Abstract
A long-unresolved question in the developmental biology of Drosophila melanogaster has been whether methyl farnesoid hormones secreted by the ring gland are necessary for larval maturation and metamorphosis. In the present study, we have used RNAi techniques to inhibit 3-Hydroxy-3-Methylglutaryl CoA Reductase (HMGCR) expression selectively in the corpora allatal cells that produce the circulating farnesoid hormones. The developing larvae manifest a number of developmental, metabolic and morphogenetic derangements. These defects included the exhibition of an “ultraspiracle” death phenotype at the 1st to 2nd larval molt, similar to that exhibited by animals that are null for the farnesoid receptor ultraspiracle. The few larvae surviving past a second lethal period at the 2nd to 3rd instar larval molt, again with “ultraspiracle” phenotype, often became developmentally arrested after either attaining a misformed puparium or after formation of the white pupa. Survival past the “ultraspiracle” lethal phenotype could be rescued by dietary provision of an endogenous dedicated precursor to the three naturally secreted methyl farnesoid hormones. In addition to these developmental and morphogenetic defects, most larvae that survived to the late second instar exhibited a posterior-originating melanization of the tracheal system. These results support the hypothesis that larval methyl farnesoid hormones are necessary for larval survival and morphogenetic transformation through the larval and pupal metamorphic processes.
Keywords: Juvenile Hormone, Methyl Farnesoate, Ecdysone, Pupation, Molt, Ultraspiracle, RXR, Metamorphosis, Melanization
Introduction
Hormonal signaling is a significant component of the regulation of cellular differentiation and tissue maturation in both vertebrates and invertebrates. In vertebrate systems, isoprene-based retinoid and steroid hormones each have been shown to affect embryonic development and reproductive maturation (Daftary and Taylor, 2006).
In the case of retinoids, several independent experimental paradigms to manipulate either the ligand, or its receptor, similarly support a role of endogenous retinoid in embryonic tissue differentiation. First, application of exogenous retinoic acid induces clear teratogenic effects on the embryonic formation of limbs and the central nervous system (Collins and Mao, 1999). Second, elegant surgical explantion techniques to detect secretion of biologically active retinoid (Sonneveld et al. ,1998) have supported the hypothesis of an endogenous paracrine contribution of retinoic acid (Maden, 2001). Third, confirmatory morphogenetic outcomes were observed when molecular genetics was used to block endogenous retinoid biosynthesis by retinaldehyde dehydrogenase 2 (Ji et al., 2006). From the other direction of experimental paradigm, i.e., manipulation of the ligand’s receptor, molecular genetics to remove the retinoic acid receptor again supported a model of paracrine activation of the retinoic acid receptor to regulate embryonic differentiation (Maden, 2006).
A similar line of investigation has been used in Drosophila melanogaster (hereafter: Drosophila) and other invertebrate model systems, to test the role of steroid and isoprene-derived farnesoid hormones in insect embryonic development and tissue differentiation. These farnesoid hormones are synthesized in a pathway that involves 3-Hydroxy-3-Methylglutaryl CoA Reductase (HMGCR, Belles et al. 2005). Classical endocrine organ (ring gland) transplantation experiments evidenced that a hormone (that was later identified as 20-hydroxy ecdysone; 20E) was necessary for both larval molting and to drive the maturation of imaginal discs at the end of larval development (Bodenstein, 1944; Jones and Jones, 2007,Vogt, 1943;). Molecular genetic experiments in Drosophila to block enzymes in the 20E biosynthetic pathway yielded early death of larvae (Henrich et al., 1993). Similarly, when biosynthesis of 20E was conditionally blocked during the metamorphic third larval instar, morphogenetic derangement of certain nerves and of imaginal structures was observed (Brennan et al., 1998; Li and Cooper, 2001). From the alternative direction of the ecdysone receptor, rather than its ligand, Drosophila null mutants of ecdysone receptor exhibit death during larval development, including an “ultraspiracle”-like failure to ecdyse the apolysed prior instar cuticle (Li and Bender, 2000).
To the considerable frustration of the field, it has been more difficult to experimentally address the role of farnesoid hormones in the larval development and morphogenetic transformation of Drosophila (Riddiford, 2008). On the basis of studies in other surgically tractable model insects, the first known farnesoid hormone in Drosophila (methyl epoxyfarnesoate, JH III) was hypothesized to function in metamorphosis by way of its decrease or absence at metamorphosis (Gilbert et al., 2000). Indeed, morphogenetic derangements can be obtained by exogenous JH treatment of Drosophila larvae at the onset of the larval to pupal transition (Postelthwait, 1974; Riddiford and Ashburner, 1991; Wozniak et al., 2004). However, for reasons of the small size of Drosophila larvae and the surgical difficulty in excising the juvenile hormone secreting cells from the ring gland, the corresponding experiment to surgically remove the endogenous hormone could not be done.
Experimental dissection later became more complicated by the discovery that the endocrine source secretes not one, but three farnesoids: methyl farnesoate, methyl epoxyfarnesoate (= JH III), and methyl bisepoxyfarnesoate (Richard et al., 1989a; hereafter referred to as ‘farnesoid hormones’ or ‘methyl farnesoids’). Yet further exacerbating the situation has been that although nuclear hormone receptors for retinoic acid and 20E were firmly identified two decades ago, identification of nuclear hormone receptors for these three Drosophila farnesoids in larvae has been comparatively delayed. Only more recently has the Drosophila ortholog of vertebrate nuclear hormone receptor RXR been show to bind methyl farnesoate with nanomolar affinity (Jones et al., 2006), while the Drosophila methoprene resistant (MET) protein has been reported to similarly bind JH III (Miura et al., 2005). However, while larvae carrying null mutation for the Drosophila RXR ortholog die with an “ultraspiracle” phenotype, most null mutant larvae for MET survive to adulthood (Wilson et al., 2006). Hence, the actual role of farnesoid hormones in the larval development and metamorphosis of Drosophila remains inadequately understood.
In the present study, we have used RNAi molecular genetic approaches targeted to the ring gland to selectively block the endogenous biosynthesis of the three methyl farnesoids secreted by the Drosophila ring gland. We report here the resultant survival, behavioral, morphogenetic and other phenotypic outcomes. These outcomes include the presentation of an apparent“ultraspiracle” death phenotype, similar to that exhibited by larvae carrying a null mutation for the Drosophila RXR. The death with “ultraspiracle” phenotype can be rescued by exogenously supplying a dedicated endogenous farnesoid precursor to the three secreted farnesoid hormones.
Methods and Materials
Fly Strains
The fly strains used in these studies were maintained on standard food medium at 25° C. The wildtype background used in the present study was the yellow white (yw) strain. In order to accomplish reduction in expression of the enzyme 3-Hydroxy-3-Methylglutaryl CoA Reductase (HMGCR) specifically in the corpora allatal cells, a binary UAS-based system was used. The fly line used here (Di11) carrying the transgene with a promoter for expression of the Gal4 protein specifically in the corpora allata cells was characterized previously (Belgacem and Martin, 2007). The specificity of the expression of this RNAi only in the corpora allatal cells ensures that the HMGCR-dependent pathways in all other larval body tissues (which lead to products other than methyl farnesoid hormones) are left unsuppressed by the RNAi. Hence, the phenotypic outcomes observed will be arising specifically from suppressing the HMGCR-dependent pathway of the corpora allatal cells that leads to the production by those cells of the three methyl farnesoid hormones. From a fly strain (10367-R3) previously reported as transformed with a UAS-driven transgene encoding expression of RNAi against HMGCR (p[UAS-RNAi-HMGCR] (a courtesy of R. Ueda, NIG, Japan, to J.R.M.) (Belgacem and Martin, 2007)) we recovered upon outcrossing to yw two independent transformant lines. Both lines performed similarly upon crossing into a background containing the Di11 transgene (the latter characterized by Belgacem and Martin 2007), so the results with only one of these (HMGR(r)) are reported here. The Di11 transgenic chromosome is homozygous lethal, but can be maintained over a chromosome 3 Ser balancer marked with an actin-driven green fluorescent protein (GFP). For subsequent collection of hemolymph samples and hormone treatment experiments, these flies were crossed to the strain homozygous for the HMGCR expression transgene. At the time of collection of newly hatched 1st instar larvae, those larvae visually expressing GFP under proper illumination were manually removed.
