Abstract
Context
Earlier studies have suggested that hearing loss, which is prevalent in more than 30% of adults >60 years, may be a risk factor for dementia, but this hypothesis has never been investigated prospectively.
Objective
To determine if hearing loss is associated with incident all-cause dementia and Alzheimer’s disease (AD).
Design, Setting, and Participants
Prospective study of 639 participants (age 36 – 90 y) of the Baltimore Longitudinal Study of Aging who had audiometric testing and who were dementia-free in 1990-1994. Hearing loss was defined by a pure-tone average of hearing thresholds at 0.5, 1, 2, and 4 kHz in the better-hearing ear (normal <25 dB [n = 455], mild loss 25-40 dB [n = 125], moderate loss 41-70 dB [n = 53], severe loss >70 dB [n = 6]). Diagnosis of incident dementia was made by consensus diagnostic conference. Cox proportional hazard models were used to model time to incident dementia according to severity of hearing loss and were adjusted for age, sex, race, education, diabetes, smoking, and hypertension.
Main Outcome Measure
Incidence of all-cause dementia and AD until May 31, 2008.
Results
During a median follow-up of 11.9 years, 58 cases of incident all-cause dementia were diagnosed of which 37 cases were AD. The risk of incident all-cause dementia increased log-linearly with the severity of baseline hearing loss (1.27 per 10 db loss, 95% CI: 1.06 – 1.50). Compared to normal hearing, the hazard ratio for incident all-cause dementia was 1.89 for mild hearing loss (95% CI: 1.00 – 3.58), 3.00 for moderate hearing loss (95% CI: 1.43 – 6.30), and 4.94 for severe hearing loss (95% CI: 1.09 – 22.4). The risk of incident AD also increased with baseline hearing loss but with a wider confidence interval (1.20 per 10 dB of hearing loss, 95% CI: 0.94 – 1.53).
Conclusions
Hearing loss is independently associated with incident all-cause dementia. Whether hearing loss is a marker for early stage dementia or is actually a modifiable risk factor for dementia deserves further study.
Introduction
The prevalence of dementia is projected to double every twenty years such that by 2050 over 100 million people or nearly 1 in 85 persons will be affected worldwide 1;2. The devastating impact of dementia on affected individuals and the burden imposed on their families and society has made the prevention and treatment of dementia a public health priority. Interventions that could merely delay the onset of dementia by one year would lead to a more than 10% decrease in the global prevalence of dementia in 2050 3. Unfortunately, there are no known interventions that currently have such effectiveness.
Epidemiologic approaches have focused on the identification of putative risk factors that could be targeted for prevention based on the assumption that dementia is easier to prevent than to reverse. Candidate factors include low involvement in leisure activities and social interactions, sedentary state, diabetes, and hypertension 4. Some researchers have also suggested that hearing loss, by reducing stimulatory input and hampering social interaction, may be associated with dementia 5;6, but this hypothesis has never been prospectively studied. Given the growing number of people with hearing loss 7 and the array of technologic interventions currently available for aural rehabilitation, understanding whether hearing loss is a risk factor for dementia is important. We performed the current study to investigate the prospective association of hearing loss with incident dementia within the cohort of the Baltimore Longitudinal Study of Aging (BLSA).
Methods
Subjects
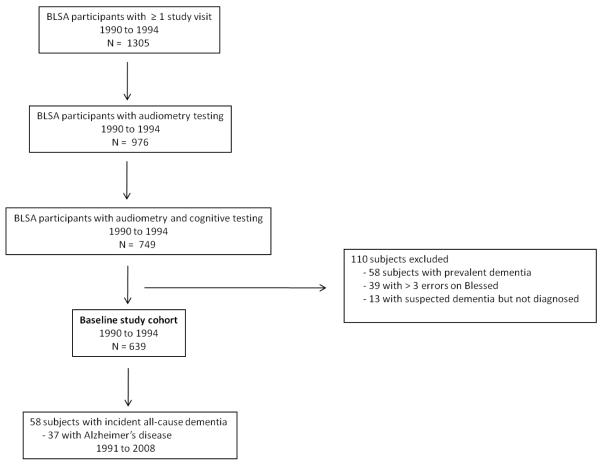
Subjects were participants in the BLSA, an ongoing prospective study of the effects of aging that was initiated in 1958 by the National Institute on Aging 8. The BLSA cohort is comprised of community-dwelling volunteers who travel to the National Institute on Aging in Baltimore biennially for 2.5 days of intensive testing. From 1990-1994, 1305 subjects participated in at least one study visit of which 976 of the participants had audiometry testing, and 749 had both audiometry and cognitive testing. Some subjects had missing audiometry or cognitive testing data because of inadequate time for testing or tester unavailability during study visits. After excluding subjects with prevalent dementia (n = 58), >3 errors on the Blessed test (n = 39), and suspected dementia (n = 13), our baseline cohort was comprised of 639 subjects who were followed until May 31, 2008 (Figure 1). For subjects with more than 1 visit during this time period, data from the first assessment were used. All participants provided written informed consent, and the BLSA study protocol was approved by the Institutional Review Board.
