Abstract
Chronic pressure-mediated baroreflex activation suppresses renal sympathetic nerve activity. Recent observations indicate that chronic electrical activation of the carotid baroreflex produces sustained reductions in global sympathetic activity and arterial pressure. Thus, we investigated the effects of global and renal specific suppression of sympathetic activity in dogs with sympathetically-mediated, obesity-induced hypertension by comparing the cardiovascular, renal, and neurohormonal responses to chronic baroreflex activation and bilateral surgical renal denervation. After control measurements, the diet was supplemented with beef fat while sodium intake was held constant. After 4 weeks on the high-fat, when body weight had increased ~a 50%, fat intake was reduced to a level that maintained this body weight. This weight increase was associated with an increase in mean arterial pressure from 100±2 to 117±3 mm Hg and heart rate from 86±3 to 130±4 bpm. The hypertension was associated with a marked increase in cumulative sodium balance despite ~ a 35% increase in GFR. The importance of increased tubular reabsorption to sodium retention was further reflected by ~ a 35% decrease in fractional sodium excretion. Subsequently, both chronic baroreflex activation (7 days) and renal denervation decreased plasma renin activity and abolished the hypertension. However, baroreflex activation also suppressed systemic sympathetic activity and tachycardia and reduced glomerular hyperfiltration while increasing fractional sodium excretion. In contrast, GFR increased further after renal denervation. Thus, by improving autonomic control of cardiac function and diminishing glomerular hyperfiltration, suppression of global sympathetic activity by baroreflex activation may have beneficial effects in obesity beyond simply attenuating hypertension.
Keywords: obesity, hypertension, baroreflex, sympathetic nervous system, renal nerves, renal function, renin-angiotensin system
Introduction
Although obesity-related hypertension has an important neurogenic component, the role of the sympathetic nervous system in mediating the cardiovascular, renal, neurohormonal, and metabolic abnormalities associated with weight gain is not completely understood.1,2 Recently, modern device technology has allowed for prolonged electrical stimulation of the carotid baroreflex in a controlled manner. This has provided a novel, non-pharmacological approach for chronically suppressing central sympathetic outflow and lowering arterial pressure.3 In pre-clinical studies, this device has been used to better understand the mechanisms that mediate long-term reductions in arterial pressure in response to suppression of sympathetic activity in both normotensive and hypertensive dogs.3 These studies have provided a strong physiological rationale for pursuing recent clinical trials evaluating the efficacy of baroreflex activation therapy in patients with resistant hypertension.4–7
To date, findings from clinical trials indicate that chronic electrical stimulation of the carotid baroreflex has sustained effects to lower arterial pressure in patients with resistant hypertension.4–7 Given the importance of the kidneys in long-term control of arterial pressure and recent observations that the natural activation of the baroreflex chronically suppresses renal sympathetic nerve activity (RSNA) and promotes sodium excretion,8–13 pre-clinical studies have focused on the mechanisms whereby chronic electrical activation of the carotid baroreflex increases renal excretory function.3 Interest in the renal nerves as a mediator of neurogenic hypertension has recently intensified following the introduction of another medical device for hypertension therapy. This device specifically targets the innervation to the kidneys.14–15 Studies using catheter-based endovascular radiofrequency ablation of the renal nerves, like those using baroreflex activation, have demonstrated impressive antihypertensive effects in patients with resistant hypertension. Furthermore, a report in a patient with resistant hypertension indicating that renal sympathetic denervation was associated with decreased systemic sympathetic activity, as well as arterial pressure, has further captured the attention of investigators.16 This observation suggests that sensory afferent signals from the kidneys to the brain contribute to central sympathetic outflow. Although this finding has not been confirmed in a patient population of sufficient size, device-based therapy has rekindled interest in the role of renal efferent nerves in mediating neurogenic hypertension and the potential role of renal afferent nerves in contributing to systemic sympathetic activation.
By comparing the effects of baroreflex activation and surgical renal denervation in the same dogs with established obesity-induced hypertension, the main goal of this study was to determine whether the cardiovascular, neurohormonal, renal, and metabolic (insulin resistance) responses to global sympathetic suppression by baroreflex activation can be achieved by direct renal denervation. This model of hypertension in the dog was chosen because it mimics many of the abnormalities of human obesity,2,17–19 and many patients with resistant hypertension are obese.20–21 Because there is little information on the relative effects of these two interventions on renal function and central sympathetic outflow, these were areas of focus in the present study. We hypothesized that by chronically suppressing central sympathetic outflow, baroreflex activation would diminish the augmented rate of sodium reabsorption, and the concomitant glomerular hyperfiltration and hypertension associated with obesity. In comparison, although renal denervation was expected to lead to some degree of blood pressure reduction, additional systemic and renal responses to removal of the afferent and efferent innervation of the kidneys were more difficult to predict.
Methods
Animal Preparation
All procedures were performed in accordance with National Institutes of Health Guidelines and approved by the Institutional Animal Care and Use Committee. Surgical procedures were performed under isoflurane anesthesia (1.5–2.0%) after pre-medication with acepromazine (0.15 mg/kg, sq) and induction with thiopental (10mg/kg, sq).
Six male dogs weighing 23–26 kg were used in this study. Arterial and venous catheters were implanted for continuous measurement of arterial pressure and blood sampling, and for continuous intravenous infusion of isotonic saline as previously described.19,22–23 In addition, stimulating electrodes were implanted around each carotid sinus and the lead bodies were connected to a pulse generator.19,22–23 The electrodes and the pulse generator were provided by CVRx, Inc. (Maple Grove, MN).
