Summary
We examined new users of osteoporosis drugs among seniors in Pennsylvania and found no evidence of healthy adherer bias on observed associations between adherence to treatment and non-vertebral fracture risk; we document fracture reduction with better adherence to bisphosphonates, yet no fracture reduction with better adherence to calcitonin or raloxifene.
Introduction
We examined the potential for “healthy adherer bias” when studying the effects of adherence to osteoporosis pharmacotherapy on fracture risk. Based on clinical trial evidence, bisphosphonates, calcitonin, and raloxifene reduce vertebral fracture risk; yet only bisphosphonates are documented to reduce non-vertebral fracture risk.
Methods
This is a cohort study of older women in Pennsylvania who initiated osteoporosis drugs between 1995 and 2005. We included new users of bisphosphonates, calcitonin, and raloxifene. Adherence was categorized based on a measure of compliance as high [proportion of days covered (PDC) ≥ 80%], intermediate (50% < PDC < 80%), or low (PDC ≤ 50%) according to a 180-day ascertainment period. Non-vertebral fracture rates within 365 days after the ascertainment period were compared between adherence categories (reference = low) using Cox proportional hazard models and adjusting for fracture risk factors. Primary and secondary prevention cohorts were examined separately. Adherence to calcitonin and ralox-ifene were control analyses.
Results
We found little difference in fracture rates between levels of adherence to calcitonin, bisphosphonates for primary prevention, or raloxifene for secondary prevention. We document lower fracture rates among high versus low adherent bisphosphonate users for secondary prevention (HR = 0.53, 95%CI = 0.38–0.74) and higher fracture rates among high versus low adherent raloxifene users for primary prevention (HR = 2.01, 95% CI = 1.04–3.87).
Conclusions
We document little evidence of healthy adherer bias when studying the association between better adherence to osteoporosis drugs and fracture risk reduction, with only better adherence to bisphosphonates reducing fracture risk. The higher fracture risk among highly adherent raloxifene users for primary prevention is likely due to residual confounding.
Keywords: Bones, Fractures, Medication adherence, Osteoporosis, Selection bias
Introduction
Osteoporosis is an important public health problem resulting in considerable fracture-related morbidity [1]. Several effective pharmacologic options exist to prevent fractures among those at high risk [2], yet fewer than half of patients treated with osteoporosis drugs adhere to therapy [3]. Prior studies that examine healthcare utilization data have found a strong positive association between better adherence to osteoporosis pharmacotherapy and fracture prevention [3–7]. These findings underscore the importance of interventions to improve treatment adherence. However, these observed positive effects may also arise, in part, from “healthy adherer bias.” Healthy adherer bias is a type of selection bias that occurs when adherence to therapy is associated with healthy patient characteristics that are not available in healthcare utilization data sources [8, 9]. For example, if patients who adhere to osteoporosis pharmacotherapy are also more likely to engage in physical activity or take over-the-counter calcium and vitamin D, then the protective effects of physical activity and vitamin supplementation may be falsely attributed to adherence to osteoporosis pharmacotherapy. Recent analysis of the placebo arm of the Women’s Health Initiative trial also identifies that better adherence to placebo reduced hip fracture risk [10]. Accurate estimates of the benefits of medication adherence are important for the cost effectiveness analyses of quality improvement interventions as well as health policy decision making [11].
A recent study found no evidence of healthy adherer bias in explaining survival after acute myocardial infarction, with only adherence to medications of proven mortality benefits (statins and β-blockers but not calcium channel blockers) being associated with survival [12]. Although these findings support the importance of maximizing adherence to therapy post-myocardial infarction, secondary prevention with pharmaceuticals may not be susceptible to healthy adherer bias because patients may generally be more motivated to adhere to therapy and engage in protective behaviors following an acute event [13–15]. Healthy adherer bias may be more likely to influence studies that examine adherence to medication for primary prevention of an asymptomatic condition, such as osteoporosis treatment to prevent fractures.