Experimental Rearing Conditions
Newly hatching 1st instar larvae (< 4 hr old) were collected and reared individually at 25° C in 10 mm × 75 mm glass vials containing standard Drosophila diet. Larvae were observed and scored periodically for survival and expression of other phenotypes described here.
For studies on dietary provision of various farnesoid-related compounds to fly larvae with expressing HMGCR-RNAi, three independent replicates were performed. Each replicate consisted of at least 11 larvae being reared individually in glass vials of food, onto which had been layered and dried 6.25 μl of an ethanolic solution containing 125 nanomoles of the given compound. Each larva was examined daily for the following parameters (a) the developmental stage of death, (b) the status of ecdysis before death, (c) the location of the body at death (in the food, on the food, on the glass wall of the vial or at the interface of the food and the glass wall) and (d) for the location of the ecdysed prior instar larval mouthparts (whether attached to the dead larva, visibly separated or not from the dead larvae by more than one body length away, or not present on either the food or the wall of the glass vial, i.e., located down in the food). When the larva died on the glass wall of the vial, it could be easily determined whether the caste prior cuticle was still attached to the new body. This status could also be observationally determined when the new larva had died on the food. However, in a minority of cases, the roughened/wet food surface prevented clear observation of the point of attachment. On the basis of some preliminary assessments, we decided that for the purpose of the studies here, it was reasonable to presume that mouthparts less than one body length away from the body of the dead larva were still attached to the larva.
For the studies on hemolymph farnesoid concentrations, wild type and experimental 3rd instar larvae were reared as respective batch cohorts on standard diet, and recovered from the diet for extraction at the time indicated in the text. Some parallel experimental larvae were reared on food containing blue dye, which confirmed the larvae were feeding during the 3rd instar up to the time of collection.
Microscopy
Photography of phenotypes relating to failed partial ecdysis, arrested puparial/pupal development and melanization of the tracheal system was performed with a Spot camera (Nikon) on a Nikon SMZ1500 binocular microscope. Photography of posterior spiracles and mandibles associated with an “ultraspiracle” phenotype was performed on a Nikon Eclipse TE300 compound microscope also fitted with a Spot Camera. Images acquired through the Spot Camera were imported into Adobe Photoshop for figure preparation.
Chemicals
The various farnesoid-related chemicals used in the feeding studies were obtained from the following sources. Sigma: Mevalonolactone (97 % pure); trans, trans-Farnesol (>95% pure); JH III (>65 % pure; calculations on dose in feeding studies adjusted for the purity). Echelon: Methyl farnesoate (>95% pure). Fluka: Farnesal (>85% pure mixed isomers). Zoecon: Methoprene (>99 % pure). Enzo: Methoprene acid (98% pure, +/- isomer mix).
Measurement of Methyl Farnesoid Concentration in Hemolymph
The three methyl farnesoids known to be synthesized in and released from the corpora allatal cells of the Drosophila larval ring gland are methyl farnesoate, methyl epoxyfarnesoate, and methyl bisepoxyfarnesoate (Richard et al. 1989a,b). Although the methods to those papers describe coextraction together of the ring gland plus culture medium, preliminary analyses of the products contained in the gland versus the secreted into the medium confirmed that the ring gland did not store any of these three newly synthesized “methyl farnesoid” products (D. Richard, pers. comm.). According to the known biosynthesis pathway for each of these compounds in Drosophila, all three derive from mevalonate, the enzymatic product of hydroxymethylglutaryl CoA reductase (Belles et al., 2005; Moshitsky and Applebaum, 1995). Hence, the RNAi that is genetically targeted against HMGCR in the corpora allatal cells would necessarily inhibit production of all three methyl farnesoids. To confirm by physiochemical means that the methyl farnesoid concentration in circulation is suppressed, we measured the methyl farnesoid concentration in normally developing and RNAi-targeted larvae.
Due to constraints in obtaining hemolymph from second instar or younger Drosophila larvae, analyses were performed on surviving mid-late 3rd instar RNAi-HMCGR larvae. As a control for potential occasion to occasion differences in developmental rates, on each occasion that a cohort of Di11; RNAi-HMGCR larvae were bled, a sibling cohort of Actin-GFP/RNAi-HMGCR (i.e., wild type) larvae were bled. The ratio between these two groups of the total circulating methyl farnesoids was then calculated.
These cohorts of batch reared larvae were washed several times with distilled water prior to extraction of hemolymph. The hemolymph obtained was by manual piercing of larvae with a fine needle, and then centrifuging (2000 g × 2 min) the carcasses against a fine mesh cloth in an 0.5 ml microfuge tube. The isolated hemolymph that had been collected into the bottom of the tube was immediately transferred into methanol in 500 ul conical glass microvials having Teflon crimp caps for subsequent determination of methyl farnesoid content.
As adapted from Teal et al. (2000), methyl farnesoids were extracted from methanolic extracts of hemolymph by injecting 100 μl of pentane containing 1pg/ul each of Z-9-tetradecenoic acid methyl ester (Z-9-14:OMe) and farnesyl acetate (FA) through the cap of the vial and then vortexing the mixture for 2min. The emulsification was broken by centrifugation at 8000 g for 5 min and the pentane layer removed to a new vial. The methanolic extract was re-extracted using only pentane (100 μl) two additional times and the pentane extracts combined. Prior to chemical analysis the samples were subjected to micro-solid phase extraction (MSPE). MSPE was performed using 3.5 mm (id) glass tubes drawn to a 1.5 mm (id) hole. A stainless steel screen (180 mesh), fused into the glass using heat at the point at which the taper was made served as a retainer for the 20 mg bed of activated silica gel (60-80 mesh). Prior to application of extracts the MSPE was washed with 2ml of pentane. After application of the pentane extract from hemolymph the MSPE was washed with another 500 μl of pentane. The farnesoids of interest were then eluted with 250 μl of pentane containing 15% redistilled ethyl acetate. Samples were concentrated to ca.15 μl under a fine stream of N2 prior to chemical analysis.
Chemical ionization mass spectroscopy (CIMS, isobutene reagent gas) was performed using an Agilent 5975B MS interfaced to an Agilent 6890N gas chromatograph (GC). Samples (1ul) were injected using a cool-on-column injector. The injector was fitted with a 10 cm length of 0.5 mm (internal diameter) deactivated fused silica tubing which was in-turn connected to a 1m × 0. 25 mm ( internal diameter) length of deactivated fused silica tubing which acted as a retention gap. The retention gap was connected to the analytical column, a 30m × 0.25 mm (id, 0.25 μm coating thickness) DB5MS (J&W Scientific) fused silica capillary column. The conditions of chromatography were: initial oven and injector temperatures = 30 °C, 5 min; oven and injector temperatures increased at 10°C/min; final temperature = 225. The MS was operated in selective ion mode (SIM) using m/z = 241 for methyl (Z)-9-tetradecenoate (Z-9-14:OME); m/z = 251, 219, 191 for methyl farnesoate; m/z = 267, 249 and 235 for methyl epoxyfarnesoate); m/z = 221 and 203 for farnesyl acetate (FA); m/z = 283, 265 and 251 for methyl bisepoxyfarnesoate).
Each day an initial analysis was performed using a synthetic blend of the compounds of interest at a concentration of 1ng/μl in the total ion acquisition mode (m/z 60-300) to determine the retention times and chromatographic properties of the compounds. If properties had changed we replaced the retention gaps and reanalyzed the standard blend. If times shifted then the sectors for monitoring selected ions for each compound were adjusted. Subsequently an injection of 50 pg of each of the standards was made in the selected ion mode to insure that the retention windows were correct and that ratios of ions for each compound were as expected. Compounds eluting during analysis of natural samples were compared for ratios of diagnostic ions and retention times with those obtained from data generated from SIM analysis of the 50 pg blend of synthetic standards run each day. Amounts of farnesoids present in samples were calculated from integrations of peak areas for principal ions for each compound (Z-9-14:OME m/z = 241; methyl farnesoate m/z =251; methyl epoxyfarnesoate m/z = 235; FA m/z =221; methyl bisepoxyfarnesoate m/z = 281). Correction factors were calculated into quantification calculations to account for differences in ion abundance for each or the ions based on intensities of ions calculated from SIM analyses of the synthetic standards run each day.