General Methods
Obesity hypertension was produced using the same protocol we and others have previously reported.18–19 In short, during a 3-week postoperative period and throughout the study, the dogs were maintained in metabolic cages, given free access to water and fed a fixed daily diet containing ~5 mmol of sodium and ~55 mmol of potassium. In addition, the dogs received a continuous intravenous infusion of isotonic saline at a rate of 350 mL/day. Thus, total daily sodium intake was ~60 mmol throughout the study. Water consumption was monitored daily and 24-hour urine samples were collected at 11 AM each day at the time of feeding. During the 3-week postoperative period, the dogs were trained to lie quietly in their cages for several hours each morning to allow blood sampling and measurement of GFR. After this 3-week period of acclimation when electrolyte and fluid balance was achieved, steady-state control measurements were made. Subsequently, cooked beef fat was added to the regular diet for the remainder of the study. During the initial 4 weeks of the high-fat feeding, the diet was supplemented with 0.6 to 0.7 kg/day fat until body weight increased to ~ 150% of control. Once this weight gain was achieved, dietary fat was reduced (on day 29) to 0.1–0.15 kg/day to maintain a constant body weight for the remainder of the study. This reduction in fat intake commenced 4 days before electrical stimulation of the carotid baroreflex on day 33 (see below).
During the control period (the days immediately preceding fat feeding) and at weekly intervals throughout the experimental periods, blood samples (~10 ml) were taken from one of the two arterial catheters and GFR was measured while the dogs were recumbent and in a resting state. Arterial pressure was sampled continuously at 100 samples/s, 24-hours/day, using a Power Lab data-acquisition system (ADInstruments) and displayed and recorded on a computer for subsequent analysis.23 The daily values for mean arterial pressure (MAP) and heart rate were averaged from the 20-hour period extending from 11:30-7:30 am.
Experimental Protocol
Control
Days 1–28, high fat (Developmental phase of obesity and hypertension)
- Days 29–60, reduced fat (Established phase of obesity hypertension)
- Days 33–40, baroreflex activation (1 week)
- Days 40–47, recovery (1 week)
- Day 47, bilateral renal denervation
- Day 60, end of study (2 weeks after renal denervation)
For the 7 days of carotid sinus stimulation (days 33–40), the pulse generator was programmed to target a reduction in arterial pressure from hypertensive to control levels. This goal was achieved by making small adjustments in voltage during days 1–2, but no changes in the parameters of activation were made after the first 48 hours of activation. Stimulation parameters were 3–6 V, 30 Hz, and 0.5-ms pulse duration. On day 47, the dogs were subjected to surgical bilateral renal denervation using procedures previously employed in our laboratory.24 To verify completeness of renal denervation, renal cortical samples for measurement of renal tissue NE were taken from the dogs at the end of the study. For comparison, samples were also taken from 6 lean dogs with innervated kidneys.
Analytical Methods
GFR was determined from the clearance of 125I-iothalamate.18 The distribution space of 125I-iothalamate was used as an index of extracellular fluid volume. Filtered load of sodium was determined from measurements of GFR and plasma sodium concentration. Fractional sodium excretion was calculated from the filtered load and urinary excretion of sodium. Plasma renin activity (PRA) and plasma levels of aldosterone, cortisol, and insulin were measured by radioimmunoassay.19,22–23 Plasma NE concentration and renal tissue levels of NE were measured by high-performance liquid chromatography (HPLC) with electrochemical detection. Approximately half of the NE determinations were made in our laboratory 25–26 and half in the laboratory of Dr. David S.Goldstein.27 Determinations of standard plasma samples in each laboratory were comparable, and plasma NE values for each dog were determined in a single assay. Plasma glucose concentration was measured with the glucose oxidation method.19 Standard techniques were used to measure hematocrit and the plasma concentrations of sodium, potassium, and protein.19,22–23
Statistical Analyses
Results are expressed as mean±SE. One-way, repeated measures ANOVA followed by either the Dunnett’s or Bonferroni post-hoc t-tests for multiple comparisons was used to compare responses to appropriate controls during the developmental (high fat) and established phases of hypertension (reduced fat), respectively. Statistical significance was considered to be P<0.05.
Results
Developmental Phase of Hypertension
Hemodynamic and Renal Responses
The hemodynamic and renal responses were similar to those reported previously in this model of obesity18–19 and are summarized in Table S1 ( please see http://hyper.ahajournals.org). During the first 4 weeks of the high-fat diet, there was a progressive increase in MAP associated with weight gain. On day 28, MAP was increased 17±3 mmHg along with the target weight gain of ~ 50%. Heart rate increased substantially during the development of the hypertension. The induction of hypertension during weight gain was associated with marked sodium retention. During the control period, sodium and potassium excretion was 60±2 and 48±3 mmol/day, respectively, reflecting the intake of these electrolytes, whereas sodium excretion averaged 49±2 mmol/day during the 4 weeks on the high-fat diet. Thus, during the initial 4 weeks of the high-fat diet, there was an average retention of~ 320 mmol of sodium, along with an increase in sodium iothalamate space of ~ 2.0–2.5L (Table S1, please see http://hyper.ahajournals.org). There was no significant change in potassium excretion during the high-fat diet. Throughout the 4 weeks of the high fat diet, there were reciprocal changes in GFR and fractional sodium excretion. On day 28, GFR increased and fractional sodium excretion decreased by ~ 35%. Therefore, the sodium retention associated with fat feeding can be accounted for by an increase in tubular reabsorption of sodium.