Figure 1.
Selection of subjects for study inclusion
Cognitive testing and diagnosis of dementia
The protocol for adjudication of dementia in the BLSA has been used continuously since 1986 and has been described previously 9. Participants ≥65 years old underwent a complete neurological and neuropsychological examination using a standard battery of tests. Participants <65 were first screened with the Blessed-Information-Memory-Concentration test and underwent further examination if they made three or more errors. Dementia diagnosis was established during a multidisciplinary consensus diagnostic conference using Diagnostic and Statistical Manual of Mental Disorders, 3rd edition revised for diagnosis of dementia 10, and National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association criteria for diagnosis of Alzheimer’s disease 11. If subjects were determined to have clinically-significant cognitive decline (typically memory) but did not meet criteria for dementia, they were classified as suspected dementia which corresponds to the current diagnosis of mild cognitive impairment 12. Subjects were initially evaluated for dementia every 2 years during their routine BLSA follow-up visits. In 1997, follow-up was shifted to a sliding-scale schedule to reduce subject burden and improve data collection. Subjects >80 years were seen annually, subjects 60-80 years were seen biennially, and subjects <60 years were seen every 4 years.
Audiometry
Audiometry was performed in the BLSA study from 1958 to 1994. Over the entire period from 1990-1994 when the baseline evaluation for this analysis was performed, hearing thresholds were measured using an automated testing device (Virtual Equipment Co., Audiometer Model 320) in a soundproof chamber under unaided conditions. A pure tone average (PTA) of air-conduction thresholds at 0.5, 1, 2, and 4 kHz was calculated for each ear, and the PTA in the better-hearing ear was used for subsequent analyses since this ear would be the principal determinant of hearing and speech perception ability on an everyday basis. PTA in dB was used as both a continuous variable and as a categorical variable defined by commonly-used levels of hearing loss: normal (PTA <25 dB), mild loss (25-40 dB), moderate loss (41-70 dB), and severe loss (>70 dB). Prior to 1990, audiometric testing was performed using a Bekesy audiometer (GSI(1701)) and these data were used in analyses of pre-baseline hearing trajectories.
Other covariates
A diagnosis of diabetes was established based on a fasting glucose >125 mg/dL, a pathologic oral glucose tolerance test, or a positive history of a physician diagnosis plus treatment with oral anti-diabetic drugs or insulin. The diagnosis of hypertension was established based on a systolic blood pressure >140 and/or diastolic blood pressure ≥90 mmHg or treatment with antihypertensive medications. Race (white/black/other), education (in years), smoking status (current/former/never), and hearing aid use were based on self-report.
Statistical analyses
Baseline characteristics of cohort subjects were compared using one-way analysis of variance for continuous variables and chi-square or Fisher exact test for categorical variables. Cox proportional hazard models were used to study time to incident all-cause dementia or Alzheimer’s disease. Subjects not diagnosed with dementia were censored at the time of their last negative cognitive evaluation. Time-on-study (i.e. time of entry into the baseline study cohort) was used as the time scale with the exception of one model that utilized age as the time scale.