Results
Larval Death In RNAi-HMGCR Animals
The enzymatic product of HMGCR (mevalonic acid) is essential for the farnesoid biosynthesis pathway. We sought to determine 2nd instar, 3rd instar, and pupal phenotypic outcomes of RNAi interference with HMGCR. We first sought to determine the periods of lethal phenotype in these “farnesoid (-)” animals. To ensure that the time of death was accurately determined, the larvae were individually reared and monitored regularly during the ensuing larval feeding and molting. As shown in Fig. 1, we observed specific physiological occasions at which larval death was concentrated. The major periods of larval death were at each occasion of larval molting, i.e., during or newly after the 1st to 2nd instar molt, during or newly after the 2nd to 3rd instar molt and, of those still surviving, finally at puparium formation or the larval-pupal molt. The greatest mortality was observed at or immediately after the 2nd to 3rd instar molt (“L2-3 intramolt” and “NM3” = newly molted 3rd instar in Fig. 1). Observations in which blue dye was included in the food confirmed that the larvae were feeding during the 1st and 2nd instar prior to their death at the intramolt periods.
Figure 1.
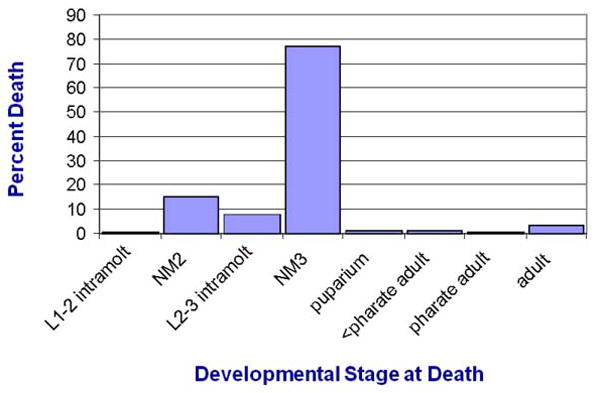
Mortality of farnesoid (-) larvae at successive developmental stages. Newly hatching 1st instar larvae expressing an RNAi that suppresses expression of HMGCR in the larval corpora allatal cells were moved to individual small diet vials and observed regularly for survival. >99% of these 1st instar larvae burrowed into the diet soon after transfer, and were then subsequently monitored for attainment of the indicated life stages (1-2 intramolt = lst instar larva exhibited incomplete ecdysis to the 2nd instar; NM2 = newly molted 2nd instar; 2-3 intramolt = 2nd instar larva exhibited incomplete ecdysis to the 3rd instar; NM3 = newly molted 3rd instar; puparium = 3rd instar larva attained puparium formation but had not everted to white pupa; < pharate adult = had attained at least white pupa stage but not attained pharate adult status). n = 341
Suppressed Methyl Farnesoid Level in Hemolymph of RNAi-HMGCR Larvae
In view of the strong larval death phenotype observed in larvae expressing RNAi-HMGCR, we sought confirmation that the level circulating farnesoid hormone was reduced. The complex and potentially shifting ratio of the three methyl farnesoids secreted by the ring gland (methyl farnesoate, methyl epoxyfarnesoate, methyl bisepoxyfarnesoate) at different stages of Drosophila larval development is not known. Hence our genetic approach here was to suppress production of all three methyl farnesoids. Due to constraints on obtaining sufficient hemolymph to measure circulating total methyl farnesoid concentration in 2nd instar larvae, we sampled hemolymph from RNAi-HMGCR larvae that had survived to the mid-late 3rd instar feeding stage. For each sampling occasion, a matched cohort of wild type larvae was also bled, and the ratio of the measurements from the two groups was calculated. For n=3 matched pairs of cohorts, the average±SE of ratio of total methyl farnesoid concentration in RNAi-HMGCR to wild type was 0.37± 0.06 (i.e., was significantly reduced to 37% of the concentration (1.7 nanomolar) in the wild type 3rd instar larvae, P < 0.006, t-statistic). These ratio data support that the RNAi-interference method we used was successful in suppressing the level of circulating methyl farnesoid hormone. Because we analysed the experimental hormone levels only in those rare RNAi-HMGCR larvae that survived to the mid-late 3rd instar, it is likely that the ratio above underestimates the level of farnesoid suppression during larval development. That is, the methyl farnesoid level in the RNAi-HMGCR larvae dying before to the mid-late 3rd instar may have been even more suppressed than in those 3rd instar individuals that had escaped the death windows at the 1st-2nd instar and 2nd-3rd instar larval molts (see also below)
“Ultraspiracle” Death Phenotype
Many larvae dying at the 1st to 2nd instar molt displayed a classical “ultraspiracle” phenotype that was reported previously for larvae with a null locus for the ultraspiracle nuclear hormone receptor (Perrimon et al., 1985). As seen in Fig. 2 A, in this phenotype, the apolysed 2nd instar posterior spiracles are juxtaposed near the prior 1st instar posterior spiracles. A similar apolysed state is seen on the anterior end of the larva, with the 2nd instar mouthparts juxtaposed near the prior 1st instar mouthparts (Fig. 2 B). The same spiracular and mouthpart phenotypes were observed for larvae which survived the first larval molt, but instead died at the next (2nd to 3rd) instar molt (Fig. 2 C).
Figure 2.
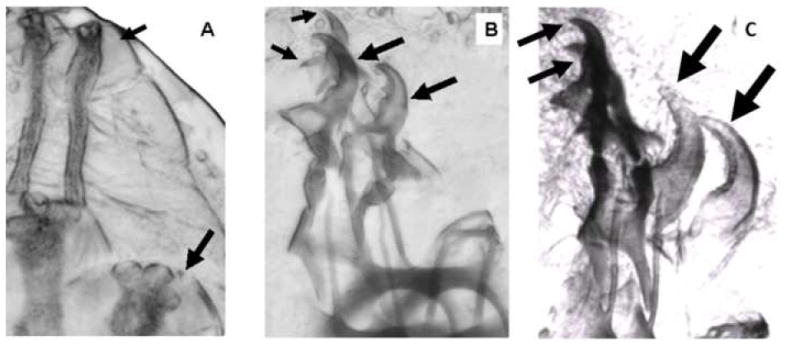
Classic “Ultraspiracle” death phenotype of farnesoid (-) larvae. Some of the larvae expressing an RNAi that suppresses expression of HMGCR in the larval corpora allatal cells were individually reared and observed to developmentally arrest and die after the 1st instar apolysis but prior to ecdysis to the 2nd instar. The view in (A) shows the juxtaposition of the new 2nd instar spiracles (large arrows) to the prior 1st instar spiracles (small arrows). The anterior end view in (B) shows the juxtaposition of the new 2nd instar mouthparts (large arrows) to the prior 1st instar mouthparts (small arrows). The corresponding phenotype was also observed in many larvae that survived to the 2nd to 3rd instar molt. Panel C shows the “double mouthhooks” of 2nd instar larvae (small arrows) and 3rd instar larvae (large arrows).
At each larval-larval molt, some other larvae progressed slightly beyond the unecdysed “ultraspiracle” phenotype, and attained an incomplete ecdysis before dying. Examples in Fig. 3 show larvae attempting to ecdyse from the 2nd to the 3rd instar. Some larvae could only split the apolysed 2nd instar cuticle along the middle of the body, and not remove the remainder of the cuticle from either the mouthparts or the posterior end (Fig. 3A). In other cases, the 2nd instar cuticle was fully ecdysed except for being caught on the mouthparts (Fig. 3B). In other (the majority of) cases the mouthparts were freed, but the old cuticle remained caught only on the posterior end and was dragged along as the dying larva attempted to move (Fig. 3C).
Figure 3.

Failed 2nd to 3rd instar ecdysis phenotype of farnesoid (-) larvae. Some of the larvae expressing an RNAi that suppresses expression of HMGCR in the larval corpora allatal cells were individually reared and were to observed to partially ecdyse but fail to completely ecdyse to the 3rd instar. The most frequently observed phenotypes of these larvae were (Panel A) the splitting of the 2nd instar exoskeleton at the middle of the body but failure to remove the exoskeleton from either of the anterior or posterior ends, (Panel B) the 2nd instar mouthparts remaining caught in the 3rd instar mouthparts, and (Panel C) the posterior end of the 2nd instar exoskeleton remaining caught on the posterior end of the 3rd instar, causing the remainder of the cast 2nd instar exoskeleton (including 2nd instar mouthparts, arrow) to be ‘dragged along.’