Neurohormonal Responses
The neurohormonal responses during the high fat diet were also similar to those we have reported previously (Table S2, please see http://hyper.ahajournals.org).19 In brief, other than an transient increase in PRA during week 1 of the high fat diet, there were no significant changes in the renin-angiotensin-aldosterone system or in plasma levels of cortisol and NE. While there were no increases in plasma glucose concentration, plasma insulin concentration was elevated throughout the entire 4 weeks of the high fat diet. This resulted in a marked fall in the glucose-to-insulin ratio from a control value of 16.4±3.1 to 4.8.±0.6 (day 28), indicating insulin resistance.
Plasma protein concentration increased progressively during the high fat diet rising from a control level of 5.8±0.2 to 6.5±0.2 g/dL on day 28. Other than a transient decrease in hematocrit (~10%) on day 7, there were no additional changes in hematocrit (control=0.43±0.02), plasma sodium (control=144±1mmol/L), or plasma potassium (control=4.0±0.1mmol/L) concentration during the development of obesity hypertension.
Established Phase of Hypertension
Responses to Baroreflex Activation
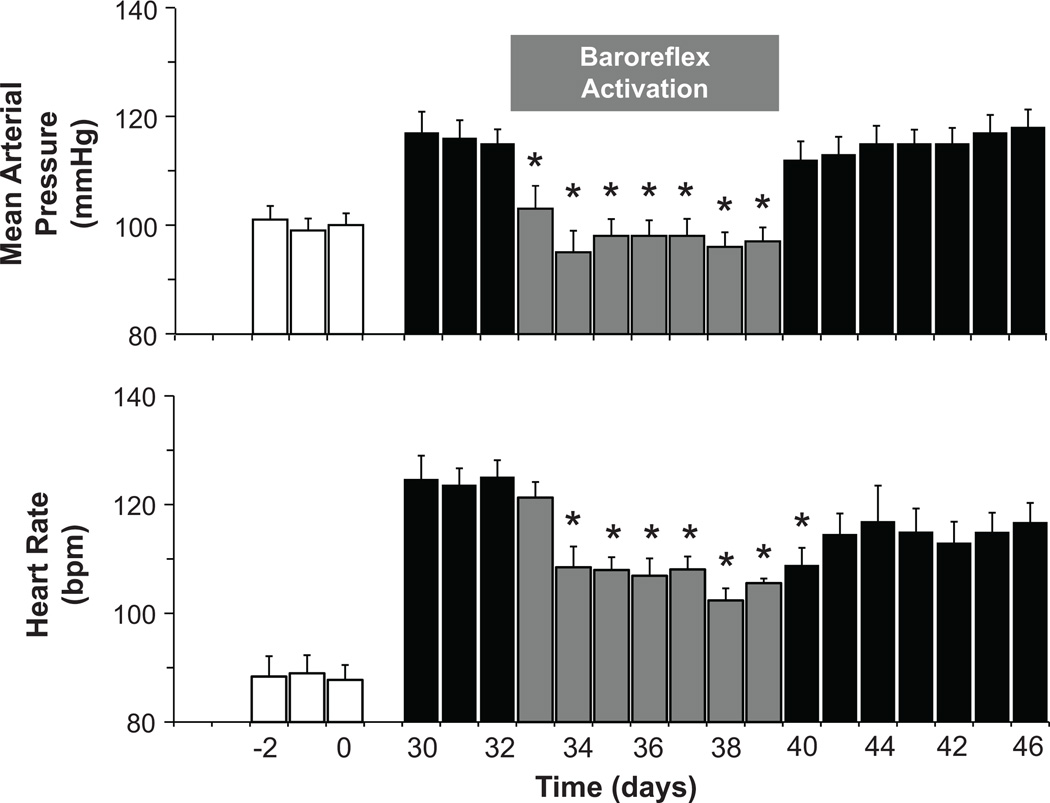
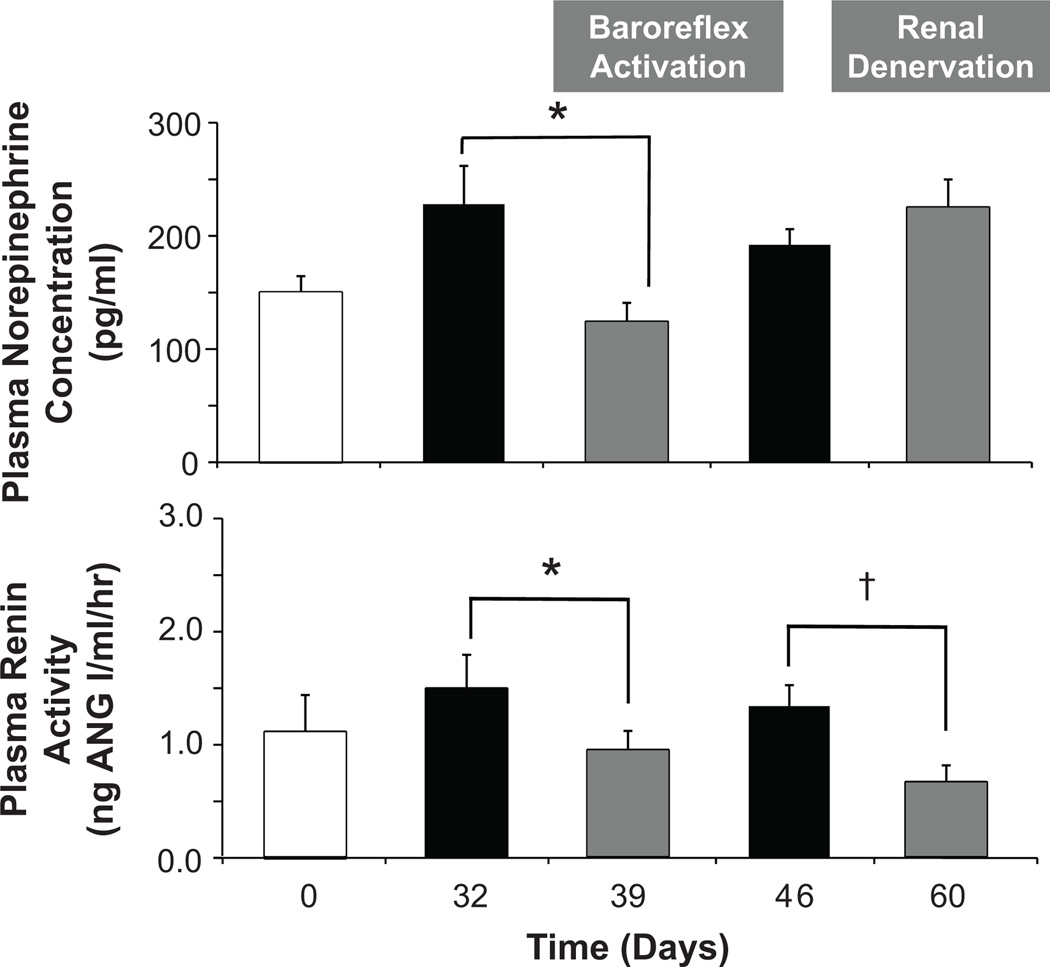
As illustrated in Figure 1, baroreflex activation completely abolished the hypertension and greatly diminished the tachycardia associated with weight gain. Further, the lowering of arterial pressure with baroreflex activation occurred concurrently with significant reductions in both plasma NE concentration and PRA (Figure 2). As reported previously,19 there were no significant changes in body weight or sodium balance during baroreflex activation when compared to pre-stimulation values (day 32); sodium-iothalamate space was also unchanged. Concomitant with increasing fractional sodium excretion toward lean values, baroreflex activation also diminished the hyperfiltration associated with obesity (Figure 3), indicating a primary reduction in the elevated rate of tubular sodium reabsorption. After terminating baroreflex activation, all of the above measures returned to values that were not significantly different from pre-stimulation levels on day 32.
