services, Centers for Disease Control and Prevention, Centers for Medicare and Medicaid Services; 2006. [Google Scholar]
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(suppl 5):S5-97–S5-101. [PubMed] [Google Scholar]
- Zittermann A, Fischer J, Schleitoff SS, Tenderich G, Fuchs U, Koerfer R. Patients with congestive heart failure and healthy controls differ in vitamin D-associated lifestyles factors. Int J Vitam Nutr Res. 2007;77:280–288. doi: 10.1024/0300-9831.77.4.280. [DOI] [PubMed] [Google Scholar]