Death at Larval-Pupal Molt
Less than 5% of the individually reared farnesoid (-) larvae survived to pupariation. Some of these larvae became developmentally arrested at pupariation stage with variation in phenotype. Most that were arrested stopped development after sclerotization of the puparium but prior to eversion to the white pupa stage (Fig. 4A, B). However, a minor proportion were observed that ceased feeding, became sessile, everted their anterior spiracles, but then failed to sclerotize a puparium. A trend was also observed that the smaller the size of the sclerotized puparium that was formed, the lesser was the browning pigmentation of the puparium.
Figure 4.

Reduced pupa inhibits expression of HMGCR in the larval corpora allatal cells survived to form puparia that then died without pupation. Typically, these larvae were delayed reaching the pupariation stage, and their puparial size (A, ventral view; B, dorsal view) was distinctly less than normal (C). In a frequent phenotype, the projected papillae were appressed together (D, E). Some 3rd instar larvae did not properly contract the body in forming a puparium, especially at the anterior end involving the operculum (G), similar to that reported in Fig. 1 of Henrich et al., (2000), reprinted here as panel F.
Abnormal Morphology of Puparium
Normally, when the body of the 3rd instar larva contracts to form the puparium, the anterior spiracles are everted, with the ends of each everted spiracle possessing papillae that splay apart (Fig. 4 D). However, many animals expressing the HMCGR-RNAi that exhibited arrested puparial development also frequently failed to fully spread the projected ends of the everted anterior spiracles (Fig. 4 E). In a series of animals examined for this purpose, significantly fewer (14%, n = 64) of the HMGCR-RNAi expressing animals showed the normal splaying of the papillae on both everted spiracles, as compared to 94.7% of sibling GFP-expressing animals (n=21) (P < 0.001, χ2 test). In the farnesoid (-) larvae that were developmentally arrested as puparia, the operculum often was misformed and not fully contracted anteriorly/posteriorly (Fig. 4 G).
Death During Pupal and Adult Stages
Of the small percentage of larvae that attained an apparently normally externally formed white pupa stage, some did not proceed beyond that stage, while others developed on to the pharate adult before arresting development. No animals were observed that appeared to have clearly ceased development between the white pupa and the pharate adult stage (e.g., reaching eye pigmentation but not progressing farther). Few animals (mostly females) survived to adult emergence (Fig. 1). Of those that did, individuals expressing a particular tracheal melanization phenotype appeared to survive a shorter time than other surviving siblings (see below).
Change in Rate of Larval Development
We were interested in whether the farnesoid (-) larvae would exhibit phenotypes other than those directly associated with morphology or death. In view of a previous report that the total time from egg hatch to pupation was increased in farnesoid (-) larvae (Belgacem and Martin 2007), we inquired as to what stages during that interval were being lengthened. Close observation disclosed that farnesoid (-) larvae attained the molt from the 2nd to the 3rd instar at most several hours later than sibling larvae not expressing the RNAi (within the margin of ± 2 hr time of collection of that starting newly hatched 1st instar larvae). We then observed that larvae surviving beyond that the 2nd to 3rd larval molt showed a sharp dichotomy into two groups with respect to the subsequent rate of development to pupariation. Under the rearing conditions we used, most control sibling 3rd instar larvae not expressing RNAi against HMGCR(r) took 2-3 more days to then develop to pupariation. The subset of farnesoid (-) larvae that successfully formed white pupae also took 2-3 days to develop to puparation after the 2nd to 3rd instar molt. However, the total larvae period up to pupariation by the other subset of farnesoid (-) larvae that became developmentally arrested at puparium formation was delayed by a day, compared to the subset of farnesoid (-) larvae that proceeded on to the white pupa stage (avg.± S.E. 6.3±0.21 days versus 5.3±.07 days, respectively, p < 0.01, t-statistic). Hence, the general appearance of a delay in larval development of farnesoid(-) animals centered primarily in a lengthed 3rd instar in the subset of animals that attained, but then developmentally arrested at, puparium formation.
Although these larvae that became developmentally arrested at pupariation exhibited a 3rd instar feeding period an average of a day longer than normal, this did not result in greater final size of the larva. As shown in Fig. 4A, B such puparial larvae instead were typically distinctly smaller in size than normal.
Behavioral Changes in Farnesoid (-) Larvae
Under the conditions of rearing larvae individually in small glass vials, nearly all control sibling larvae not expressing the RNAi pupated at the surface of the food, typically at the interface of the edge of the food and the glass vial (Fig. 5). However, farnesoid (-) larvae exhibited a distinctly different behavior. Beginning with those that died at the 1st to 2nd instar molt, some of these larvae moved up the side of the glass vial before they died at that location. Surviving larvae that went on to attain the 2nd to 3rd instar intramolt, or even to the newly molted 3rd instar, respectively, as their stage of death, exhibited a significantly increased tendency to move up the side of the glass vial before they died on the glass (Fig. 5).
Figure 5.
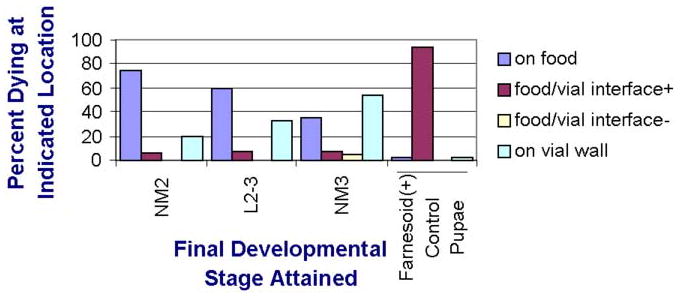
Behavioral death phenotype of farnesoid (-) larvae, which exhibited a significantly less incidence of dying at the glass vial/food interface than was the incidence of control animals pupating at that location (P 0 < 0.0001, χ2). Those farnesoid (-) larvae that progressed to L2-3 intramolt before death exhibited a significantly greater incidence of moving up the side of the glass rearing vials just before death than those than died in 2nd instar (P < 0.0001, χ2), and those that attained the newly molted 3rd instar before dying exhibited yet significantly more greater incidence than those dying at L2-3 intramolt, P < 0001, χ2). NM2 = newly molted 2nd instar; L2-3= intramolt from 2nd to 3rd instar; NM3 = newly molted 3rd instar. Interface + = body orientation with head upward onto glass wall and posterior down onto food (see Fig. 10 A). Interface - = body orientation with head downward onto the food and with the posterior upward onto the glass. (n= 400).
The above data prompted an even closer observation regime to detect the actual movement that preceded death on the side of the glass rearing vial. On several occasions we observed that newly molted 3rd instar larvae would suddenly and quickly move from the food to the side of the vial, and within a very short time of aggressive move many (>10) body lengths up the side of the vial away from the food. At some point, the movement would change to moving laterally along the vial, and the larva would soon cease moving, and subsequently engage in feeble movements. Within several hours the larva would be dead, often not having moved far (e.g., less than 2-3 body lengths), though sometimes the larva would be found back down on the food, dead or moribund.
In other cases, farnesoid (-) larvae were observed at the 2nd to 3rd instar intramolt stage that would similarly move quickly up the side of the vial. A signature behavior of these larvae would be to rear their head and first several body segments up off the glass, ‘cobra-like’. The larvae would appear to be exerting considerable strain and tension within the body (giving the impression that the larvae were trying to ecdyse). These larvae frequently died on the side of the glass vial, sometimes with the cast 2nd instar exoskeleton nearby on the side of the glass vial.
Sensitivity of Farnesoid (-) Larvae to Exogenously-Supplied Farnesoids
We tested the effect of single exogenously supplied farnesoid-like compounds on larvae with suppressed HMGCR. Larvae supplied in the diet with only control EtOH carrier died at either the L2-3 intramolt (= ultraspiracle phenotype) or at attainment of the newly molted 3rd instar. However, an even greater incidence of death prior to attainment of the newly molted third instar occurred when the larvae were supplied instead with only JH III (P < 0.0001) or only methyl farnesoate (P < 0.0001), or both (P < 0.0001) in the diet (Fig. 6; χ2 .tests). Thus, when the biosynthetic pathway leading to all three naturally secreted methyl farnesoids is suppressed, adding only one of the farnesoids (JH III or methyl farnesoate) back to the larva is even more developmentally adverse than is the case of just suppression of the biosynthetic pathway.