Figure 1.
Mean arterial pressure and heart rate responses to prolonged baroreflex activation. Values are mean±SEM (n=6). *P<0.05 versus day 32. Lean control values (open bars) are also presented for reference.
Figure 2.
Neurohormonal responses to prolonged baroreflex activation and bilateral renal denervation. Values are mean±SEM (n=6). *P<0.05 versus day 32; †P<0.05 versus day 46. Lean control values (open bars) are also presented for reference.
Figure 3.
Effects of prolonged baroreflex activation and bilateral renal denervation on glomerular filtration and tubular function. Values are mean±SEM (n=6). *P<0.05 versus day 32; †P<0.05 versus day 46. Lean control values (open bars) are also presented for reference.
Other than the reductions in PRA and plasma NE concentration, there were no other significant changes in the plasma levels of any measured hormones, glucose, electrolytes or in hematocrit. Consequently, there was no change in the glucose-insulin-ratio from day 32 before (5.5±0.7) to day 39 of baroreflex activation (5.8±0.6).
Responses to Renal Denervation
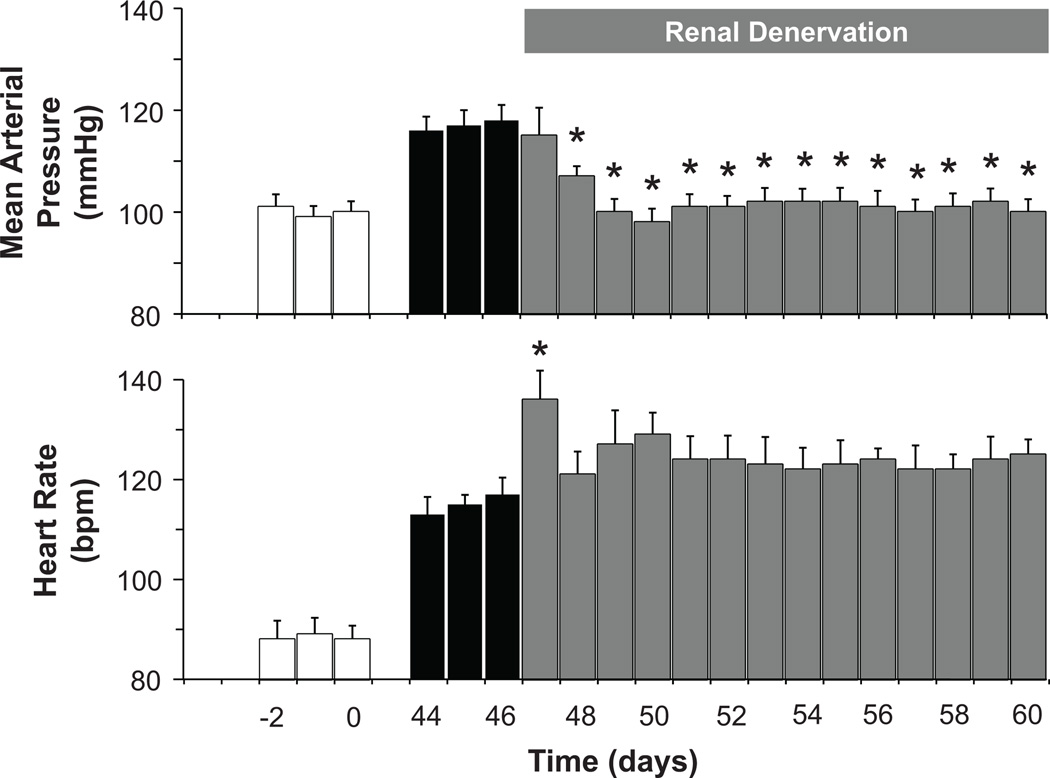
Similar to baroreflex activation, renal denervation abolished obesity-induced hypertension (Figure 4) along with suppressing PRA (Figure 2). These reductions in arterial pressure and PRA were not significantly different from those occurring during baroreflex activation. However, unlike baroreflex activation, renal denervation did not lower plasma NE concentration or attenuate the tachycardia associated with weight gain. There were no significant changes in body weight, sodium balance, or sodium-iothalamate space after renal denervation. In addition, in contrast to the reduction in GFR with baroreflex activation, GFR actually increased further after renal denervation; fractional sodium excretion remained suppressed (Figure 3).