All Cox models included covariates of sex, age, race, education, diabetes, smoking, and hypertension. Diabetes and hypertension were included as covariates in the analysis because they have been found to be risk factors for dementia 4. Additional models included baseline Blessed scores (residual variability in cognition after definition of the baseline cohort) and hearing aid use. All covariates were treated as time-constant variables. Cox model proportionality assumptions and the linear association between hearing loss and dementia were tested using the Schoenfield residuals method. To examine the graphical association between hearing threshold and dementia, we utilized a smoothing spline for the hearing threshold and age in the proportional hazard model 13. A loess smoother was then applied to the exponential of the partial residuals derived from the hazard model against the hearing threshold. A bootstrap procedure was utilized to generate 10000 datasets that were then used to estimate the 95% confidence interval for the loess smoother. Analysis of hearing loss trajectories pre-baseline was performed using a random effects analysis and adjusted for age. Population attributable risk (PAR) was calculated by 14: PAR = Pexposed(RR – 1)/[1+ Pexposed(RR-1)] where Pexposed was the prevalence of baseline hearing loss ≥25 dB and RR was the rate ratio (hazard ratio) of dementia risk associated with hearing loss.. Subjects with missing data were excluded from analyses, and this represented < 0.2% of the study sample (1 subject) for all analyses except for analyses incorporating hearing aid use where there was more extensive missing data (typically among normal hearing subjects who did not respond). Significance testing for all analyses was 2-sided with a type I error of 0.05. The statistical software used was R version 2.9.1 (www.r-project.org).
Results
Baseline demographic characteristics of participants by hearing loss category are presented in Table 1. In general, subjects with greater hearing loss were more likely to be older, male, and hypertensive. Blessed scores did not differ by hearing loss category (p = .08), although the range of errors was narrow (0-3) because subjects with >3 errors were excluded from the study cohort at baseline.
Table I.
Demographic and clinical characteristics of baseline study cohort by hearing loss status
| Hearing loss statusa | |||||
|---|---|---|---|---|---|
| Normal (n = 455) |
Mild (n = 125) |
Moderate (n = 53) |
Severe (n = 6) |
P value |
|
| Male sex | 225 (50) | 94 (75) | 36 (68) | 5 (83) | <.001 |
| Age, mean (SD), y | 59.9 (12.2) | 71.1 (8.6) | 77.0 (8.4) | 77.7 (4.8) | <.001 |
| Race | .17 | ||||
| White | 404 (89) | 121 (97) | 49 (92) | 6 (100) | |
| Black | 44 (10) | 4 (3) | 4 (8) | 0 (0) | |
| Other | 7 (2) | 0 (0) | 0 (0) | 0 (0) | |
| Education, mean (SD), y | 16.6 (2.8) | 16.2 (3.0) | 16.7 (3.6) | 16.2 (4.0) | .74 |
| Diabetes | 62 (14) | 20 (16) | 12 (23) | 1 (17) | .27 |
| Smoking | .05 | ||||
| Current | 19 (4) | 1 (1) | 1 (2) | 0 (0) | |
| Former | 244 (54) | 85 (68) | 35 (66) | 3 (50) | |
| Never | 192 (42) | 39 (31) | 17 (32) | 3 (50) | |
| Hypertension | 204 (45) | 79 (63) | 38 (72) | 6 (100) | <.001 |
| Hearing aid useb | 6 (2) | 14 (12) | 39 (78) | 4 (67) | <.001 |
| Blessed Information-Memory- Concentration test |
.08 | ||||
| 0 | 265 (58) | 65 (52) | 31 (58) | 0 (0) | |
| 1 | 112 (25) | 32 (26) | 13 (25) | 2 (33) | |
| 2 | 49 (11) | 19 (15) | 6 (11) | 3 (50) | |
| 3 | 29 (6) | 9 (7) | 3 (6) | 1 (17) | |
| Subjects developing all-cause dementia during follow-up |
20 (4.4) | 21 (16.8) | 15 (28.3) | 2 (33.3) | <.001 |
Values are expressed as number (percentage) unless otherwise indicated. Hearing loss categories are defined by the pure tone average (PTA) of hearing thresholds at 500, 1000, 2000, and 4000hz with tones presented by air-conduction to the better hearing ear: Normal (PTA < 25dB), Mild (25-40 dB), Moderate (41-70 dB), and Severe (>70 dB).
Data on hearing aid use were missing for 72 subjects. Subjects with hearing aid use data per hearing loss category were normal (n=393), mild (n=118), moderate (n=50), severe (n=6).
Baseline covariates associated with an increased risk of incident all-cause dementia are hearing loss, age, hypertension, hearing aid use, and Blessed score (Table II). Independent of age, in the 15 years prior to baseline assessment (n = 520 with 2678 observations), participants who later developed incident dementia lost on average 0.52 dB PTA/year (95% CI: 0.34 – 0.70) compared to 0.27 dB PTA/year (95% CI: 0.21 – 0.33) in those who remained non-demented.