Figure 6.
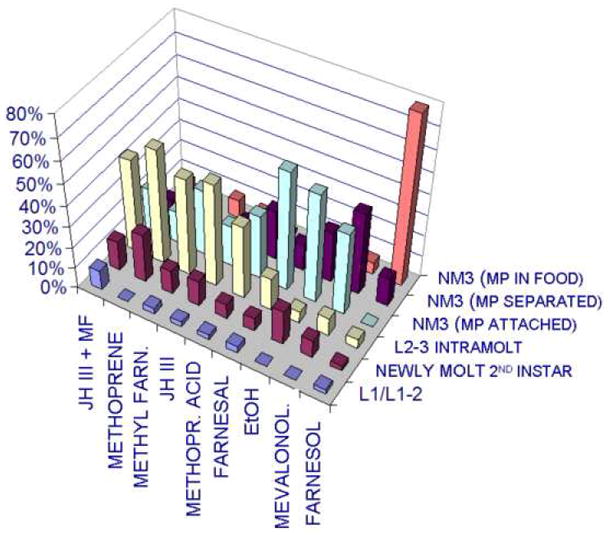
Effect (expressed as % death) of provision of farnesoid-like compounds in the larval diet of farnesoid (-) larva. The indicated compounds were layered onto the surface of the larval diet, providing both topical and dietary exposure. Control ethanol-treated larvae did not successfully ecdyse the 2nd instar cuticle, or if they did so, performed the ecdysis on the (abnormal location) above the food surface. However, nearly all larvae exposed to farnesol successfully ecdysed the 2nd instar cuticle to a free 3rd instar, and they performed that ecdysis with the cast 2nd instar mouthparts remaining under the surface of the food, as normal. MF = methyl farnesoate; JH III = methyl epoxyfarnesoate; Methyl Farn. = methyl farnesoate; Methopr. Acid = methoprene acid; Mevalonol. = mevalonolactone; L1 = death during 1st instar; L1-2 = death during 1st instar to 2nd instar intramolt stage prior to ecdysis; L2-3 = death during 2nd instar to 3rd instar intramolt stage prior to ecdysis; NM3 = newly molted 3rd instar; MP attached = 2nd instar mouthparts visible less than one body length away from the dead body of the 3rd instar larva; MP separated = 2nd instar mouthparts visibly located (on food surface or glass vial) more than one body length away from the dead body of the 3rd instar larva; MP in food = 2nd instar mouthparts were not visible on food surface or up on wall of glass vial above food. Dissection of food in several vials confirmed the 2nd instar mouthparts and cuticle were buried under the food surface.
The newly molted 3rd instar larvae that died in the EtOH carrier treated controls exhibited a distinct phenotype in which 2nd instar cuticle, although nearly fully ecdysed, most often remained attached at a narrow point to the body of the new 3rd instar. Most commonly the point of remaining attachment was at the posterior spiracles (e.g., Fig. 3 C). However, farnesol, a dedicated precursor to the synthesis of all three methyl farnesoids normally secreted by the ring glands (Belles et al., 2005), exerted a rescue effect. Nearly all farnesol treated larvae were rescued to fully ecdyse and release the second instar cuticle. An additional phenotype of rescue by farnesol was also observed. When individually reared wild type 2nd instar larvae ecdyse to the 3rd instar, the ecdysis typically takes place under the food surface, so that the case 2nd instar mouthparts cannot be observed by external examination of the vial and food. The farnesol-treated farnesoid (-) larvae not only fully ecdysed the 2nd instar cuticle, but they also did so under the food surface. Thus, the inclusion of farnesol in the diet (1) rescued the larval molting biochemistry to enable full ecdysis of the 2nd instar cuticle, and (2) rescued the wild type behavior to conduct that ecdysis under the food surface.
Melanization of Tracheal System
The larvae expressing RNAi against HMGCR exhibited a striking and fully penetrant phenotype of melanization of the tracheal system, especially the two main dorsal tracheal trunks (Fig. 7). This phenotype most often began at the posterior end of the two main dorsal tracheae (Fig. 7 B, arrow), becoming apparent at the approach or onset of the 2nd to 3rd larval molt. However, in a minority of larvae, the phenotype would begin earlier, during the feeding stage of the 2nd instar, as one or several small black dots at the posterior end of the larva. Only in very rare cases did a 2nd instar feeding larva that exhibited these spots then fail to proceed on to body-wide manifestation of the melanization shown in Fig. 7 A-C.
Figure 7.
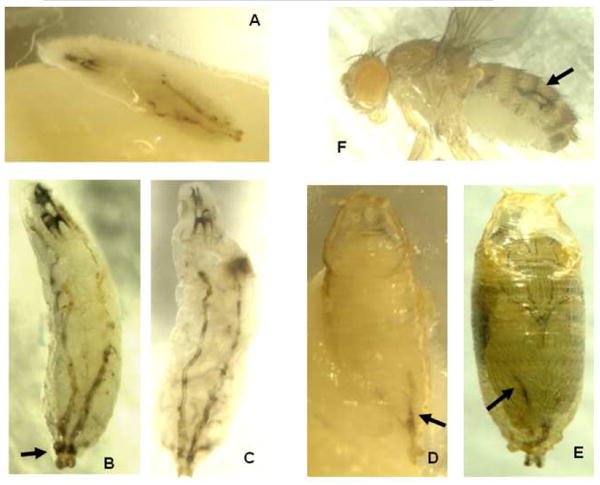
Abnormal melanization of tracheae in farnesoid (-) larvae. Most of the individually reared larvae expressing an RNAi that blocks expression of HMGCR in the corpora allatal cells were to observed exhibit tracheal melanization. The melanization started at the posterior end of the 2 main dorsal tracheal trunks, and rapidly spread anteriorly at or shortly after the 2nd instar to 3rd instar apolysis (A, B). In some cases, there seemed to be a positional periodicity to foci of the most darkened areas (C). Rarely, larvae that exhibited a lesser amount of melanization survived to pupate, and exhibit melanized streaks in the abdomen (D, E), and even more rarely, those pupae would survive to emerge as adults that still exhibited the tracheal melanization (F).
Once the 2nd to 3rd instar larval molt had been reached, the melanization spread anteriorly up each tracheal trunk over the next several hours. During this anterior spread, the pattern in many larvae would pass through a phase in which the melanization first seemed localized to periodically distributed foci, often paralleling each other on each tracheal trunk (Fig. 7 A). The melanization would then spread to the intervening areas of the tracheal trunks, making a pair of continguous stripes anteriorly up the body of the larva (Fig. 7 B, C).
The great majority of RNAi-expressing larvae that exhibited these melanization stripes would become moribund within several hours of the 2nd to 3rd instar molt. However, a very small percentage would attain pupariation (Fig. 4 A, B), and an even smaller proportion would reach pupation. A minor melanization “stripe” could still be seen in these white pupae (Fig. 7 D), and in those surviving to the pharate adult (Fig. 7 E) and even in the very few adults that emerged from such afflicted pupae (Fig. 7 F). These melanization phenotypes were not observed in the sibling farnesoid (+) larvae. The majority of RNAi-HMCGR larvae surviving to the mid-late 3rd instar that were bled for measurement of circulating methyl farnesoids (above) possessed only a single minor melanization stripe on one side (such as shown in Fig. 7 D), while the remainder did not show any melanization. When this condition is contrasted with the much heavier melanization in larvae that died at the 2nd to 3rd instar intramolt (Fig. 7 A-C), its suggests that those larvae surviving to the mid-late 3rd instar (for bleeding) may have possessed less effective suppression of the farnesoid pathway than did those dying at the 2nd to 3rd instar intramolt.
Discussion
An objective of our studies here was to identify endocrine-related developmental phenotypes in Drosophila larvae that have suppressed endogenous farnesoid hormones. We report here a number of morphogenetic abnormalities that appear to represent misfunction of the metamorphic endocrine axes. Some of these abnormalities could be rescued by exogenous treatment with a farnesoid that is a dedicated precursor to the three methyl farnesoid compounds normally secreted from the larval ring gland.