Figure 4.
Mean arterial pressure and heart rate responses to bilateral renal denervation. Values are mean±SEM (n=6). *P<0.05 versus day 46. Lean control values (open bars) are also presented for reference.
Other than the reduction in PRA, there were no significant changes in the plasma levels of any measured hormones, glucose, electrolytes, or in hematocrit. Consequently, there was no change in the glucose-to-insulin ratio from day 39 proceeding (4.9±0.4) to day 60 following renal denervation (4.5±0.5).
In dogs with intact innervation, levels of NE in the renal cortex were 564±113 ng/g tissue. In the obese denervated dogs, tissue levels of NE were markedly reduced to 22±8 ng/g tissue. These values in innervated and denervated kidneys reflect the completeness of renal denervation.24
Discussion
Dogs fed a high-fat diet for several weeks exhibit many of the hemodynamic, neurohormonal, renal, and metabolic changes associated with obesity in human subjects including weight gain, sodium retention, hyperfiltration, hypertension, tachycardia, hyperinsulinemia, insulin resistance, and activation of the sympathetic and renin-angiotensin systems.2,17–19 In the present study, both baroreflex activation and renal denervation abolished obesity-induced hypertension in dogs, highlighting the importance of RSNA in mediating the increase in arterial pressure. Furthermore, in both instances, the reduction in arterial pressure occurred concurrently with suppression of the renin-angiotensin system suggesting that neurally-induced renin release contributes significantly to the maintenance of the hypertension. However, beyond these analogous responses, the effects of baroreflex activation and renal denervation on systemic sympathetic activity, as well as cardiac and renal function were significantly different. These functional differences may have relevance to the progression of cardiovascular disease in obesity. Finally, these interventions did not affect postabsorptive levels of insulin or glucose, indicating that the insulin resistance associated with weight gain may be independent of increased sympathetic activity.
There is considerable evidence for increased activation of the sympathetic nervous system throughout the evolution of obesity-related hypertension.1,2,28–32 Furthermore, measurements of renal NE spillover in both the early prehypertensive and advanced hypertensive stages of obesity in human subjects indicate increased RSNA is consistent with the hypothesis that the renal nerves provide the critical link between increased central sympathetic outflow and impaired renal function that leads to and sustains the hypertension.31–32 Therefore, because the natural activation of the baroreflex in hypertension has sustained effects to suppress RSNA and promote sodium excretion,10–13 it was not surprising that baroreflex activation had pronounced blood pressure lowering effects in this experimental model of obesity-induced hypertension. In fact, baroreflex activation completely abolished the hypertension, confirming our previous findings.19 Furthermore, complete elimination of the hypertension by abrogating the sympathetic drive to the kidneys by renal denervation emphasizes the importance of increased RSNA in sustaining the hypertension. The finding that the renal nerves are of paramount importance in sustaining obesity-induced hypertension is consistent with the report that bilateral renal denervation prior to weight gain prevented the development of obesity-related hypertension in dogs fed a high-fat diet.33 It should be noted that the absence of a significant increase in plasma NE concentration during the development of obesity hypertension in the present study is not inconsistent with sympathetic activation. Such an increase in plasma NE concentration was likely masked by increases in extracellular fluid volume (Table S1) and cardiac output associated with the weight gain,18 which would be expected to lower circulating levels of NE by dilution and increased tissue extraction, respectively.34
While not directly evaluated, the results of the present study suggest that activation of the renin-angiotensin system, via neurally-induced renin secretion, contributes importantly to obesity hypertension. It should be pointed out, however, that while increases in PRA have been reported in experimental animals and in human subjects during the evolution of obesity-induced hypertension,2,18,28,35 this has not been a consistent finding.19,33,36–37 In the present study, PRA was increased above control levels after one week of fat feeding, but not in the subsequent developmental or established phases of the hypertension. The temporal changes in PRA during the development of obesity hypertension in the present study suggest one possible explanation for the variable PRA findings reported by others. That is, although the progression from the initial to the more advanced stages of obesity hypertension may be associated with persistent neural stimulation of renin secretion, the neural drive to increase renin release may be partially offset in time by the progressive increase in arterial pressure. More specifically, as a result of its actions to promote sodium reabsorption in the proximal tubule and loop of Henle, one way increased RSNA stimulates renin secretion--in addition to directly stimulating juxtaglomerular cells--is by reducing sodium chloride delivery to the macula densa. In time, however, the rise in arterial pressure, concomitant with salt and water retention and expansion of extracellular fluid volume, would be expected to increase sodium delivery to the macula densa and, thus, suppress renin secretion. This would partially counteract the direct tubular and juxtaglomerular actions of the renal nerves favoring renin secretion. Thus, the more established phases of obesity hypertension may be associated with only subtle increases in renin secretion, not easily discerned by intermittent measurements of PRA. Nonetheless, this does not discount the importance of the renin-angiotensin system in the hypertensive process. One could argue that for the level of volume expansion and the prevailing degree of hypertension associated with obesity, PRA should be depressed, not normal. Moreover, in the present study, substantial reductions in PRA and arterial pressure occurred in parallel with both systemic and renal-specific inhibition of sympathetic activity, pointing to the importance of neurally-induced renin secretion in contributing to the hypertension. Finally, despite the equivocal levels of PRA reported in experimental and clinical studies, chronic administration of ANG II receptor antagonists or angiotensin converting enzyme inhibitors is effective in reducing elevated blood pressure in obese animals and humans,2,38–39 indicating the importance of the renin-angiotensin system in the pathogenesis of the hypertension.