Our main objective was to examine the potential for healthy adherer bias in studies that examine the effect of adherence to osteoporosis drugs on fracture prevention. We compared adherence to different osteoporosis drug classes (bisphosphonates, nasal calcitonin, raloxifene) and subsequent fracture risk. Although each drug class is proven effective in reducing vertebral fracture risk, only the bisphosphonates alendronate and risedronate have good evidence to suggest that they also reduce non-vertebral fracture risk—such as hip fracture risk [2]. Therefore, based on clinical trial evidence, in the absence of healthy adherer bias, adherence to calcitonin and raloxifene would not be associated with non-vertebral fracture prevention. To better understand the potential for healthy adherer bias when studying the association between adherence to therapy and fracture prevention, we examined primary prevention and secondary prevention cohorts separately. We hypothesized that adherence to therapy for primary prevention would be more susceptible to healthy adherer bias than adherence to therapy for secondary prevention. We therefore expected to see stronger associations between adherence to drugs with no proven benefit (calcitonin and raloxifene) in preventing non-vertebral fractures in primary prevention than in secondary prevention cohorts.
Materials and methods
Study cohort
The study population was identified from pharmaceutical claims for enrollees in the Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE). This state-wide program provides drug coverage without restriction for low-income residents aged 65 or more years with annual household income above the Medicaid threshold. We studied three separate cohorts of new users identified from pharmacy claims between July 1, 1995 and March 31, 2005: (1) oral bisphosphonates [alendronate (5, 10, 35, and 70 mg), risedronate (5 and 35 mg)], (2) nasal calcitonin, and (3) raloxifene. The first relevant dispensing since July 1, 1995 identified the prescription index date. New use was defined as having no use of any bisphosphonate, calcitonin, or raloxifene in the 365 days prior to index date. Study eligibility was limited to female patients with one or more claims in both Medicare and PACE in each of the three 6-month intervals preceding the index date. This was to ensure complete drug coverage and Medicare coverage during the 1-year baseline (lookback) period used to define covariates and prior use of osteoporosis medication. In particular, we were concerned that requiring pharmacy claims only within the two preceding 6-month intervals would only ensure complete coverage to 6 months and 1 day prior to index date. Given our objective of studying healthy adherer bias, it was important to limit our study to incident users identified as no use within the 1-year lookback period. Nursing home residents, for whom prescription data may not be complete, and patients with a Medicare claim for Paget’s diagnosis (ICD-9-CM 731.0) were excluded. We also excluded patients taking combination therapy, defined by a dispensing of more than a single osteoporosis agent on the initiation date. We planned to include patients treated with ibandronate and exclude those treated with teriparatide; however, no such patients were identified. Our data included all PACE beneficiaries that met eligibility criteria. At the time of analysis, we had complete Medicare data from January 1, 1994 through to December 31, 2005.
Cohorts were subdivided into primary and secondary prevention according to diagnostic codes identified within the year prior to treatment initiation. We classified patients as being treated for secondary prevention if they had a diagnostic code for fracture (ICD-9-CM codes 733.1x, 800. xx–829.xx) in the prior year.
Adherence
We defined adherence to therapy based on pharmacy claims according to an ascertainment period of 180 days. Prior evidence suggests that non-adherence to osteoporosis drugs occurs shortly after treatment initiation and is relatively stable thereafter [3]. We examined adherence using a measure of compliance [4], classified based on the proportion of days covered (PDC) into: low (PDC ≤ 50%), medium (50% < PDC < 80%), and high compliance (PDC ≥ 80%). PDC is calculated as the total number of days supplied in the observation period, divided by the total number of days in the observation period, and capped to 1% or 100% and is therefore synonymous with the medication possession ratio (MPR) capped at 100%. The International Society for Pharmacoeconomics and Outcomes Research recognizes both PDC and MPR, yet does not recommend the use of one term over the other [16, 17]. We use PDC in our study because we believe that “proportion of days covered” more clearly describes what is being measured, and in the literature, PDC is consistently capped at 1% or 100% [18].
A cut point of ≥80% has commonly been used as an indicator of high/good compliance in primary or secondary analyses that examined the impact of adherence to bisphosphonates on fracture risk [4]. However, there is less consistency in defining low compliance. We selected ≤50% as the indicator for low compliance, which, over a 180-day ascertainment period, corresponds to fewer than 3 months of treatment—the length of time suggested before bisphosphonates are effective in reducing non-vertebral fracture risk [19]. To account for potential immeasurable time bias [20], we subtracted the number of days in hospital (including long-term care) from the denominator (PDC = total days supply/[total number of days evaluated – number of days in hospital], with a maximum of 1.0). We also excluded patients having 50% or more days in hospital during the ascertainment period, patients who switched therapies during the ascertainment period, and those experiencing a hip, humerus, radius, or ulna fracture during the ascertainment period. Switching between bisphosphonates was not considered a switch. We acknowledge that some patients may discontinue medication if it is poorly tolerated. Regardless of the reason for treatment discontinuation, we assumed that patients required pharmacotherapy and thus would ideally return to their physician for a new medication in the context of adverse drug effects or poor drug tolerance.
