Highly active anti-retroviral therapy (HAART) was an important breakthrough in HIV treatment, significantly reducing morbidity and mortality due to HIV infection (Carpenter et al., 2000; UNAIDS/WHO, 2009). HAART, however, still suffers from the emergence of multi-drug resistant virus strains, drug-drug interactions, and adherence to the prescribed regimens. We have been actively developing 2,4 (1H, 3H)-pyrimidinedione (PYD) derivatives, which target two early steps of HIV replication including virus entry and reverse transcription (Buckheit et al., 2008). The parent compound, IQP-0410 (SJ-3366; 1-(3-cyclopenten-1-ylmethyl)-5-ethyl-6-(3,5-dimethylbenzoyl)-2,4(1H,3H)-pyrimidinedione) is a member of the HEPT (6-substituted acyclouridine derivative) class of molecules (Baba et al., 1994) and has been reported as a highly potent NNRTI with an extended range of action, including HIV-2 (Buckheit et al., 2008). Cell-based evaluations with both HIV-1 and HIV-2 suggest that the PYDs inhibit virus entry through recognition of a pre-fusion conformational structure involving both envelope and Gag determinants (manuscript in preparation). The dual mechanism of action of the PYD congeners renders these compounds ideal for continued development. From the series of seventy-four PYDs those with cyclopropyl, phenyl, and 1- or 3-cyclopenten-1-yl substitutions at the N-1, a methyl linker between the cyclic moiety and the N-1, and a benzoyl group at the C6 of the PYD possessed the greatest antiviral activity (Buckheit et al., 2001). IQP-0405, IQP-0406, IQP-0407, IQP-0528, IQP-0558, IQP-0410 and IQP-1187 (Table 2) were defined as highly potent inhibitors of HIV-1 with EC50 values ranging from 0.6 to 2 nM in a CEM-SS-based CPE assay, 11 to 20 nM against the subtype B (HT/92/599) HIV in PBMCs, 2 to 7 nM against the subtype C (ZA/97/003) HIV in PBMCs, and inhibit HIV entry to uninfected target cells with EC50 values ranging from 0.17 to 2 μM. Each of the seven lead candidates possess anti-HIV activity that is greater than other HEPT-like inhibitors reported in the literature (Requejo, 2006) and no other HEPT-like NNRTI has been reported to demonstrate HIV-2 inhibitory activity. The dual mechanism of action resulting in inhibition of virus entry and reverse transcription suggests that each of these congeners might be expected to represent a potential candidate for further development with an additional rationale to be defined by their interaction with other approved HIV inhibitors. We have evaluated the lead pyrimidinedione congeners (Table 1; Buckheit et al., 2008) using in vitro anti-HIV combination therapy assays using both CEM-SS-based CPE inhibition assays and PBMC-based virus replication inhibition assays with other HIV inhibitors targeting virus entry and fusion (Chicago Sky Blue, Cyanovirin, ISIS 5320, and T20), reverse transcriptase (Efavirenz, UC781, AZT and Tenofovir), and protease (Ritonavir), to evaluate their combination antiviral interactions.
Table 2.