While both baroreflex activation and renal denervation suppressed renin secretion and blood pressure, these manipulations differed substantially in their ability to diminish the systemic sympathoexcitation of obesity-hypertension. The pronounced fall in plasma NE concentration during baroreflex activation indicates a potent sustained effect of this reflex to attenuate the high levels of central sympathetic outflow in obesity. In contrast, renal denervation failed to reduce the high circulating levels of NE associated with weight gain, suggesting that sensory afferent signals from the kidneys do not contribute importantly to chronic sympathetic overactivity in obesity hypertension. Thus, this finding does not support the hypothesis that renal afferents add to the systemic sympathetic activation in patients with resistant hypertension,14–16 despite obesity being a common feature in these individuals.20–21 Experimental evidence favoring this hypothesis is based on observations in one patient with resistant hypertension.16 In this subject, chronic reductions in central sympathetic outflow occurred following catheter-based renal sympathetic denervation. It will be of interest to determine whether this observation is confirmed in a larger patient population with resistant hypertension treated with multiple medications. Further, because impaired renal function is a common finding in patients with resistant hypertension,20–21 sensory afferent signals originating from the kidneys and leading to systemic sympathetic activation may be dependent upon the degree of renal dysfunction. This interesting possibility merits investigation.
In the present study, baroreflex activation and renal denervation also had disparate effects on heart rate. The tachycardia associated with weight gain was substantially diminished by baroreflex activation, but not by renal denervation. While it is reasonable to suggest that the decrease in heart rate with baroreflex activation might be related to suppression of sympathetic activity, it is relevant that the tachycardia of obesity hypertension is associated with impaired baroreflex control of both sympathetic and vagal outflow.29 Furthermore, despite increases in sympathetic activity to the kidneys and the skeletal muscle vasculature, there is no clear increase in sympathetic outflow to the heart in obesity hypertension,1,32 clearly illustrating the regional differences in sympathetic responses. More specific to the present model of obesity hypertension, studies using autonomic blockade have demonstrated that the increased heart rate in dogs fed a high-fat diet is primarily due to decreased vagal tone rather than to increased sympathetic activity.40–41 In addition, studies in normotensive dogs indicated that chronic baroreflex activation augmented spontaneous baroreflex control of heart rate over time scales consistent with vagal activity.23 Nonetheless, regardless of relative contributions of sympathetic suppression and parasympathetic activation in diminishing the tachycardia of obesity, by improving autonomic balance to the heart, baroreflex activation may prevent and arrest life-threatening arrhythmias.
The findings in the present study also indicated significant differences between the renal responses to baroreflex activation and renal denervation. Specifically, two of the most pronounced responses to weight gain were sodium retention and glomerular hyperfiltration. The glomerular hyperfiltration associated with both sodium retention and reduced fractional sodium excretion (increased fractional sodium reabsorption) during weight gain indicates that the increase in GFR was a compensatory response to achieve sodium balance. One possibility to explain the increased GFR in obesity is that increased RSNA had a primary effect to increase sodium reabsorption prior to the macula densa. This is in keeping with the progressive response to increased stimulation of the renal nerves, with modest increases in adrenergic activity leading to increased sodium reabsorption directly and indirectly by stimulating renin secretion (higher levels of activation having vascular effects to decrease GFR and blood flow).42 Increased sodium reabsorption in the proximal tubule and/or loop of Henle, sites of action of the renal nerves and angiotensin II, would increase fractional sodium reabsorption (decrease fractional sodium excretion) and decrease sodium chloride delivery to the macula densa.43 This, in turn, would elicit a tubuloglomerular feedback signal to dilate the afferent arteriole and increase GFR. By inhibiting RSNA, there is an increase in fractional sodium excretion (decrease in fractional sodium reabsorption), which attenuates the high rate of sodium reabsorption. This increase in sodium chloride delivery to the macula densa would be expected to lead to a tubuloglomerular feedback mediated constriction of the afferent arteriole, which is a likely explanation for the fall in GFR with baroreflex activation. If this is indeed the primary mechanism that diminishes hyperfiltration during baroreflex activation, then constriction of the afferent arteriole may reduce glomerular injury and the progressive hypertension in the more chronic phases of obesity.