Outcomes
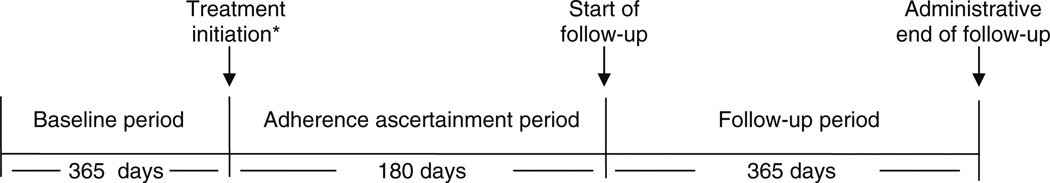
Our primary outcome was non-vertebral fracture (hip, humerus and radius or ulna) within 365 days after the adherence ascertainment period (Fig. 1). We defined fractures using previously validated criteria requiring diagnostic and procedural codes: occurring in hospital and within 7 days for hip fracture or any diagnostic and relevant procedural code occurring within 30 days for fractures of the humerus, radius, and ulna [21, 22]. These codes have been validated against medical records as the reference standard, with an estimated sensitivity of at least 90% [21]. We examined hip fracture, the most costly consequence of untreated osteoporosis, as a secondary outcome. Observation time began after the adherence ascertainment period, to a maximum of 365 days of follow-up (Fig. 1).
Fig. 1.
Study design. *Index date; at the time of analysis, we had complete Medicare data from January 1, 1994 through to December 31, 2005. Inclusion was limited to those with at least one Medicare and pharmacy claim in each of the three 6-month intervals prior to treatment initiation (system activity); therefore, July 1, 1995 is the first possible date of treatment initiation. Baseline period: 1-year lookback to define new users and to assess covariates. Adherence ascertainment period: Eligibility restricted to new users with complete drug coverage during the ascertainment period (drug coverage, survive, community-dwelling). Patients experiencing a hip, humerus, radius, or ulna fracture or spending 50% or more days in hospital during the ascertainment period were excluded. Adherence based on a measure of compliance and calculated as proportion of days covered, adjusting for number of days in hospital [20] = total days supply/(total number of days evaluated – number of days in hospital); capped at 1.0. Follow-up for outcome assessment: maximum length of follow-up was 365 days from the end of the 180-day ascertainment period or December 31, 2005
To test the hypothesis that osteoporosis medication adherence is a marker of health-seeking behaviors, we examined the association between adherence to therapy and subsequent vaccination (influenza and pneumoccocal) and non-osteoporosis-related preventive healthcare testing [mammography and Papanicolaou (PAP) testing]. Diagnostic and procedural codes for each follow-up event are summarized in Table 3 in Appendix.
Table 3.
Study outcome coding (diagnostic and procedural codes)
| Variable | Definition |
|---|---|
| Primary outcomea | |
| Hip fracture | ICD-9-CM diagnostic codes: 820.xx, 733.14 during hospitalization AND at least one of the following procedural codes during the same hospitalization AND within 7 days of the diagnostic code, ICD-9-CM procedural codes: 78.55, 79.05, 79.15, 79.25, 79.35, 79.65; CPT-4 codes 27230–27248 |
| Secondary outcome | |
| Hip, humerus, radius, or ulnaa | Hip (defined above) Humerus: ICD-9-CM diagnostic codes: 812.xx, 733.11 AND at least one of the following procedural codes within 30 days of the diagnostic code, ICD-9-CM procedural codes: 78.52, 79.01, 79.11, 79.21, 79.31, 79.61, CPT-4 codes 23600, 23605, 23610, 23615, 23620, 23625, 23630, 23665, 23670, 23675, 23680, 24500, 24505, 24506, 24510, 24515, 24530, 24531, 24535, 24536, 24538, 24540, 24542, 24545, 24560, 24565, 24570, 24575–24581, 24583, 24585–24588, 24516 Radius, ulna: ICD-9-CM diagnostic codes: 813.xx, 733.12 AND at least one of the following procedural codes within 30 days of the diagnostic code, ICD-9-CM 78.53, 79.02, 79.12, 79.22, 79.32, 79.62; CPT-4 codes 24620, 24625, 24635, 24650, 24655, 24660, 24665, 24666, 24670, 24675, 24680, 24685, 25500, 25505, 25510, 25515, 25530, 25535, 25540, 25545, 25560, 25565, 25570, 25575, 25600, 25605, 25610, 25611, 25615, 25620, 25650 |