Anti-HIV-1 Efficacy of PYD Congeners and Antiviral Agents of Varying MOA
| COMPOUND | ANTIVIRAL ASSAYa | ||
|---|---|---|---|
| CEM-SS/HIV-1RF EC50 (μM) | PBMC/HIV-1 Clade BHT/92/599 EC50 (μM) | PBMC/HIV-1 Clade CZA/97/003 EC50 (μM) | |
| IQP-0405 | 0.001 | 0.013 | 0.007 |
| IQP-0406 | 0.0009 | 0.012 | 0.004 |
| IQP-0407 | 0.002 | 0.019 | 0.002 |
| IQP-0528 | 0.0002 | 0.014 | 0.005 |
| IQP-0558 | 0.0005 | 0.02 | 0.006 |
| IQP-0410 | 0.0002 | 0.011 | 0.005 |
| IQP-1187 | 0.0004 | 0.02 | 0.003 |
| T20 | 0.002 | 0.016 | 0.003 |
| Chicago Sky Blue (μg/mL) | 3.5 | 5.4 | 4.6 |
| Cyanovirin (μg/mL) | 0.002 | 0.7 | 0.7 |
| UC781 (ng/mL) | 0.01 | 1.5 | 0.06 |
| ISIS 5320 | 0.7 | 0.9 | 2.0 |
| Tenofovir | 1.2 | 0.02 | 0.7 |
| AZT | 0.005 | 0.01 | 0.002 |
| Efavirenz | 0.0009 | 0.002 | 0.1 |
The results presented were obtained from representative antiviral assays with appropriate control compounds evaluated in parallel selected from a minimum of three antiviral assays. HIV cytoprotection were obtained by XTT dye reduction endpoint and virus replication in PBMCs with clinical HIV isolates was measured by RT incorporation. We have demonstrated that the standard error among multiple antiviral assays averaged less than 10% of the respective mean EC50 and TC50. In each individual assay, mean efficacy and toxicity values are derived from a minimum of three replicate wells.
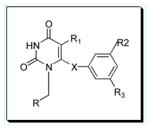
Table 1.
Chemical Structure of Lead PYD Congeners.
| Structure | IQP # | R1 | R2 | R3 | X | R |
|---|---|---|---|---|---|---|
|
0405 | Et | Me | Me | O | Cyclopropyl |
| 0406 | iPr | Me | Me | O | Cyclopropyl | |
| 0407 | Et | Me | Me | C=O | Cyclopropyl | |
| 0528 | iPr | Me | Me | C=O | Cyclopropyl | |
| 0558 | iPr | Me | Me | C=O | 1-Cyclopenten-1-yl | |
| 0410 | Et | Me | Me | C=O | 3-Cyclopenten-1-yl | |
| 1187 | iPr | Me | Me | C=O | 3-Cyclopenten-1-yl |
HIV-1 inhibitory activity of the compounds was evaluated in CEM-SS cells and fresh human PBMCs as described previously (Nara et al., 1987; Foley et al. 1965) in microtiter assays using the RF laboratory strain of HIV-1 and CXCR4-tropic low passage subtype B HT/92/599 and CCR5-tropic subtype C ZA/97/003 clinical isolates (AIDS Research and Reference Reagent Program, NIAID). Antiviral and toxicity data are reported as the quantity of drug required to inhibit 50% of virus-induced cell killing or virus production [EC50] and the quantity of drug required to reduce cell viability by 50% [TC50]. The standard combination anti-HIV assays (Buckheit et al., 1995) were performed using 5 concentrations of test compound A tested in all possible combinations with 9 concentrations of test compound B in a checkerboard pattern and analyzed for compound interactions at the 95% and 99% confidence intervals using the Prichard and Shipman MacSynergy II software program (Prichard and Shipman, 1990). Our extensive experience with the evaluation of combination assay data has resulted in the following interpretations of synergy plot data: >100 μM2% is defined as a highly synergistic interaction; 51 to 99 μM2% is defined as synergistic; 0 to 50 μM2% and −50 to 0 μM2% is defined as additive; −99 to −51 μM2% is defined as antagonistic; and <−100 μM2% is defined as highly antagonistic.