“Ultraspiracle” Phenotype
It is well established that larval molting and metamorphosis in Drosophila is endocrinologically regulated, with the indispensible participation of two heterodimerizing nuclear hormone receptors: the ecdysone receptor (EcR) and “ultraspiracle”(USP) (Henrich, 2005). Genetic experiments to test specifically for the phenotype that occurs when either EcR or USP is missing during larval development have yielded respectively similar results.
Originally, USP was named on the basis of a phenotype of lethal failed ecdysis at the 1st to 2nd instar molt, wherein unecdysing larvae retain the 1st instar posterior spiracles juxtaposed near the underlying 2nd instar spiracles (Perrimon et al., 1985). Null mutants for USP also exhibit failure to ecdyse the 1st instar mouthparts, resulting in juxtaposition of the 1st and 2nd instar mouthparts (i.e., “double mouthparts,” Li and Bender, 2000). Tests for the effect of loss of larval EcR function yielded a similar double spiracle/double mouthparts phenotype at either of the first two larval instars (Li and Bender, 2000). These similarities in loss of larval function phenotypes for both USP and the EcR support the model that both receptors are necessary components of their heterodimer for the purposes of passage through the first two larval molts, perhaps as part of a larger complex containing the methoprene-tolerant (MET) protein and other transcription factors (Bitra and Palli, 2009; Li et al., 2007).
While the above ‘joint phenotypes’ support a model of the joint necessity of each receptor in larval molting, there remains the consideration as to the necessity, joint or otherwise, of respective ligands of the two receptors. Classical in vivo endocrine transplantation studies of Bodenstein (1944) showed that larval cuticle deprived of endocrine product of the ring gland (a major source of larval ecdysone, the precursor to 20-OH ecdysone) fails to molt, but that restoration of that endocrine source restores the larval molt. In a later genetic approach, some mutations that rendered deficiency in 20-OH ecdysone, (the ligand for EcR) also rendered the phenotypes the double mouthhooks (Gaziova et al., 1994; Henrich et al., 1993) and double spiracles (Henrich, pers. comm.) at the failed larval-larval ecdysis.
In the present study, we report that many larvae with suppressed ring gland production of all three of the known secreted methyl farnesoid products also exhibit a double mouthpart/double spiracle phenotype. Distinctively, Henrich et al. (1993) also reported that some larvae suppressed for the EcR ligand underwent a partial larval-larval ecdysis exhibiting the attachment of the cast cuticle at the posterior body end, just as we observed here for farnesoid (-) larvae. These results are significant in that at least one of these methyl farnesoids in physical assay can bind the receptor USP with nanomolar affinity (ca. 50 nM, Jones et al. 2006). Hence, under certain conditions, this same “ultraspiracle” (failed ecdysis) phenotype is exhibited by larvae that are (1) suppressed for expression of either of the two heterodimerizing hormone receptors, or (2) suppressed for either endogenous circulating compounds that exhibit nanomolar affinity in physical assay for the two respective receptors. The specific biochemical lesion that culminates in the double mouthpart/double spiracle phenotype in each of these four respective scenarios remains to be established.
Farnesoid Rescue/Exacerbation of Failed L2-3 Ecdysis
Our analysis of the circulating methyl farnesoids found that the interfering RNA approach used here suppressed the level of methyl farnesoids. The compound farnesol is a product in the endogenous pathway that leads from HMGCR to biosynthesis, in the ring gland, of the three secreted methyl farnesoids. Because farnesol is ‘dedicated’ to the production of methyl farnesoids, reduced farnesol production in turn results in reduced production of the three downstream methyl farnesoid hormones. The present study found that the “ultraspiracle” phenotype of failed L2-3 ecdysis (that occurs when the farnesoid pathway in the ring gland is blocked) can be rescued by topical/dietary provision of farnesol. So far as we are aware, these results are the first report that any failed larval-larval molting process in Drosophila can be rescued to successful ecdysis by provision of exogenous farnesoid. Because farnesol is a pathway component that is upstream of the steps yielding the three secreted methyl farnesoid hormones, if the exogenously supplied farnesol rescues the remaining downstream biosynthetic steps, it would be predicted to rescue the production of all three secreted methyl farnesoid hormones.
An exacerbation of the larval lethal phenotype of suppressed farnesoid production was seen when individual methyl farnesoid hormones (methyl farnesoate or JH III) were supplied exogenously to farnesoid (-) larvae. This may at first seem paradoxical – provision of missing farnesoid hormone might be hypothesized to rescue, not exacerbate, the lethal phenotype. One possible explanation could be that a proper total concentration and proper ratio of all three secreted farnesoid hormones is necessary to effectuate nonredundant larval functions of these three hormones. A detailed titering of the circulating concentrations of these three hormones during larval development is urgently needed.
Misformation of Puparium
An endocrine-regulated morphogenetic feature of the Drosophila metamorphosis is the formation of a puparium from the 3rd instar cuticle. The shape of the puparium arises, in part, from a contraction of the larval body after it has ceased feeding and attached itself to substrate. Classical endocrine transplantation studies of Vogt (1943) and Possompes (1953) demonstrated that surgical interference with normal ring gland secretory function during the 3rd instar can result in failure of the larval body to contract into its more compact puparial shape, especially anteriorly. More recent genetic analysis of mutant nuclear hormone receptors implicated a mechanistic role in this phenotype for the ligand binding domain of USP (Henrich et al., 2000). Indeed, the morphology of the puparial operculum shown in Fig. 4 G here looks very similar to the aberrant puparial operculum obtained by Henrich et al. (2000) with their USP ligand binding domain mutant (see their Fig. 1, that is reproduced in part here as Fig. 4 F).
Relatedly, Vogt (1943) observed that in larvae made farnesoid (-) (by ligation) before commitment to wandering behavior, the isolated abdominal epidermis exhibited a distinct sensitivity to endocrine induction to formation of a puparium. The isolated abdominal cuticle could be induced to form a puparium by implantation of the fragments (“thighs”; shenkelzellen) of the ring gland that produce ecdysone. However, formation of a puparium was accelerated by implantation from wandering stage larvae of both the portion of the gland secreting ecdysone and the gland fragment containing the corpora allatal cells (that secrete the three methyl farnesoids). Subsequent in vitro ring gland culture experiments observed a surge in secretion of all three methyl farnesoid hormones at the end of the third instar, after a pre-wandering commitment to the subsequent wandering behavior (Jones and Jones, 2007; Richards et al., 1989a). That is, the stage of the larval ring gland where the secretion of corpora allatal cells exhibited a puparium-formation activity in bioassay is also a larval stage at which a surge occurs in secretion of methyl farnesoids by cultured glands (and in increase in total circulating methyl farnesoids, data not shown).
These data provide endocrine evidence of a regulatory separation of the commitment for wandering behavior, versus the subsequent and discrete induction of puparium formation. In our study here, larvae genetically made farnesoid (-) also had difficulty in puparium formation (sclerotization and pigmentation). Thus, the results of the present study using molecular genetics complement the indication of earlier endocrine work that a surge in methyl farnesoids after wandering commitment but before pupariation has a role to enhance the puparium-formation signal.
Altered Larval Movement
In an earlier study using this same HMGCR-RNAi system to suppress methyl farnesoid production, it was observed that farnesoid (-) adults exhibited less walking behavior than normal (Belgacem and Martin, 2007). However, the effect of suppressed farnesoids on larval movement was not examined in that study. Our present study has identified three forms of larval movement that are altered under conditions of suppressed production of methyl farnesoids.
When feeding wild type larvae are stimulated to molt from the 2nd to 3rd larval instar, under our conditions the larvae typically perform cuticle ecdysis under the surface of the food, so that the cast 2nd instar mouthparts are left under the food surface. However, the farnesoid (-) larvae instead exhibited the behavior to perform the 2nd to 3rd instar larval molt above the surface of the food. Interestingly, exogenous exposure of these larvae to farnesol restored the behavior of the larvae to ecdyse under the food surface.