| Control outcome—preventive healthcare services (vaccinations) | |
| Influenza | HCPCS code G0008; CPT-4 codes 90655, 90656, 90657, 90658, 90660; ICD-9-CM code V04.81 |
| Pneumoccocal | HCPCS code G0009; CPT-4 code 90732 |
| Control outcome—preventive healthcare services (screening) | |
| Papanicolaou (PAP) testing | HCPCS codes G0123, G0124, G0141, G0143, G0144, G0145, G0147, G0148, P3000, P3001, Q0091; ICD-9-CM codes V76.2, V76.47 |
| Mammography | HCPCS codes G0202, G0203, G0204, G0206; CPT-4 codes 76082, 76083, 76092, 76090, 76091, 3014F; ICD-9-CM codes V76.1x) |
CPT-4 Current Procedural Terminology, 4th edition; HCPCS Healthcare Common Procedure Coding System; ICD-9-CM International Classification of Diseases, Ninth Revision, Clinical Modification
Validated codes[21] updated to include new codes, e.g., that replace previously validated codes
Covariates
Patient demographics were determined at the time of index prescription and other variables by medical and pharmacy claims within the year prior to index prescription date. In our main analysis of fracture risk, we controlled for potential confounding by including factors plausibly related to fracture risk, such as age, prior fracture, comorbidity, and prior drug use (Table 4 in Appendix). Covariates included in the adjusted analysis for vaccination and preventive healthcare testing outcomes included: age groups, hospitalization, comorbidity score quartiles, and number of drugs during the 365-day period prior to treatment initiation categorized into quintiles, as well as respective vaccination or testing during the 365-day baseline period and the 180-day adherence ascertainment period.
Table 4.
Coding of covariates in baseline period—all included when studying fracture risk
| Variable | Definition | Coding |
|---|---|---|
| Medicare enrolment information at time of index prescription | ||
| Age | Age in years | Categorical, groupings: 65–69, 70–74, 75–79, 80–84, 85–89, and 90 or more years |
| Race | Caucasian race | Dichotomous (yes/no) |
| Medicare claims within 365 days prior to index osteoporosis drug prescription | ||
| Hospitalization within prior year | Any | Dichotomous (yes/no) |
| Comorbidity score | Charlson comorbidity score (17, 18) | Ordinal (quartiles, 3 dummies) |
| Osteoporosis related | ||
| Osteoporosis | ICD-9-CM: 733.0x | Dichotomous (yes/no) |
| Kyphosis | ICD-9-CM: 737.1x, 737.41, 737.3x | Dichotomous (yes/no) |
| Prior fracture—vertebral | Vertebral (ICD-9-CM: 733.13, 805.xx) | Dichotomous (yes/no) |
| Prior fracture—non-vertebral | Hip (ICD-9-CM: 820.xx, 733.14), humerus (ICD-9-CM: 812.xx, 733.11), and/or radius/ulna (ICD-9-CM: 813.xx, 733.12) | Dichotomous (yes/no) |
| Prior fracture—other | Any fracture (ICD-9-CM: 733.1x, 800.xx-829.xx) other than vertebral / non-vertebral defined above | Dichotomous (yes/no) |
| Comorbidities | ||
| Alzheimer’s/other dementia | ICD-9-CM: 290.xx, 294.xx, 330.xx, 331.xx | Dichotomous (yes/no) |