A summary of the results of combination assay evaluations are presented in Table 3 (CEM-SS), Table 4 (fresh human PBMCs with subtype B HT/92/599) and Table 5 (fresh human PBMCs with subtype C ZA/97/003). At effective concentrations of these two drug combinations, no synergistic toxicity was observed with the exception of toxicity at a single combination concentration of IQP-1187 and either ISIS 5320 or AZT (data not shown). Importantly, no antagonistic antiviral interactions of the tested compounds were observed. Though there were significant differences in the defined interactions of the compounds depending on the anti-HIV assay employed (CEM-SS cells versus PBMCs), as well as differences observed in the PBMC assays when using a subtype B or subtype C clinical isolate, several two-drug combinations stood out as being highly synergistic. Specifically, tenofovir in combination with six of the seven PYD congeners yielded synergistic interactions when evaluated versus the subtype C (ZA/97/003) in PBMCs, but primarily additive interactions versus the subtype B (HT/92/599) isolate. Of the seven lead molecules evaluated, IQP-0405 yielded the most synergistic interactions in combination with the tested anti-HIV inhibitors followed by IQP-0407 and IQP-0528. Interestingly, each of these three congeners possess a cyclopropyl substitution at the N-1 of the PYD. Though additive to slightly synergistic in drug combinations with the seven lead compounds versus the subtype C isolate, AZT yielded synergistic interactions with all seven compounds in both the CEM-SS CPE assay and the subtype B PBMC assay. In the CEM-SS CPE assay and subtype B PBMC based assay, IQP-0405 demonstrated moderate to highly synergistic interactions with the entry inhibitors Chicago Sky Blue, Cyanovirin, ISIS 5320, the fusion inhibitor T20, the NRTI AZT and the PI Ritonavir. These studies suggest the combination of the NNRTI PYD derivatives with HIV entry inhibitors, NRTIs or PIs would each represent an effective combination therapeutic product. These results would also suggest that the prioritization of combination therapeutic strategies for infected individuals in the United States (predominantly subtype B strains) would be different from strategies employed for the treatment of subtype C infections in sub-Saharan Africa. Subtype C is the most rapidly expanding HIV-1 subtype and predominates in Eastern and Southern Africa and India, accounting for almost 60% of HIV cases worldwide (Requejo, 2008). However, no significant differences have been observed in the disease progression or pathogenicity of infection in individuals infected by subtype C versus patients infected by other group M subtypes (Taylor et al., 2008). Despite relative conservation of the RT sequence among the HIV-1 subtypes, studies have shown that viruses carrying RT from subtype C isolates had decreased levels of viral cDNA accumulation, which correlated with reduced integration and lower levels of virus replication (Grossman et al., 2001; Iordanskiy et al., 2010). Since subtype C HIV isolates predominantly use the CCR5 co-receptor for viral entry, even in late infection, this may reduce the viral cytopathogenicity and affect the spread of virus. The decrease in overall replication of subtype C viruses may contribute to differences observed in the combination assays using a subtype B or subtype C clinical isolate. It has also been reported that differences in the selection of resistant viruses occurs when subtype C viruses are utilized, suggesting other important biological differences between subtype C and subtype B viruses which need to be considered when designing combination therapies (Martinex-Cajas et al., 2009).
Table 3.
Combination Anti-HIV Therapy Results Using CEM-SS Cells Infected with the RF Strain of HIV-1