Table II.
Demographic and clinical characteristics of baseline study cohort by incident dementia
| No Dementiaa n = 581 |
Dementiaa n = 58 |
Univariate Hazard (95% CI) |
|
|---|---|---|---|
| Hearing loss, mean (SD), PTAb | 18.8 (13.9) | 32.6 (17.0) | 1.06 (1.04, 1.07) c |
| Hearing lossd | |||
| Normal | 435 (75) | 20 (34) | 1 |
| Mild | 104 (18) | 21 (36) | 4.9 (2.6, 8.8) |
| Moderate | 38 (7) | 15 (26) | 12.1 (6.2, 23.9) |
| Severe | 4 (1) | 2 (3) | 21.9 (5.1, 94.2) |
| Male sex | 327 (56) | 33 (57) | 1.09 (0.65, 1.82) |
| Age, mean (SD), y | 62.2 (12.3) | 78.3 (6.4) | 1.20 (1.15, 1.24) |
| Race | |||
| White | 523 (90) | 57 (98) | 1 |
| Black | 51 (9) | 1 (2) | 0.17 (.02, 1.25) |
| Other | 7 (1) | 0 (0) | -- |
| Education, mean (SD), y | 16.5 (3.0) | 16.6 (3.0) | 1.01 (0.92, 1.10) |
| Diabetes | 84 (14) | 11 (19) | 1.57 (0.87, 3.01) |
| Smoking | |||
| Current | 20 (3) | 1 (2) | 1 |
| Former | 333 (57) | 34 (59) | 2.33 (0.32, 17.0) |
| Never | 228 (39) | 23 (40) | 2.07 (0.28, 15.3) |
| Hypertension | 286 (49) | 41 (71) | 2.92 (1.66, 5.15) |
| Hearing aid usee | 47 (9) | 16 (30) | 5.3 (2.9, 9.6) |
| Blessed Information-Memory Concentration test |
|||
| 0 | 338 (58) | 23 (40) | 1 |
| 1 | 140 (24) | 19 (33) | 2.12 (1.15, 3.89) |
| 2 | 64 (11) | 13 (22) | 2.84 (1.44, 5.61) |
| 3 | 39 (7) | 3 (5) | 1.23 (0.37, 4.08) |
Values are expressed as number (percentage) unless otherwise indicated.
Pure tone average (PTA) of hearing thresholds at 0.5, 1, 2, and 4 kHz with tones presented by air-conduction to the better hearing ear
Hazard per 1 dB of PTA.
Normal (PTA < 25dB), Mild (25-40 dB), Moderate (41-70 dB), and Severe (>70 dB).
Data on hearing aid use were missing for 72 subjects. Subjects with hearing aid use data per dementia category were no dementia (n = 514) and dementia (n = 53).
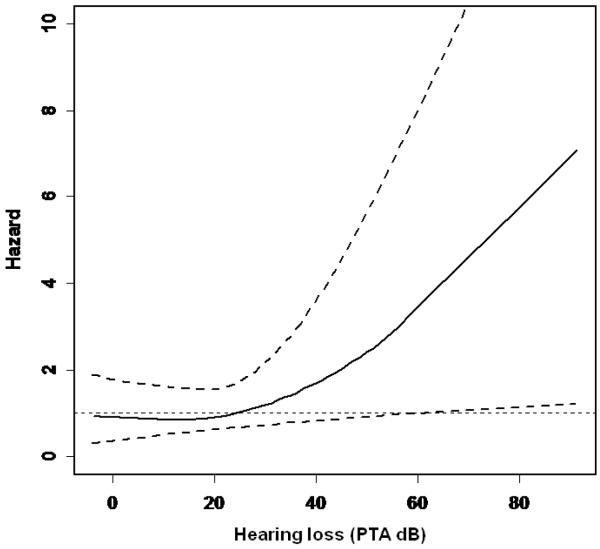
In Cox proportional hazard models adjusted for sex, age, race, education, diabetes, smoking, and hypertension (base model), the excess risk of incident dementia per 10 dB of hearing loss was 1.27 (95% confidence interval [CI]: 1.06 – 1.50) (Table III). The risk of incident dementia becomes evident for hearing loss > 25 dB and thereafter increases log-linearly with more severe loss (Figure 2). This association remained significant after censoring individuals who developed dementia within a 2, 4, or 6 year washout period from baseline (p = 0.008, 0.003, and 0.04, respectively).