| Asthma/chronic obstructive pulmonary disease | ICD-9-CM: 493.xx, 490, 491.xx, 492.xx, 494.xx, 496.xx, 506.4x | Dichotomous (yes/no) |
| Cataracts | ICD-9-CM: 366.xx | Dichotomous (yes/no) |
| Crohn’s/gastroenteritis | ICD-9-CM: 555.xx, 556.xx, 558.xx | Dichotomous (yes/no) |
| Depression | ICD-9-CM: 293.83, 296.2x. 296.3x, 298.0x, 300.4x, 309.0x, 309.1x, 309.28, 311.xx | Dichotomous (yes/no) |
| Diabetes mellitus | ICD-9-CM: 1 hospitalization discharge of 250.xx or 2 outpatient claims with 250.xx | Dichotomous (yes/no) |
| Falls history, syncope, or gait abnormality | ICD-9-CM: E885, E885.9x, E888.xx, 780.2x, 458.0x, 781.2x, 782.3x | Dichotomous (yes/no) |
| Hyperthyroidism | ICD-9-CM: 242.0x-242.9x | Dichotomous (yes/no) |
| Hyperparathyroidism | ICD-9-CM: 252.0x | Dichotomous (yes/no) |
| Ischemic stroke | ICD-9-CM: 434.xx, 436.xx | Dichotomous (yes/no) |
| Liver disease | ICD-9-CM: 571.4x, 571.6x, 571.8x, 571.9x, 573.xx, 070.xx | Dichotomous (yes/no) |
| Malignant neoplasm | ICD-9-CM: 140.xx-208.xx | Dichotomous (yes/no) |
| Overweight or obese | ICD-9-CM: 278, 278.0x | Dichotomous (yes/no) |
| Parkinson’s | ICD-9-CM: 332.xx, 333.0x or use of antiparkinsonian drug | Dichotomous (yes/no) |
| Renal disease | ICD-9-CM: 250.4x, 403.xx, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93 | Dichotomous (yes/no) |
| Rheumatoid arthritis | ICD-9-CM: 714.xx | Dichotomous (yes/no) |
| Pharmacy claims within 365 days prior to index osteoporosis drug prescription | ||
| Number of generics | Count | Ordinal (quintiles, 4 dummies) |
| Anti-epileptic | Any pharmacy claim | Dichotomous (yes/no) |
| Beta-blocker | Any pharmacy claim | Dichotomous (yes/no) |
| Benzodiazepines | Any pharmacy claim | Dichotomous (yes/no) |
| Gastroprotective | Any pharmacy claim | Dichotomous (yes/no) |
| Glucocorticoids | Any pharmacy claim | Dichotomous (yes/no) |
| Hormone therapy | Any pharmacy claim | Dichotomous (yes/no) |
| Selective Cox-2 inhibitor | Any pharmacy claim | Dichotomous (yes/no) |
| Other nonsteroidal anti-inflammatory drug | Any pharmacy claim | Dichotomous (yes/no) |
| Selective serotonin reuptake inhibitors (SSRI) | Any pharmacy claim | Dichotomous (yes/no) |
| Non-SSRI antipsychotic | Any pharmacy claim | Dichotomous (yes/no) |
| Statin therapy | Any pharmacy claim | Dichotomous (yes/no) |
| Thiazide diuretic | Any pharmacy claim | Dichotomous (yes/no) |
| Thyroid drugs | Any pharmacy claim | Dichotomous (yes/no) |
| Miscellaneous sleep/hypnotic/barbiturates | Any pharmacy claim | Dichotomous (yes/no) |
Statistical analysis
We used Cox proportional hazard models to compare event rates between levels of adherence, adjusting for risk factors for fracture. Observation time began after the 180-day ascertainment period and ended on the first of death date, 365 days of follow-up, or December 31, 2005. All rates were expressed as the number of events per 100 person-years. We tested proportional hazard assumptions by including an interaction term between exposure and the log of time. No violations of the proportional hazard assumptions were identified except for secondary prevention with bisphosphonates and hip fracture risk among those in the intermediate adherence group.
The Partners HealthCare Institutional Review Board approved this project. Data Use Agreements are in place from the Centers for Medicare and Medicaid Services and PACE.