| IQP Compound Tested in Combination with: | IQP-0405 | IQP-0406 | IQP-0407 | IQP-0528 | IQP-0558 | IQP-0410 | IQP-1187 |
|---|---|---|---|---|---|---|---|
| Synergy Volumea | Synergy Volume | Synergy Volume | Synergy Volume | Synergy Volume | Synergy Volume | Synergy Volume | |
| Chicago Sky Blue (EI) | 171.7+/−80.9 | 38.9+/−47.8 | 91.6+/−45.5 | 124.9+/−67.7 | 31.4+/−19.7 | 12.8+/−0.3 | 27.5+/−3.4 |
| Cyanovirin (EI) | 146.9+/−101.5 | 42.0+/−32.6 | 173.5+/−101.8 | 140.4+/−87.1 | 102.6+/−21.6 | 24.9+/−35.0 | 99.8+/−8.0 |
| Efavirenz (NNRTI) | 16.5+/−5.7 | 63.3+/−73.8 | 113.5+/−28.3 | 106.8+/−49.6 | 19.6+/−8.7 | 35.4+/−8.6 | 86.2+/−14.4 |
| ISIS 5320 (EI) | 182.1+/−114.1 | 68.2+/−80.2 | 206.4+/−96.3 | 90.9+/−47.3 | 151.7+/−51.5 | 65.3+/−17.2 | 125.5+/−39.5 |
| UC781 (NNRTI) | 59.8+/−8.6 | 17.5+/−26.4 | 28.9+/−8.2 | 63.3+/−9.7 | 30.2+/−4.0 | 39.8+/−13.8 | 18.6+/−13.3 |
| T20 (FI) | 139.2+/−88.7 | 25.6+/−37.1 | 25.3+/−12.1 | 15.3+/−18.2 | 13.1+/−18.7 | 44.4+/−3.2 | 15.3+/−13.4 |
| AZT (NRTI) | 153.2+/−54.0 | 88.7+/−66.8 | 232.9+/−16.3 | 188.1+/−173.4 | 133.7+/−89.6 | 179.0+/−89.4 | 83.5+/−9.5 |
| Tenofovir (NRTI) | 20.7+/−5.9 | 16.9+/−19.7 | 118.3+/−22.7 | 30.2+/−0.7 | 5.3+/−7.5 | 35.0+/−10.9 | 7.8+/−11.0 |
| Ritonavir (PI) | 74.6+/−16.5 | 18.7+/−21.1 | 69.1+/−14.2 | 12.9+/−7.3 | 20.5+/−8.6 | 6.4+/−8.6 | 28.2+/−17.4 |
Synergy volumes were calculated by the Prichard and Shipman MacSynergy II program at the 95% confidence interval. The results of the MacSynergy II evaluation quantify the volume of the synergistic, additive, or antagonistic surface and are expressed in units of uM2% (or uMug/mL% for Chicago Sky Blue and Cyanovirin). Synergistic results are highlighted in bold. The mean and standard deviation from at least two replicate results are presented for each combination of products.
Table 4.
Combination Anti-HIV Therapy Results Using PBMCs Infected with the Subtype B (HT/92/599) HIV-1 Strain
| IQP Compound Tested in Combination with: | IQP-0405 | IQP-0406 | IQP-0407 | IQP-0528 | IQP-0558 | IQP-0410 | IQP-1187 |
|---|---|---|---|---|---|---|---|
| Synergy Volumea | Synergy Volume | Synergy Volume | Synergy Volume | Synergy Volume | Synergy Volume | Synergy Volume | |
| Chicago Sky Blue (EI) | 19.6+/−14.5 | 109.4+/−123.7 | 19.9+/−23.6 | 75.3+/−12.9 | 16.1+/−18.5 | 13.3+/−6.0 | 32.1+/−20.3 |
| Cyanovirin (EI) | 107.8+/−57.8 | 7.8+/−12.2 | 32.3+/−8.4 | 84.6+/−25.7 | 27.7+/−27.2 | 78.5+/−33.7 | 81.1+/−41.6 |
| Efavirenz (NNRTI) | 71.6+/−12.1 | 12.6+/−20.0 | 56.7+/−5.9 | 31.1+/−22.7 | 27.7+/−27.2 | 19.0+/−8.9 | 177.4+/−0.2 |
| ISIS 5320 (EI) | 28.0+/−1.3 | 146.0+/−143.3 | 100.0+/−44.5 | 235.9+/−149.9 | 87.0+/−24.3 | 24.6+/−34.6 | 15.1+/−11.7 |
| UC781 (NNRTI) | 102.4+/−18.4 | 41.0+/−32.3 | 117.5+/−39.8 | 30.8+/−25.7 | 15.9+/−12.2 | 13.3+/−13.7 | 102.6+/−44.3 |
| T20 (FI) | 139.1+/−47.1 | 150.7+/−203.1 | 13.2+/−6.0 | 98.8+/−42.4 | 14.1+/−19.9 | 84.4+/−45.5 | 83.3+/−39.7 |
| AZT (NRTI) | 77.5+/−7.8 | 317.6+/−194.4 | 111.8+/−30.1 | 206.0+/−70.9 | 81.7+/−1.4 | 216.1+/−9.1 | 75.6+/−5.1 |
| Tenofovir (NRTI) | 125.0+/−6.4 | 23.9+/−11.0 | 146.9+/−2.8 | 16.8+/−16.5 | 31.4+/−1.4 | 14.9+/−6.7 | 23.0+/−9.4 |
| Ritonavir (PI) | 96.0+/−36.6 | 51.6+/−50.0 | 103.2+/−5.4 | 123.9+/−34.0 | 56.9+/−5.0 | 132.7+/−53.7 | 91.8+/−10.7 |
Synergy volumes were calculated by the Prichard and Shipman MacSynergy II program at the 95% confidence interval. The results of the MacSynergy II evaluation quantify the volume of the synergistic, additive, or antagonistic surface and are expressed in units of uM2% (or uMug/mL% for Chicago Sky Blue and Cyanovirin). Synergistic results are highlighted in bold. The mean and standard deviation from at least two replicate results are presented for each combination of products.