In contrast to the effect of baroreflex activation to diminish glomerular hyperfiltration, GFR actually increased further after renal denervation. Increased GFR in the absence of a change in fractional sodium reabsorption (Figure 3) may reflect a prominent vasodilatory response in preglomerular vessels due to greater renal adrenergic suppression than achieved with baroreflex activation.43 The increase in GFR in the absence of a change in fractional sodium excretion may reflect the balance between the primary vascular and tubular effects of renal sympathetic denervation, actions that decrease and increase fractional sodium excretion, respectively.43
Although controversial, it has been suggested that activation of the sympathetic nervous system contributes to insulin resistance.44 While the mechanism was not addressed, a recent report indicated that insulin sensitivity improved after renal sympathetic denervation in patients with resistant hypertension and insulin resistance.45 In contrast, in the present study, neither renal denervation nor baroreflex activation lowered the elevated postabsorptive levels of insulin or altered plasma glucose concentration. Thus, the present study does not support the contention that either renal-specific or systemic inhibition of sympathetic activity improves insulin sensitivity in obesity hypertension.
Due to the nature of this study, a potential limitation was that randomization of interventions was not possible. That is, baroreflex activation had to precede renal denervation, and time dependent control studies in obese dogs were not performed. However, dietary intake and body weight were constant throughout the established phase of obesity hypertension and recovery values one week after baroreflex activation were similar to those measured before carotid sinus stimulation. Furthermore, unpublished data from one of the authors (JRH) indicate that blood pressure and heart rate are stable for at least 5 weeks after establishment of obesity hypertension. Therefore, we believe that time-dependent changes were insignificant and unlikely to have influenced the findings. An additional limitation is that this experimental model of obesity-induced hypertension does not reproduce all the abnormalities present in resistant hypertension and, therefore, extrapolation of the current findings to this complex disease state should be made with caution.
Perspectives.
The technology to chronically stimulate the carotid baroreflex has provided a unique experimental tool for understanding the mechanisms that not only mediate the blood pressure lowering effects of the baroreflex, but also the cardiovascular responses to suppression of central sympathetic outflow in general. The mechanistic insight from experimental studies is particularly relevant to the current fascination with device-based therapy for the treatment of resistant hypertension because it helps to identify subsets of this heterogeneous population who stand to benefit the most. Although impressive reductions in arterial pressure have been reported in patients with resistant hypertension when treated with device-based therapy, little is known about the renal hemodynamic effects of these novel interventions. As glomerular hypertension and hyperfiltration are precursors of progressive renal injury in obesity,2 and obesity is commonly present in patients with resistant hypertension,20–21 an especially important finding in the present study is that baroreflex activation may reduce glomerular hyperfiltration by constricting the afferent arteriole through the normal physiological action of tubuloglomerular feedback. Therefore, this may add to the clinical benefit of lowering arterial pressure during baroreflex activation in patients with drug-resistant hypertension. However, this possibility will require careful temporal determinations of renal function in this patient population with diverse degrees of kidney impairment.
Supplementary Material
Acknowledgements
We thank David S. Goldstein and Patti Sullivan (NIH/NINDS) for plasma and renal tissue NE determinations.
We also thank Jamie Beckman for his technical assistance.
Sources of Funding
National Heart, Lung, and Blood Institute Grant HL-51971.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Thomas E. Lohmeier Consultant fees, Scientific Advisory Board, CVRx
Eric D. Irwin Consultant fees, Scientific Advisory Board, CVRx
In addition to providing the electrodes and pulse generators, CVRx, Inc. has been the sponsor of several clinical trials using carotid sinus stimulation for the treatment of resistant hypertension.3
References
- 1.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE, da Dilva AA, Brandon E, Stec DE, Ying Z, Jones DW. Parhophysiology of obesity-induced hypertension and target organ damage. In: Lip GYH, Hall JE, editors. Comprehensive Hypertension. New York: Elsevier; 2007. pp. 447–468. [Google Scholar]
- 3.Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation. Mechanisms and potential for hypertension therapy. Hypertension. 2011;57:880–886. doi: 10.1161/HYPERTENSIONAHA.108.119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, Schmidli J, Allemann Y, Delacretaz E. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension. 2009;54:530–536. doi: 10.1161/HYPERTENSIONAHA.109.134023. [DOI] [PubMed] [Google Scholar]
- 5.Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, Peters T, Sweep FCGJ, Haller H, Pichlmaier, Luft F, Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55:619–626. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 6.Scheffers IJM, Kroon AA, Schmidli J, Jordan J, Tordoir JJM, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, Ceral J, Eckert S, Erglis A, Narkiewicz K, Philipp T, de Leeuw PW. Novel baroreflex activation therapy in resistant hypertension. J Am Coll Cardiol. 2010;56:1254–1258. doi: 10.1016/j.jacc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 7.Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal trial. J Am Coll Cardiol. 2011;58:765–773. doi: 10.1016/j.jacc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Guyton AC. Arterial Pressure and Hypertension. Philadelphia, PA: Saunders; >1980. [Google Scholar]
- 9.Cowley AW., Jr Long-term control of arterial blood pressure. Physiol Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- 10.Lohmeier TE, Drummond HA. The baroreflex in the pathogenesis of hypertension. In: Lip GYH, Hall JE, editors. Comprehensive Hypertension. New York: Elsevier; 2007. pp. 265–279. [Google Scholar]