Results
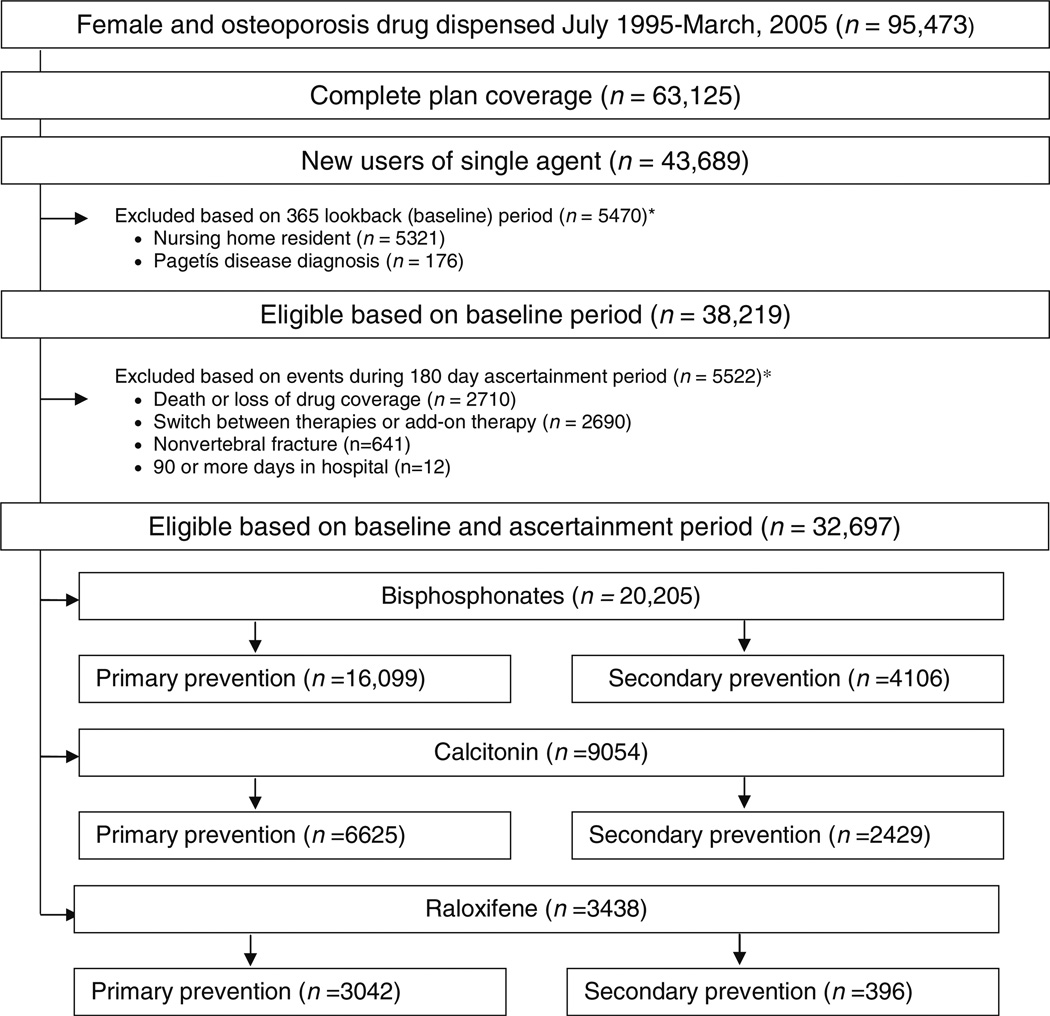
We identified 32,697 (20,205 bisphosphonate, 9,054 calcitonin, and 3,438 raloxifene) eligible new users between July 1, 1995 and March 31, 2005 (Fig. 3 in Appendix). Bisphosphonate users had a mean age of 79 years (SD = 6.3), calcitonin users a mean age of 81 years (SD = 6.7), and raloxifene users a mean age of 77 years (SD = 6.4). Table 1 summarizes cohort characteristics by drug class and level of adherence. The proportion within each level of adherence was similar between primary and secondary prevention cohorts (data not shown). Patients in high adherence groups tended to have a higher prevalence of osteoporosis and more often had used statins, mammography, and bone mineral density (BMD) testing.
Fig. 3.
Flow diagram of participant inclusion—enrollees in the Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE). *subcategories for exclusion are not mutually exclusive
Table 1.
Characteristics of study cohorts, new users of osteoporosis medication in Pennsylvania from 1995 to 2005, by level of adherence
| Bisphosphonate (N = 20,205) | Calcitonin (N = 9,054) | Raloxifene (N = 3,438) | ||||
|---|---|---|---|---|---|---|
| Adherence levela | Low | High | Low | High | Low | High |
| No. within each adherence level | 7,220 | 10,057 | 5,950 | 936 | 1,178 | 1,808 |
| Proportion within each adherence level | 35.7 | 49.8 | 65.7 | 10.3 | 34.3 | 52.6 |
| Mean age in years (SD) | 79.0 (6.4) | 78.7 (6.2) | 80.8 (6.6) | 79.8 (6.6) | 77.4 (6.3) | 77.4 (6.3) |
| Primary prevention (%) | 80.7 | 79.0 | 73.9 | 73.9 | 88.9 | 88.1 |
| Hospitalization within prior year (%) | 23.2 | 21.8 | 32.8 | 31.1 | 18.2 | 19.1 |
| Caucasian (%) | 94.8 | 96.7 | 97.1 | 97.6 | 95.3 | 96.3 |
| Diagnoses | ||||||
| Alzheimer’s/other dementia (%) | 4.0 | 4.9 | 7.5 | 5.3 | 3.4 | 5.0 |
| Asthma/chronic obstructive pulmonary disease (%) | 21.0 | 18.4 | 24.1 | 21.3 | 17.1 | 17.0 |
| Cataracts (%) | 36.4 | 39.4 | 36.9 | 37.9 | 37.9 | 38.3 |
| Crohn’s/gastroenteritis (%) | 4.5 | 4.3 | 5.5 | 6.3 | 4.6 | 5.3 |
| Depression (%) | 9.8 | 8.3 | 12.9 | 11.2 | 9.3 | 8.7 |
| Diabetes mellitus (%) | 21.6 | 21.0 | 22.9 | 20.5 | 20.6 | 24.4 |
| Falls history, syncope, or gait abnormality (%) | 15.7 | 15.0 | 19.6 | 19.1 | 11.6 | 13.2 |
| Fracture (%) | 19.3 | 21.0 | 26.1 | 26.1 | 11.1 | 11.9 |
| Hyperthyroidism (%) | 2.9 | 3.8 | 3.9 | 3.7 | 3.2 | 3.3 |
| Hyperparathyroidism (%) | 0.8 | 1.1 | 1.0 | 0.7 | 0.4 | 0.7 |
| Ischemic stroke (%) | 4.7 | 4.9 | 6.7 | 6.1 | 4.8 | 4.8 |