Table 5.
Combination Anti-HIV Therapy Results Using PBMCs Infected with the Subtype C (ZA/97/003) HIV-1 Strain
| IQP Compound Tested in Combination with: | IQP-0405 | IQP-0406 | IQP-0407 | IQP-0528 | IQP-0558 | IQP-0410 | IQP-1187 |
|---|---|---|---|---|---|---|---|
| Synergy Volumea | Synergy Volume | Synergy Volume | Synergy Volume | Synergy Volume | Synergy Volume | Synergy Volume | |
| Chicago Sky Blue (EI) | 25.0+/−32.7 | 20.5+/−40.0 | 23.9+/−24.0 | 25.2+/−13.7 | 11.5+/−15.8 | 35.1+/−13.2 | 40.3+/−3.7 |
| Cyanovirin (EI) | 84.6+/−24.7 | 29.9+/−27.1 | 85.9+/−32.0 | 100.6+/−51.1 | 12.2+/−1.4 | 60.9+/−4.0 | 5.9+/−6.0 |
| Efavirenz (NNRTI) | 41.3+/−4.2 | 86.2+/−117.4 | 23.8+/−3.8 | 11.9+/−16.8 | 74.1+/−0.6 | 74.0+/−26.2 | 116.2+/−20.5 |
| ISIS 5320 (EI) | 22.7+/−18.0 | 1.0+/−0 | 11.1+/−15.7 | 5.6+/−0.1 | 6.8+/−7.1 | 2.3+/−0.9 | 9.4+/−12.6 |
| UC781 (NNRTI) | 18.7+/−16.1 | 22.3+/−12.1 | 68.0+/−6.3 | 24.7+/−29.6 | 23.5+/−31.1 | 87.4+/−11.5 | 92.5+/−54.3 |
| T20 (FI) | 73.3+/−33.0 | 88.1+/−56.6 | 11.4+/−16.1 | 63.4+/−1.8 | 1.1+/−0.2 | 29.0+/−28.1 | 186.6+/−41.1 |
| AZT (NRTI) | 48.0+/−2.5 | 109.7+/−50.5 | 34.6+/−4.9 | 16.2+/−0 | 61.1+/−4.2 | 28.3+/−4.0 | 32.6+/−18.3 |
| Tenofovir (NRTI) | 88.3+/−41.1 | 227.7+/−308.1 | 210.7+/−107.8 | 259.2+/−244.9 | 272.3+/−273.4 | 96.4+/−21.3 | 32.7+/−22.1 |
| Ritonavir (PI) | 127.3+/−82.2 | 130.0+/−112.7 | 82.6+/−31.0 | 132.9+/−51.2 | 13.4+/−17.4 | 62.2+/−13.4 | 55.9+/−6.5 |
Synergy volumes were calculated by the Prichard and Shipman MacSynergy II program at the 95% confidence interval. The results of the MacSynergy II evaluation quantify the volume of the synergistic, additive, or antagonistic surface and are expressed in units of uM2% (or uMug/mL% for Chicago Sky Blue and Cyanovirin). Synergistic results are highlighted in bold. The mean and standard deviation from at least two replicate results are presented for each combination of products.