Table III.
Cox proportional hazard models for incident all-cause dementia per 10 decibel (dB) of hearing loss
| N | Hazard per 10 dB of Hearing Lossa (95% CI) |
P value | |
|---|---|---|---|
| Base Model b | 638 | 1.27 (1.06 – 1.50) |
.008 |
|
Base + Blessed
Score |
638 | 1.24 (1.04 – 1.48) |
.01 |
|
Base with age
as time scale |
638 | 1.29 (1.08 – 1.53) |
.005 |
|
Base + hearing
aid use |
566 | 1.33 (1.07 – 1.64) |
.008 |
Hearing loss defined by the pure tone average (PTA) of air-conduction hearing thresholds at 0.5, 1, 2, and 4 kHz in the better-hearing ear.
Base model covariates include sex, age, race, education, diabetes, smoking, and hypertension
Figure 2.
Risk of incident all-cause dementia by baseline hearing loss after adjustment for age, sex, race, education, diabetes, smoking, and hypertension. Hearing loss is defined by the pure tone average (PTA) of 0.5, 1, 2, and 4 kHz tones presented by air-conduction in the better hearing ear. Upper and lower dashed lines correspond to the 95% confidence interval.
Confirmatory analyses from models including baseline Blessed error score (to account for baseline cognitive function) or using age as the time-scale rather than time-on-study (to account for residual confounding between age and hearing loss) produced virtually unchanged findings (Table III). Restricting the analytical cohort to participants ≥65 y at baseline (n = 315) or excluding participants at baseline with a prior history of stroke or transient ischemic attack (n = 19) also did not substantially change the main findings (cf. Table III). There was no evidence to suggest that self-reported hearing aid use was associated with a reduction in dementia risk (HR = 0.97, p = .92).
In subsequent analyses we categorized hearing loss according to commonly-accepted levels of hearing loss severity. Compared to normal hearing, participants with mild hearing loss had a hazard ratio [HR] for incident dementia of 1.89 [95% CI: 1.00 – 3.58, p = 0.049], those with moderate hearing loss had a HR of 3.00, [95% CI: 1.43 – 6.30, p = 0.004], and those with severe hearing loss (n=6) had a HR of 4.94 [95% CI: 1.09 – 22.4, p = 0.04].
When the outcome of the analysis was restricted to incident Alzheimer’s disease (37 of the 58 dementia cases), hearing loss was associated with an excess risk of 1.20 per 10 dB of hearing loss (95% CI: 0.94 – 1.53). This result is comparable to the risk seen for all-cause dementia (cf. Table III) but with a wider confidence interval, possibly due to the smaller sample size.
We estimated the proportion of incident all-cause dementia risk that was attributable to hearing loss for participants >60 years in our cohort assuming that hearing loss could be causally associated with dementia. Hearing loss ≥25 dB in the better-hearing ear was present in 43% of this sub-cohort, and the relative risk (hazard ratio) of dementia associated with hearing loss was 2.32 (95% CI: 1.32- 4.07). Thus, the attributable risk of dementia associated with hearing loss in this sub-cohort was 36.4% (95% CI: 12.8 – 58.6).
Comment
In this study we found that hearing loss was independently associated with incident all-cause dementia after adjustment for sex, age, race, education, diabetes, smoking, and hypertension, and our findings were robust to multiple sensitivity analyses. The risk of all-cause dementia increased log-linearly with hearing loss severity, and for individuals >60 years in our cohort, over one-third of the risk of incident all-cause dementia was associated with hearing loss.
Our findings contribute significantly to the discussion in the literature on whether hearing loss is a risk factor for dementia. Previous studies suggested that individuals with hearing loss are more likely to have a diagnosis of dementia 5;6 and poorer cognitive function 15. Supporting this hypothesis, smaller prospective studies have observed that hearing loss is associated with accelerated cognitive decline in individuals with prevalent dementia 16;17. Although, a prospective study of cognitively-normal elderly volunteers failed to find any meaningful association between hearing loss at study entry and later cognitive function, the results of this study are questionable because of the short (5-year) follow-up and a 50% dropout rate 18. In our study, hearing loss, a condition that is highly prevalent in older adults and often remains untreated 19, was strongly and prospectively associated with incident dementia.