Related to this altered location of the ecdysis to the 3rd larval instar was our observation of an increased activity of larvae to moveup the side of the glass vial at the time of the 2nd to 3rd instar ecdysis. This behavior to vigorously walk up the walls of the glass vial at or near the time of the next larval-larval molt (and die rapidly thereafter) was not observed in the wild type sibling controls. This behavior has apparently not been described as a phenotype of suppressed ecdysone ligand or of suppressed ecdysone receptor.
During normal development near the end of the feeding stage, 3rd instar larvae are induced (apparently by 20E) to cease feeding and walk vigorously for hours, until ceasing at the final location for the larval-pupal molt. In mass culture wandering larvae are frequently observed to move up the sides of the rearing chamber and form a puparium up off of the food. We observed here that when reared individually in the glass vials, the final position of wild type puparium formation was nearly always in the food near the food/vial interface . Early endocrine studies showed that in the absence of the ecdysone-producing fragments of the ring gland, feeding 3rd instar larvae never initiated wandering behavior (Burtt 1938; Possompes 1953). The endocrine studies by Possompes (1953) reported that hormonal secretion of the corpora allatal region of the ring gland during the third (last) instar of Calliphora delayed the exhibition of the “wandering” behavior. Supporting that observation was his demonstration that adult corpora allata explanted into 3rd instar larvae also delayed the larval wandering behavior. More recently, natural methyl farnesoid hormones and synthetic structural analogs have been reported to prolong the feeding stage of third instar Drosophila larvae (Riddiford and Ashburner 1981). These data have led to a current paradigm that at least during the 3rd (last) larval instar of Drosophila and other cyclorraphous flies, methyl farnesoid secreted by the corpora allatal cells of the ring gland prolongs the feeding stage and delays wandering behavior.
On the basis of physical protein-protein binding interactions, Li et al., (2007) hypothesized that the MET protein (that was reported in physical assay to bind JH III; Miura et al., 2005) itself binds in a protein complex that contains two other receptors: USP (that binds methyl farnesoate, Jones et al., 2006)) and EcR (that binds 20E). Perhaps with implications for the present study is that overexpression of the MET protein (which can also form homodimers, Godlewski et al., 2006) caused 1st instar larvae to notably die on the side of the food vial (Barry et al., 2008). Although it was not reported that the death occurred at the 1st to 2nd instar larval molt per se, the death was reported to occur about 24 hr after egg hatch, which is close to the time when the molt would normally occur. Barry et al. (2008) hypothesized that the observed effects of MET protein overexpression arose by shifting dynamic equilibrium toward MET homodimer formation, which in turn then disrupted the normal function of the USP/EcR/MET complex. Whether the underlying mechanism of the behavioral alterations observed here, or by Barry et al. (2008), represents a precocious induction of wandering behavior per se, or is the outcome of some other independent effect, remains unknown.
A third form of altered locomotory behavior observed in our studies concerns the wandering behavior prior to puparium formation. A number of those farnesoid (-) larvae surviving the molt to the 3rd instar then exhibited a distinct delay in the exhibition of wander behavior. This altered behavior was most associated with those animals that developmentally arrested after formation of the puparium. Both delayed wandering behavior and arrested development at the puparium stage have generally been considered as a symptom of suppressed ecdysteroid action. As reviewed above, there is evidence that the initiation of wandering behavior is modulated by methyl farnesoid secretion from the ring gland during the larval feeding stage. For the subsequent occurrence of puparium formation, it is relevant to consider that Richard et al. (1989a) detected a significant secretion of methyl farnesoids in culture by ring glands that had been excised from animals prior to puparium formation. Collectively the above data warrant the consideration that the delayed wandering behavior and the developmentally arrested puparia observed here both arise indirectly from an effect on the production of 3rd instar ecdysteroids. However, there are binding sites for the USP/EcR heterodimer at genes such as E75A, that is involved in regulating ecdysone biosynthesis (Bernado et al., 2009; Bialecki et al., 2002), offering the USP/EcR heterodimer as a possible molecular site of the effect observed here when endogenous farnesoids are suppressed.
Tracheal Melanization
One of the most striking phenotypes observed in this study was the melanization of the larval tracheal trunks. This melanization typically accelerated in its spread anteriorly in the several hours before death occurred, and further increased after the larvae became moribund/deceased. This phenotype appears different from the long-known formation of melanotic tumor masses that appear in the hemolymph (rather than the trachea) of larvae receiving pharmacological overdose of farnesoid compounds (Bryant and Sang, 1969). Several lines of evidence suggest that the tracheal melanization phenotype observed here arises from a triggering or release of immune response. During the course of our studies reported here, Tang et al., (2008) reported that a tracheal melanization phenotype was observed after relaxation of the inhibition over the proteolytic cascade that culminates in melanin formation (compare Fig. 7 here, to Fig. 1 of Tang et al., 2008). Those authors also reported that an end product of the melanization reaction in turn activates transcription of a gene for an antimicrobial peptide (Drosomycin).
Recently, Flatt et al., (2008) have reported evidence that, at least in adult Drosophila, the methyl epoxyfarnesoate (JH III) can suppress the immune response, apparently through suppression of transcription of both the gene encoding Drosomycin and the gene for Diptericin, another antimicrobial peptide. Those authors also observed that in their cell transfection, promoter assay system, methyl farnesoate at nanomolar concentrations also suppresses activation of the Diptericin promoter (Flatt, pers. comm.). Our data here, and those reported by Tang et al. and Flatt et al., suggest a future line of study to test the possibility of a methyl farnesoid effect on immune response at the upstream level of the melanization cascade, in addition to the methyl farnesoid regulation of transcription of downstream antimicrobial peptide(s).
Acknowledgments
Supported by NIH grant GM075248 to GJ and DJ. We are grateful to R. Ueda NIG, Japan, to provide the P[UAS-HMGCR-RNAi] line. Y.H.B. and J.R.M. were supported by the French Ministry of Research and Education (ACI: Action Concertée Incitative: Biologie du Développement et Physiologie Intégrative) and by the CNRS (ATIPE-Neurobiologie), France.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Barry J, Wang S, Wilson TG. Overexpression of Methoprene-tolerant, a Drosophila melanogaster gene that is critical for juvenile hormone action and insecticide resistance. Insect Biochem Mol Biol. 2008;38:346–53. doi: 10.1016/j.ibmb.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgacem YH, Martin JR. Hmgcr in the corpus allatum controls sexual dimorphism of locomotor activity and body size via the insulin pathway in Drosophila. PLoS ONE. 2007;2(1):e187. doi: 10.1371/journal.pone.0000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellés X, Martín D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annu Rev Entomol. 2005;50:181–99. doi: 10.1146/annurev.ento.50.071803.130356. [DOI] [PubMed] [Google Scholar]
- Bernardo TJ, Dubrovskaya VA, Jannat H, Maughan B, Dubrovsky EB. Hormonal regulation of the E75 gene in Drosophila: identifying functional regulatory elements through computational and biological analysis. J Mol Biol. 2009;387:794–808. doi: 10.1016/j.jmb.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Bialecki M, Shilton A, Fichtenberg C, Segraves WA, Thummel CS. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev Cell. 2002;3:209–20. doi: 10.1016/s1534-5807(02)00204-6. [DOI] [PubMed] [Google Scholar]