The most common medications given in first-line HAART treatment are the NNRTIs in combination with two NRTIs in HIV-infected individuals without prior exposure to antiretroviral therapy or in patients whom experienced virological breakthroughs on at least one prior PI-based regimen (Boyd, 2011; Mbuagbaw et al., 2010). Second generation NNRTIs, Delavirdine, Etravirine and Rilpivirine, have demonstrated a higher genetic barrier to resistance than Efavirenz and Nevirapine, in addition to long plasma half-lives, low pill burdens, increased tolerability, and the ability to be safely combined with other commonly used medications (Azijn et al., 2010; De Clercq, 2009; Seminari et al., 2008). In vitro combination studies of FDA approved NNRTIs with other classes of HIV inhibitors typically result in additive to synergistic interactions (Azijn et al., 2010; De Clercq, 2004; Seminari et al., 2008). Many variations in HAART regimens are available, some of which differ in toxicity, adverse effects, suppression of virus replication, development of viral resistance, and patient adherence (Deek and Perry, 2010; Pallela et al., 1998; Yazdanpanah et al., 2004). The two-drug combination anti-HIV-1 results indicate PYD congeners, which alone target two steps in virus replication, may be clinically promising as additions to primary or salvage HAART therapy. A co-formulated product of a PYD with an approved HIV drug essentially yields three possible mechanisms of antiviral action in one product with potentially fewer drug-drug interactions. The PYDs with a cyclopropyl R group (IQP-0405, IQP-0407 and IQP-0528) have been identified as lead candidates in combination therapy evaluations, as well as range of action evaluations in PBMCs, selection of drug resistant virus, cross resistance evaluations, metabolic stability in liver microsomes and stability in fresh human hepatocytes.
Highlights.
The PYD congeners are highly potent dual-acting inhibitors of HIV-1 and HIV-2.
The PYDs interact in an additive to synergistic manner with other anti-HIV agents.
Antagonism or synergistic toxicity was not observed with the PYD combinations.
The optimal PYD combinations are distinct for subtype B and subtype C HIV.
PYDs with cyclopropyl substitutions appear to represent the best lead compounds.
Acknowledgments
This work was supported in part by funding from the National Institutes of Health Microbicide Innovation Program (NIAID grant 8R21A1076967-02) and Small Business Innovative Research Program (NIAID grant 2R44A1067047-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azijn H, Tirry I, Vingerhoets J, Pierre de Bethune M, Kraus G, Boven K, Jochmans D, Van Craenenbroeck E, Picchio G, Rimsky L. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother. 2010;54(2):718–27. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M, Shigeta S, Tanaka H, Miyasaka T, Ubasawa M, Umezu K, Walker RT, Pauwels R, De Clercq E. Preclinical evaluation of MKC-442, a highly potent and specific inhibitor of human immunodeficiency virus type 1 in vitro. Antimicrob Agents Chemother. 1994;38:688–692. doi: 10.1128/aac.38.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SD. Management of HIV infection in treatment-naïve patients: a review of the most current recommendations. Am J Health Syst Pharm. 2011;68(11):991–01. doi: 10.2146/ajhp100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckheit RW, Jr, Kinjerski TL, Fliakas-Boltz V, Russell JD, Stup TL, Pallansch LA, Brouwer WG, Dao DC, Harrison WA, Schultz RJ, Bader JP, Yang SS. Structure-activity and cross-resistance evaluations of a series of human immunodeficiency virus type 1-specific compounds related to oxathiin carboxanilide. Antimicrob Agents Chemother. 1995;39:2718–2727. doi: 10.1128/aac.39.12.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckheit RW, Jr, Watson K, Fliakas-Boltz V, Russell J, Loftus TL, Osterling MC, Turpin JA, Pallansch LA, White EL, Lee JW, Lee SH, Oh JW, Kwon HS, Chung SG, Cho EH. SJ-3366, a unique and highly potent nonnucleoside reverse transcriptase inhibitor of human immunodeficiency type 1 (HIV-1) that also inhibits HIV-2. Antimicrob Agents Chemother. 