| Liver disease (%) | 2.1 | 2.1 | 2.5 | 2.4 | 3.2 | 2.7 |
| Malignant neoplasm (%) | 14.2 | 14.8 | 13.6 | 15.2 | 14.3 | 13.3 |
| Osteoporosis (%) | 49.1 | 55.1 | 43.0 | 47.8 | 39.5 | 45.3 |
| Overweight or obese (%) | 2.2 | 1.9 | 2.2 | 1.1 | 2.6 | 1.7 |
| Parkinson’s (%) | 1.3 | 1.5 | 2.5 | 1.5 | 1.6 | 1.1 |
| Renal disease (%) | 1.0 | 1.0 | 1.2 | 1.4 | 0.9 | 1.1 |
| Rheumatoid arthritis (%) | 6.5 | 6.4 | 7.4 | 6.4 | 4.2 | 4.5 |
| Drug use | ||||||
| Anti-epileptic (%) | 0.7 | 0.7 | 0.8 | 0.9 | 1.0 | 0.9 |
| Beta-blocker (%) | 32.8 | 32.7 | 31.7 | 30.0 | 33.3 | 32.4 |
| Benzodiazepines (%) | 18.6 | 15.1 | 20.5 | 19.9 | 18.3 | 15.8 |
| Gastroprotective (%) | 34.4 | 31.0 | 44.5 | 47.6 | 34.5 | 37.6 |
| Glucocorticoids (%) | 12.1 | 10.3 | 12.4 | 13.1 | 9.4 | 7.4 |
| Hormone therapy (%) | 7.2 | 8.8 | 7.0 | 6.9 | 15.6 | 19.7 |
| Selective Cox-2 (%) | 14.8 | 14.9 | 11.1 | 12.1 | 16.2 | 18.9 |
| Other nonsteroidal anti-inflammatory drug (%) | 24.0 | 23.4 | 27.9 | 26.8 | 21.8 | 21.6 |
| Selective serotonin reuptake inhibitors (SSRI, %) | 13.5 | 12.1 | 16.1 | 14.0 | 12.4 | 14.3 |
| Non-SSRI antipsychotic (%) | 10.0 | 9.0 | 12.2 | 11.3 | 9.8 | 11.4 |
| Statin (%) | 26.1 | 29.7 | 18.1 | 20.6 | 30.0 | 35.2 |
| Thiazide diuretic (%) | 15.2 | 14.5 | 13.2 | 13.5 | 12.9 | 16.6 |
| Thyroid drugs (%) | 18.7 | 20.7 | 19.0 | 20.2 | 17.6 | 20.5 |
| Miscellaneous sleep/hypnotic/barbiturates (%) | 7.2 | 5.9 | 8.7 | 7.5 | 6.8 | 6.3 |
| Preventive health services | ||||||
| Flu shot (%) | 20.6 | 24.5 | 14.4 | 15.9 | 23.3 | 24.2 |
| Pneumococcal vaccination (%) | 7.4 | 7.6 | 7.5 | 8.0 | 6.7 | 7.0 |
| Mammography (%) | 31.9 | 39.3 | 24.8 | 34.3 | 45.2 | 48.4 |
| Papanicolaou (PAP) test (%) | 12.2 | 15.5 | 9.6 | 12.2 | 22.9 | 23.8 |
| Bone mineral density test (%) | 28.4 | 38.5 | 13.7 | 20.1 | 28.9 | 35.6 |
| Lipid testing (%) | 25.6 | 26.1 | 21.2 | 24.5 | 27.9 | 28.5 |
Adherence determined using a 180-day ascertainment period and defined using a measure of compliance, the PDC; low: PDC ≤ 50%, high: PDC ≥ 80%. Characteristics among intermediate adherence groups generally fall in between low and high adherence groups
Adjusting for covariates, better adherence to bisphosphonates significantly reduced the risk for non-vertebral fractures (high versus low adherence HR = 0.53, 95%CI = 0.38–0.74), and hip fracture specifically (high versus low adherence HR = 0.44, 95%CI = 0.27–0.70) among the secondary prevention cohort. However, no evidence of fracture benefit was observed among the primary prevention cohort