A number of mechanisms may be theoretically implicated in the observed association between hearing loss and incident dementia. There may be an over-diagnosis of dementia in individuals affected by hearing loss, or vice versa an over-diagnosis of hearing loss in individuals with cognitive impairment at baseline. An over-diagnosis of dementia in our study is unlikely because the diagnostic protocol for incident dementia relied on a consensus conference that examined information from multiple sources. We also conducted sensitivity analyses censoring individuals diagnosed with dementia during a 6-year washout period from baseline that did not affect our results. In such an analysis, individuals would already have had several “normal” cognitive exams with hearing loss before being diagnosed with dementia, likely making the dementia diagnosis not confounded by poor communication. Hearing loss (short of profound deafness) also minimally impairs face-to-face communication in quiet environments (i.e. during cognitive testing) particularly in the setting of testing by experienced examiners who are accustomed to working with older adults 20.
An over-diagnosis of hearing loss is also unlikely since there is no evidence that mild cognitive impairment would affect the reliability of audiometric testing. Behaviorally, pure-tone audiometry has been performed even in children as young as 5 years. We also excluded any individuals with recognized cognitive impairment at baseline (mild cognitive impairment or Blessed > 3), and our results were robust to models controlling for baseline Blessed scores.
Another possibility is that both hearing loss and progressive cognitive impairment are caused by a common neuropathologic process, possibly the same that leads to Alzheimer’s disease. However, pure tone audiometry is typically considered a measure of the auditory periphery because detection of pure tones relies solely on cochlear transduction and neuronal afferents to brainstem nuclei and the primary auditory cortex. Perception of pure tones do not require higher levels of auditory cortical processing 21, and auditory brainstem response testing of these pathways is usually normal in AD patients 22. In contrast, central auditory nuclei required for higher order auditory processing can be affected by AD neuropathology 23-25, and tests of central auditory function have been found to be associated with Alzheimer’s disease 26.
The likelihood of another neurobiological process such as vascular disease or factors related to family history (e.g. ApoE status) causing both hearing loss and dementia also cannot be fully excluded. However, risk factors for vascular disease such as diabetes, smoking, and hypertension were adjusted for in our models, and a preliminary study has not found a positive association between ApoE status and hearing hearing loss 27. Other variables such as mental and leisure activities were not included as covariates in our models since these variables would not be expected to cause hearing loss and act as meaningful confounders in our models. Our results were also robust to excluding individuals at baseline who had a prior history of stroke or TIA.
Finally, hearing loss may be causally related to dementia, possibly through exhaustion of cognitive reserve, social isolation, environmental deafferentation, or a combination of these pathways. Cognitive reserve reflects inter-individual differences in neurocognitive processing that allow some individuals to cope better with neuropathology than others 28. Functional MRI studies showing inter-individual variation in efficiency of task-related neural processing provide some evidence for this concept 29;30. Cognitive reserve has also been used to explain discrepancies between the extent of neuropathology seen at autopsy and clinical expression of dementia 31. The potential effect of hearing loss on cognitive reserve is suggested by studies demonstrating that under conditions where auditory perception is difficult (i.e. hearing loss), greater cognitive resources are dedicated to auditory perceptual processing to the detriment of other cognitive processes such as working memory 32;33. This reallocation of neural resources to auditory processing could deplete the cognitive reserve available to other cognitive processes and possibly lead to the earlier clinical expression of dementia neuropathology 34.
Communication impairments caused by hearing loss can also lead to social isolation in older adults 35;36, and epidemiologic37;38 and neuroanatomic studies39 have demonstrated associations between poor social networks and dementia. Our results would also seem to support this possible pathway because the risk of dementia associated with hearing loss only appeared to increase at hearing thresholds >25 dB which is considered the threshold at which hearing loss begins to impair verbal communication 40. Finally, a hypothetical mechanism by which hearing loss could directly affect Alzheimer’s neuropathology is suggested by animal studies demonstrating that environmental enrichment (possibly analogous in humans to having access to auditory and environmental stimuli) can reduce β-amyloid levels in transgenic mouse models 41. This hypothesis is also supported by studies showing that individuals who remain engaged in leisure activities have a lower risk of dementia 42.