- 11.Lohmeier TE, Hildebrandt DA, Warren S, May PJ, Cunningham JT. Recent insights into the interactions between the baroreflex and the kidneys in hypertension. Am J Physiol Regulatory Integrative Comp Physiol. 2005;288:R828–R836. doi: 10.1152/ajpregu.00591.2004. [DOI] [PubMed] [Google Scholar]
- 12.Lohmeier TE, Lohmeier JR, Haque A, Hildebrandt DA. Baroreflexes prevent neurally-induced sodium retention in angiotensin hypertension. Am J Physiol Regulatory Integrative Comp Physiol. 2000;279:R1437–R1448. doi: 10.1152/ajpregu.2000.279.4.R1437. [DOI] [PubMed] [Google Scholar]
- 13.Barrett CJ, Guild S-J, Ramchandra R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension. 2005;46:1–5. doi: 10.1161/01.HYP.0000168047.09637.d4. [DOI] [PubMed] [Google Scholar]
- 14.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 15.Simplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment resistant hypertension (The Symplicity HTN-2 Trial): a randomized controlled trial. Lancet. 2011;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 16.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl JMed. 2009;361:932–934. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 17.Rocchini AP, Moorehead CP, DeRemer S, Bondie D. Pathogenesis of weight-related changes in blood pressure in dogs. Hypertension. 1989;13:922–928. doi: 10.1161/01.hyp.13.6.922. [DOI] [PubMed] [Google Scholar]
- 18.Hall JE, Brands MW, Dixon WN, Smith MJ., Jr Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993;22:292–299. doi: 10.1161/01.hyp.22.3.292. [DOI] [PubMed] [Google Scholar]
- 19.Lohmeier TE, Dwyer TM, Irwin ED, Rossing MA, Kieval RS. Prolonged activation of the baroreflex abolishes obesity-induced hypertension. Hypertension. 2007;49:1307–1314. doi: 10.1161/HYPERTENSIONAHA.107.087874. [DOI] [PubMed] [Google Scholar]
- 20.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 21.Persell SD. Prevalence of resistant hypertension in the United States: 2003–2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 22.Lohmeier TE, Irwin ED, Rossing MA, Sedar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43:306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 23.Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol. 2010;299:H402–H409. doi: 10.1152/ajpheart.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohmeier TE, Reinhart GA, Mizelle HL, Montani J-P, Hester RL, Hord CE, Jr, Hildebrandt DA. Influence of the renal nerves on sodium excretion during progressive reductions in cardiac output. Am J Physiol Regulatory Integrative Comp Physiol. 1995;269:R678–R690. doi: 10.1152/ajpregu.1995.269.3.R678. [DOI] [PubMed] [Google Scholar]
- 25.Reinhart GA, Lohmeier TE, Hord CE., Jr Hypertension induced by chronic renal adrenergic stimulation is angiotensin dependent. Hypertension. 1995;25:940–949. doi: 10.1161/01.hyp.25.5.940. [DOI] [PubMed] [Google Scholar]
- 26.Lohmeier TE, Dwyer TM, Hildebrandt DA, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension. 2005;46:1194–1200. doi: 10.1161/01.HYP.0000187011.44201.2e. [DOI] [PubMed] [Google Scholar]
- 27.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–138. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- 28.Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, Giannattasio C, Brunani A, Cavagnini F, Mancia M. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- 29.Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- 30.Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, Farley G, Paranjape SY, Davis SN, Biaggioni I. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 31.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 32.Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, Wiesner GH, Brunner-La Rocca HP, Esler MD. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17:1125–1133. doi: 10.1097/00004872-199917080-00012. [DOI] [PubMed] [Google Scholar]
- 33.Kassab S, Kato T, Wilkins C, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 34.Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- 35.Grassi G, Facchini A, Trevano FQ, Dell’Oro R, Arenare F, Tana F, Bolla G, Monzani A, Robuschi M, Mancia G. Obstructive sleep apnea-dependent and –independent adrenergic activation in obesity. Hypertension. 2005;46:321–325. doi: 10.1161/01.HYP.0000174243.39897.6c. [DOI] [PubMed] [Google Scholar]
- 36.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regulatory Integrative Comp Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 37.Grassi G, Seravalle G, Quarti-Trevano F, Dell’Oro R, Bolla G, Mancia G. Effects of hypertension and obesity on the sympathetic activation of heart failure patients. Hypertension. 2003;42:873–877. doi: 10.1161/01.HYP.0000098660.26184.63. [DOI] [PubMed] [Google Scholar]
- 38.Reisen E, Weir MR, Falkner B, Hutchinson HG, Anzalone DA, Tuck ML. Lisinopril versus hydrochlorothiazide in obese hypertensive patients. Hypertension. 1997;30:140–145. doi: 10.1161/01.hyp.30.1.140. [DOI] [PubMed] [Google Scholar]
- 39.Boustany CM, Brown DR, Randall DC, Cassis LA. At1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regulatory Integrative Comp Physiol. 2005;289:R181–R186. doi: 10.1152/ajpregu.00507.2004. [DOI] [PubMed] [Google Scholar]
- 40.Van Vliet BN, Hall JE, Mizelle HL, Montani J-P, Smith MJ., Jr Reduced parasympathetic control of heart rate in obese dogs. Am J Physiol Heart Circ Physiol. 1995;269:H629–H637. doi: 10.1152/ajpheart.1995.269.2.H629. [DOI] [PubMed] [Google Scholar]
- 41.Truett AA, Borne AT, Poincot MA, West DB. Autonomic control of blood pressure and heart rate in obese hypertensive dogs. Am J Physiol Regulatory Integrative Comp Physiol. 1996;270:R541–R549. doi: 10.1152/ajpregu.1996.270.3.R541. [DOI] [PubMed] [Google Scholar]
- 42.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 43.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol. 2003;286:F8–F15. doi: 10.1152/ajprenal.00208.2003. [DOI] [PubMed] [Google Scholar]
- 44.Hall JE, Summers RL, Brands MW, Keen H, Alonso-Galicia M. Resistance to metabolic cations of insulin and its role in hypertension. Am J Hypertens. 1994;7:772–788. doi: 10.1093/ajh/7.8.772. [DOI] [PubMed] [Google Scholar]
- 45.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Bohm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension. A pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.