2001;45:393–400. doi: 10.1128/AAC.45.2.393-400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckheit RW, Jr, Hartman TL, Watson KM, Chung SG, Cho EH. Comparative Evaluation of the Inhibitory Activities of a Series of Pyrimidinedione Congeners That Inhibit Human Immunodeficiency Virus Types 1 and 2. Antimicrob Agents Chemother. 2008;52(1):225–236. doi: 10.1128/AAC.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, Montamer JS, Richman DD, Saag MS, Schechter M, Schooley RT, Thompson MA, Vella S, Yeni PG, Volberding PA. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2000;283(3):381–90. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): past, present, and future. Chem Biodivers. 2004;1:44–64. doi: 10.1002/cbdv.200490012. [DOI] [PubMed] [Google Scholar]
- De Clercq E. The history of antiretrovirals: key discoveries over the past 25 years. Rev Med Virol. 2009;19(5):287–99. doi: 10.1002/rmv.624. [DOI] [PubMed] [Google Scholar]
- Deek ED, Perry CM. Efavirenz/emtricitabine/tenofovir disoproxil fumarate single tablet regimen (Atripla®): a review of its use in the management of HIV infection. Drugs. 2010;70(17):2315–38.12. doi: 10.2165/11203800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Foley GE, Lazarus H, Farber S, Uzman BG, Boone BA, McCarthy RE. Continuous culture of human lymphoblasts from peripheral blood of a child with acute leukemia. Cancer. 1965;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Vardinon N, Chemtob D, Alkan ML, Bentwich Z, Burke M, Gottesman G, Istomin V, Levi I, Maayan S, Shahar E, Schapiro JM. Genotypic variation of HIV-1 reverse transcriptase and protease: comparative analysis of clade C and clade B. AIDS. 2001;15(12):1453–60. doi: 10.1097/00002030-200108170-00001. [DOI] [PubMed] [Google Scholar]
- Iordanskiy S, Waltke M, Feng Y, Wood C. Subtype –associated differences in HIV-1 reverse transcriptase affect the viral replication. Retrovirology. 2010;7:85. doi: 10.1186/1742-4690-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinex-Cajas JL, Pai NP, Klein MB, Wainberg MA. Differences in resistance mutations among HIV-1 non-subtype B infections: a systematic review of evidence (1996 – 2008) J Intl AIDS Society. 2009;12(11) doi: 10.1186/1758-2652-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbuagbaw LC, Irlam JH, Spaulding A, Rutherford GW, Siegfried N. Efavirenz or nevirapine in three-drug combination therapy with two nucleoside-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals. Cochrane Database Syst Rev. 2010;12:CD004246. doi: 10.1002/14651858.CD004246.pub3. [DOI] [PubMed] [Google Scholar]
- Nara PL, Hatch WC, Dunlop NM, Robey WG, Arthur LO, Gonda MA, Fischinger PJ. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retrovir. 1987;3:283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr, Delaney KM, Moorman AC. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- Prichard MN, Shipman C., Jr A three-dimensional model to analyze drug-drug interactions. Antivir Res. 1990;14:181–206. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- Requejo HI. Worldwide molecular epidemiology of HIV. Rev Saude Publica. 2006;40:331–45. doi: 10.1590/s0034-89102006000200023. [DOI] [PubMed] [Google Scholar]
- Seminari E, Castagna A, Lazzarin A. Etravirine for the treatment of HIV infection. Expert Rev Anti Infect Ther. 2008;6(4):427–33. doi: 10.1586/14787210.6.4.427. [DOI] [PubMed] [Google Scholar]
- Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med. 2008;358:1590–02. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS/WHO. UNAIDS (Joint United Nations Programme on HIV/AIDS) and World Health Organization (WHO) UNAIDS; Geneva, Switzerland: 2009 AIDS epidemic update/Revised HIV estimates: December 2009. http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. [Google Scholar]
- Yazdanpanah Y, Sissoko D, Egger M, Mouton Y, Zwahlen M, Chene G. Clinical efficacy of antiretroviral combination therapy based on protease inhibitors or non-nucleoside analogue reverse transcriptase inhibitors: indirect comparison of controlled trials. BMJ. 2004 Jan; doi: 10.1136/bmj.37995.435787.A6. doi:10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]