In the current study, self-reported hearing aid use was not associated with a significant reduction in dementia risk, but data on other key variables (e.g. type of hearing aid used, hours worn per day, number of years used, characteristics of subjects choosing to use hearing aids, use of other communicative strategies, adequacy of rehabilitation, etc) that would affect the success of aural rehabilitation and affect any observed association were not gathered. Consequently, whether hearing advices and aural rehabilitative strategies could have an effect on cognitive decline and dementia remains unknown and will require further study.
Our study has limitations. First, only the severity of hearing loss at baseline was considered in the analysis, and information was not available on the trajectory of hearing loss after baseline assessment or the possible etiology of the hearing loss . However, it is unlikely that this limitation substantially biased our findings given that reversible hearing loss is rare, and hearing loss tends to only worsen with time. Residual confounding by other environmental, genetic, or neuropathologic processes is also plausible but speculative based on our current knowledge of established risk factors for hearing loss and dementia. Given the very close association between age and both hearing loss and dementia, there is a possibility of unaccounted residual confounding. This is unlikely, however, because we also confirmed our findings in a statistical model using age rather than time-on-study as the time-scale to account for non-linear effects of age on hearing and cognition 43. Our findings were also unchanged after restricting our cohort to participants ≥65 years at baseline.
Finally, caution must be applied when generalizing the results of our current study because the BLSA is a volunteer cohort of subjects of high socioceconomic status. Further confirmation of our results will need to be performed in larger studies using more representative, community-based samples. This potential limitation to broad generalizability, however, could strengthen the internal validity of our findings given the relative homogeneity of the study cohort in both observed and likely unobservable characteristics.
If confirmed in other independent cohorts the findings of our study could potentially have substantial implications for individuals and public health. Hearing loss in older adults may be preventable 44 and can be practically addressed with current technology (digital hearing aids, cochlear implants) and with other rehabilitative interventions focused on optimizing social and environmental conditions for hearing. With the increasing number of people with hearing loss, research into the mechanistic pathways linking hearing loss with dementia and the potential of rehabilitative strategies to moderate this association are critically needed.
Acknowledgements
Funding/Support: This work was support by the intramural research program of the National Institute on Aging
Role of the Sponsor: The National Institute on Aging funded the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation and review of the manuscript, but was not involved in manuscript approval.
Footnotes
Author contributions: Drs. Metter and Ferrucci had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lin, Metter, Ferrucci
Acquisition of data: Metter, Resnick, Zonderman, Ferrucci
Analysis and interpretation of data: Lin, Metter, O’Brien, Resnick, Zonderman, Ferrucci
Drafting of the manuscript: Lin, Ferrucci
Critical revision of the manuscript for important intellectual content: Lin, Metter, O’Brien, Resnick, Zonderman, Ferrucci
Statistical analysis: Lin, Metter, Ferrucci
Obtained funding: Ferrucci
Study supervision: Ferrucci
Financial disclosures: None
Reference List
- (1).Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Prince M, Jackson J, editors. Alzheimer’s Disease International. World Alzheimer Report 2009. 2009.
- (3).Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- (4).Coley N, Andrieu S, Gardette V, et al. Dementia prevention: methodological explanations for inconsistent results. Epidemiol Rev. 2008;30:35–66. doi: 10.1093/epirev/mxn010. [DOI] [PubMed] [Google Scholar]
- (5).Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261:1916–1919. [PubMed] [Google Scholar]
- (6).Ives DG, Bonino P, Traven ND, Kuller LH. Characteristics and comorbidities of rural older adults with hearing impairment. J Am Geriatr Soc. 1995;43:803–806. doi: 10.1111/j.1532-5415.1995.tb07056.x. [DOI] [PubMed] [Google Scholar]
- (7).Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- (8).Shock N, Greulich R, Andres R, et al. Normal human aging: the Baltimore Longitudinal Study of Aging. US Government Printing Office; Washington, D.C.: 1984. NIH Publication 84-2450. [Google Scholar]
- (9).Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- (10).APA . Diagnostic and statistical manual of mental disorders. 3rd ed American Psychiatric Assoiciation; Washington, D.C.: 1987. [Google Scholar]
- (11).McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- (12).Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- (13).Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. Springer; New York: 2000. [Google Scholar]