- Bitra K, Palli SR. Interaction of proteins involved in ecdysone and juvenile hormone signal transduction. Arch Insect Biochem Physiol. 2009;70:90–105. doi: 10.1002/arch.20281. [DOI] [PubMed] [Google Scholar]
- Bodenstein D. The induction of larval molts in Drosophila. Biol Bull. 1944;86:113–124. [Google Scholar]
- Brennan CA, Ashburner M, Moses K. Ecdysone pathway is required for furrow progression in the developing Drosophila eye. Development. 1998;125:2653–64. doi: 10.1242/dev.125.14.2653. [DOI] [PubMed] [Google Scholar]
- Bryant PJ, Sang JH. Physiological genetics of melanotic tumors in Drosophila melanogaster. VI. The tumorigenic effects of juvenile hormone-like substances. Genetics. 1969;62:321–36. doi: 10.1093/genetics/62.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtt ET. On the corpora allata of dipterous insects II. Proc Roy Soc London B. 1938;126:210–223. [Google Scholar]
- Collins MD, Mao GE. Teratology of retinoids. Annu Rev Pharmacol Toxicol. 1999;39:399–430. doi: 10.1146/annurev.pharmtox.39.1.399. Review. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. 2006;27:331–55. doi: 10.1210/er.2005-0018. Review. [DOI] [PubMed] [Google Scholar]
- Flatt T, Heyland A, Rus F, Porpiglia E, Sherlock C, Yamamoto R, Garbuzov A, Palli SR, Tatar M, Silverman N. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J Exp Biol. 2008;211:2712–24. doi: 10.1242/jeb.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LI, Granger NA, Roe RM. The juvenile hormones: historical facts and speculations on future research directions. Insect Biochem Mol Biol. 2000;30:617–644. doi: 10.1016/s0965-1748(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Gaziova I, Bonnette PC, Henrich VC, Jindra M. Cell-autonomous roles of the ecdysoneless gene in Drosophila development and oogenesis. Development. 2004;131:2715–25. doi: 10.1242/dev.01143. [DOI] [PubMed] [Google Scholar]
- Godlewski J, Wang S, Wilson TG. Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem Biophys Res Commun. 2006;342:1305–1311. doi: 10.1016/j.bbrc.2006.02.097. [DOI] [PubMed] [Google Scholar]
- Hall BL, Thummel CS. The RXR homolog ultraspiracle is an essential component of the Drosophila ecdysone receptor. Development. 1998;125:4709–17. doi: 10.1242/dev.125.23.4709. [DOI] [PubMed] [Google Scholar]
- Henrich VC, Livingston L, Gilbert LI. Developmental requirements for the ecdysoneless (ecd) locus in Drosophila melanogaster. Dev Genet. 1993;14:369–77. doi: 10.1002/dvg.1020140506. [DOI] [PubMed] [Google Scholar]
- Henrich VC. The ecdysteroid steroid receptor. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Chapter 3.5. Elsevier; 2005. pp. 243–285. online edition. [Google Scholar]
- Henrich VC, Vogtli ME, Antoniewski C, Spindler-Barth M, Przibilla S, Noureddine M, Lezzi M. Developmental effects of a chimeric ultraspiracle gene derived from Drosophila and Chironomus. Genesis. 2000;28:25–33. doi: 10.1002/1526-968x(200011/12)28:3/4<125::aid-gene50>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- Ji SJ, Zhuang B, Falco C, Schneiderm A, Schuster-Gosslerm K, Gosslerm A, Sockanathanm S. Mesodermal and neuronal retinoids regulate the induction and maintenance of limb innervating spinal motor neurons. Dev Biol. 2006;297:249–61. doi: 10.1016/j.ydbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Jones D, Jones G. Farnesoid secretions of dipteran ring glands: what we do know and what we can know. Insect Biochem Mol Biol. 2007;37:771–98. doi: 10.1016/j.ibmb.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Jones G, Jones D, Teal P, Sapa A, Wozniak M. The retinoid-X receptor ortholog, ultraspiracle, binds with nanomolar affinity to an endogenous morphogenetic ligand. FEBS J. 2006;273:4983–96. doi: 10.1111/j.1742-4658.2006.05498.x. [DOI] [PubMed] [Google Scholar]
- Li T, Bender M. A conditional rescue system reveals essential functions for the ecdysone receptor (EcR) gene during molting and metamorphosis in Drosophila. Development. 2000;127:2897–905. doi: 10.1242/dev.127.13.2897. [DOI] [PubMed] [Google Scholar]
- Li H, Cooper RL. Effects of the ecdysoneless mutant on synaptic efficacy and structure at the neuromuscular junction in Drosophila larvae during normal and prolonged development. Neuroscience. 2001;106:193–200. doi: 10.1016/s0306-4522(01)00263-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang Z, Robinson GE, Palli SR. Identification and characterization of a juvenile hormone response element and its binding proteins. J Biol Chem. 2007;282:37605–17. doi: 10.1074/jbc.M704595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. Retinoids and spinal cord development. J of Neurobiol. 2006;66:726–738. doi: 10.1002/neu.20248. [DOI] [PubMed] [Google Scholar]
- Maden M. Role and distribution of retinoic acid during CNS development. Int Rev Cytol. 2001;209:1–77. doi: 10.1016/s0074-7696(01)09010-6. Review. [DOI] [PubMed] [Google Scholar]
- Miura K, Oda M, Makita S, Chinzei Y. Characterization of the Drosophila Methoprene tolerant gene product. Juvenile hormone binding and ligand-dependant gene regulation. FEBS J. 2005;272:1169–1178. doi: 10.1111/j.1742-4658.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Moshitzky P, Applebaum SW. Pathway and regulation of JHIII-Bisepoxide biosynthesis in adult Drosophila melanogaster corpus allatum. Arch Insect Biochem Physiol. 1995;30:225–237. doi: 10.1002/arch.940300211. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Engstrom L, Mahowald AP. Developmental genetics of the 2C-D region of the Drosophila X chromosome. Genetics. 1985;111:23–41. doi: 10.1093/genetics/111.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possompes B. Sur le determinisme de la metamorphose de Calliphora erythrocephala meig. Arch Zool Exer Gener. 1953;89:203–364. [Google Scholar]
- Postlethwait JH. Juvenile hormone and the adult development of Drosophila. Biol Bull. 1974;147:119–135. doi: 10.2307/1540573. [DOI] [PubMed] [Google Scholar]
- Richard DS, Applebaum SW, Gilbert LI. Developmental regulation of juvenile hormone biosynthesis by the ring gland of Drosophila. J Comp Physiol [B] 1989;159:383–387. doi: 10.1007/BF00692410. [DOI] [PubMed] [Google Scholar]
- Richard DS, Applebaum SW, Sliter TJ, Baker FC, Schooley DA, Reuter CC, Henrich VC, Gilbert LI. Juvenile hormone bisepoxide biosynthesis in vitro by the ring gland of Drosophila: a putative juvenile hormone in the higher Diptera. Proc Natl Acad Sci USA. 1989;86:1421–1425. doi: 10.1073/pnas.86.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford LM. Hormones and Drosophila development. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. pp. 899–939. [Google Scholar]
- Riddiford LM. Juvenile hormone action: a 2007 perspective. J Insect Physiol. 2008;54:895–901. doi: 10.1016/j.jinsphys.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Ashburner M. Effects of juvenile hormone mimics on larval development and metamorphosis of Drosophila melanogaster. Gen Comp Endocrinol. 1991;82:172–183. doi: 10.1016/0016-6480(91)90181-5. [DOI] [PubMed] [Google Scholar]
- Sonneveld E, van den Brink CE, van der Leede BJ, Maden M, van der Saag PT. Mouse embryonal carcinoma cell lines stably transfected with retinoic acid receptor B2 promoter-1acZ: Sensitive system for measuring levels of active retinoids. Exp Cell Res. 1998;250:284–297. doi: 10.1006/excr.1999.4513. [DOI] [PubMed] [Google Scholar]
- Tang H, Kambris Z, Lemaitre B, Hashimoto C. A serpin that regulates immune melanization in the respiratory system of Drosophila. Dev Cell. 2008;15:617–26. doi: 10.1016/j.devcel.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal PE, Proveaux AT, Heath RR. Analysis and quantitation of insect juvenile hormones using chemical ionization ion-trap mass spectrometry. Anal Biochem. 2000;277:206–13. doi: 10.1006/abio.1999.4377. [DOI] [PubMed] [Google Scholar]
- Vogt M. Zur production und bedeutung metamorphose forderender hormone wahrend der larvaenentwicklung von Drosophila. Biol Zblt. 1943;63:395–446. [Google Scholar]
- Wilson TG, Wang S, Beno M, Farkas R. Wide mutational spectrum of a gene involved in hormone action and insecticide resistance in Drosophila melanogaster. Mol Gen Genom. 2006;276:294–303. doi: 10.1007/s00438-006-0138-4. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Chu Y, Fang F, Xu Y, Riddiford L, Jones D, Jones G. Alternative farnesoid structures induce different conformational outcomes upon the Drosophila ortholog of the retinoid X receptor, ultraspiracle. Insect Biochem Mol Biol. 2004;34:1147–62. doi: 10.1016/j.ibmb.2004.07.006. [DOI] [PubMed] [Google Scholar]