- (14).Northridge ME. Public health methods--attributable risk as a link between causality and public health action. Am J Public Health. 1995;85:1202–1204. doi: 10.2105/ajph.85.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cacciatore F, Napoli C, Abete P, Marciano E, Triassi M, Rengo F. Quality of life determinants and hearing function in an elderly population: Osservatorio Geriatrico Campano Study Group. Gerontology. 1999;45:323–328. doi: 10.1159/000022113. [DOI] [PubMed] [Google Scholar]
- (16).Peters CA, Potter JF, Scholer SG. Hearing impairment as a predictor of cognitive decline in dementia. J Am Geriatr Soc. 1988;36:981–986. doi: 10.1111/j.1532-5415.1988.tb04363.x. [DOI] [PubMed] [Google Scholar]
- (17).Uhlmann RF, Larson EB, Koepsell TD. Hearing impairment and cognitive decline in senile dementia of the Alzheimer’s type. J Am Geriatr Soc. 1986;34:207–210. doi: 10.1111/j.1532-5415.1986.tb04204.x. [DOI] [PubMed] [Google Scholar]
- (18).Gennis V, Garry PJ, Haaland KY, Yeo RA, Goodwin JS. Hearing and cognition in the elderly. New findings and a review of the literature. Arch Intern Med. 1991;151:2259–2264. [PubMed] [Google Scholar]
- (19).Popelka MM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R. Low prevalence of hearing aid use among older adults with hearing loss: the Epidemiology of Hearing Loss Study. J Am Geriatr Soc. 1998;46:1075–1078. doi: 10.1111/j.1532-5415.1998.tb06643.x. [DOI] [PubMed] [Google Scholar]
- (20).Gordon-Salant S. Hearing loss and aging: new research findings and clinical implications. J Rehabil Res Dev. 2005;42:9–24. doi: 10.1682/jrrd.2005.01.0006. [DOI] [PubMed] [Google Scholar]
- (21).Pickles JO. An introduction to the physiology of hearing. Emerald Group Publishing; Bingley, UK: 2008. [Google Scholar]
- (22).Grimes AM, Grady CL, Pikus A. Auditory evoked potentials in patients with dementia of the Alzheimer type. Ear Hear. 1987;8:157–161. doi: 10.1097/00003446-198706000-00005. [DOI] [PubMed] [Google Scholar]
- (23).Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer’s disease. Neurology. 1993;43:779–785. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- (24).Parvizi J, Van Hoesen GW, Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann Neurol. 2001;49:53–66. doi: 10.1002/1531-8249(200101)49:1<53::aid-ana30>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- (25).Baloyannis SJ, Mauroudis I, Manolides SL, Manolides LS. Synaptic alterations in the medial geniculate bodies and the inferior colliculi in Alzheimer’s disease: a Golgi and electron microscope study. Acta Otolaryngol. 2009;129:416–418. doi: 10.1080/00016480802579074. [DOI] [PubMed] [Google Scholar]
- (26).Gates GA, Beiser A, Rees TS, D’Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc. 2002;50:482–488. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- (27).O’Grady G, Boyles AL, Speer M, DeRuyter F, Strittmatter W, Worley G. Apolipoprotein E alleles and sensorineural hearing loss. Int J Audiol. 2007;46:183–186. doi: 10.1080/14992020601145294. [DOI] [PubMed] [Google Scholar]
- (28).Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Holtzer R, Rakitin BC, Steffener J, Flynn J, Kumar A, Stern Y. Age effects on load-dependent brain activations in working memory for novel material. Brain Res. 2009;1249:148–161. doi: 10.1016/j.brainres.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiol Aging. 2007;28:784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]
- (31).Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- (32).Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24:761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am. 1995;97:593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- (34).Boyle PA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Processing resources reduce the effect of Alzheimer pathology on other cognitive systems. Neurology. 2008;70:1534–1542. doi: 10.1212/01.wnl.0000304345.14212.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. 2000;40:320–326. doi: 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- (36).Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25:593–599. doi: 10.1044/jshr.2504.593. [DOI] [PubMed] [Google Scholar]
- (37).Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355:1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- (38).Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63:2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- (39).Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- (40).Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43:661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- (41).Lazarov O, Robinson J, Tang YP, et al. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- (42).Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- (43).Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- (44).Zhan W, Cruickshanks KJ, Klein BE, et al. Generational differences in the prevalence of hearing impairment in older adults. Am J Epidemiol. 2010;171:260–266. doi: 10.1093/aje/kwp370. [DOI] [PMC free article] [PubMed] [Google Scholar]