of new bisphosphonate users (Table 2). We also document little difference in fracture rates between levels of adherence to calcitonin (primary or secondary prevention) or raloxifene for secondary prevention. Contrary to our hypothesis that we would document lower rates of fracture with better adherence to raloxifene for primary prevention, we document significantly higher rates of non-vertebral fractures among high versus low adherent raloxifene users (adjusted HR = 2.01, 95%CI = 1.04–3.87) (Table 2).
Table 2.
Association between adherence to pharmacotherapy for primary versus secondary prevention among new users of bisphosphonates, calcitonin, or raloxifene on rates of non-vertebral fracture
| Primary prevention | Secondary prevention | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Unadjusted | Adjustedb | Events | Unadjusted | Adjustedb | |||||||||
| N | n | Ratea | HR | 95%CI | HR | 95%CI | N | n | Ratea | HR | 95%CI | HR | 95%CI | |
| Bisphosphonate | 16,099 | 160 | 1.1 | 4,106 | 168 | 4.4 | ||||||||
| Low | 5,826 | 59 | 1.1 | 1.00 | Reference | 1.00 | Reference | 1,394 | 79 | 6.3 | 1.00 | Reference | 1.00 | Reference |
| Intermediate | 2,326 | 18 | 0.8 | 0.76 | 0.45–1.29 | 0.77 | 0.54–1.11 | 602 | 24 | 4.3 | 0.69 | 0.44–1.10 | 0.71 | 0.44–1.12 |
| High | 7,947 | 83 | 1.1 | 1.02 | 0.73–1.43 | 1.07 | 0.85–1.35 | 2,110 | 65 | 3.3 | 0.52 | 0.38–0.73 | 0.53 | 0.38–0.74 |
| Calcitonin | 6,625 | 209 | 3.4 | 2,429 | 129 | 5.8 | ||||||||
| Low | 4,396 | 145 | 3.5 | 1.00 | Reference | 1.00 | Reference | 1,554 | 84 | 5.9 | 1.00 | Reference | 1.00 | Reference |
| Intermediate | 1,537 | 43 | 2.9 | 0.84 | 0.59–1.17 | 0.90 | 0.60–1.20 | 631 | 31 | 5.3 | 0.90 | 0.60–1.36 | 0.90 | 0.60–1.40 |
| High | 692 | 21 | 3.2 | 0.91 | 0.57–1.43 | 1.00 | 0.60–1.50 | 244 | 14 | 6.3 | 1.05 | 0.60–1.86 | 1.20 | 0.70–2.10 |
| Raloxifene | 3,042 | 56 | 1.9 | 396 | 19 | 5.1 | ||||||||
| Low | 1,047 | 13 | 1.3 | 1.00 | Reference | 1.00 | Reference | 131 | –c | 4.9 | 1.00 | Reference | 1.00 | Reference |
| Intermediate | 402 | –c | 1.8 | 1.40 | 0.56–3.52 | 1.53 | 0.60–3.88 | 50 | –c | 4.2 | 0.85 | 0.17–4.23 | 0.49 | 0.07–3.56 |
| High | 1,593 | 36 | 2.3 | 1.83 | 0.97–3.44 | 2.01 | 1.04–3.87 | 215 | 11 | 5.4 | 1.11 | 0.41–2.99 | 1.23 | 0.33–4.63 |
Non-vertebral fracture rate/100 person-years of observation
Adjusted for all variables listed in Table 4 in Appendix
Number of events fewer than 11 are not reported to adhere with Centers for Medicare and Medicaid Services data reporting guidelines
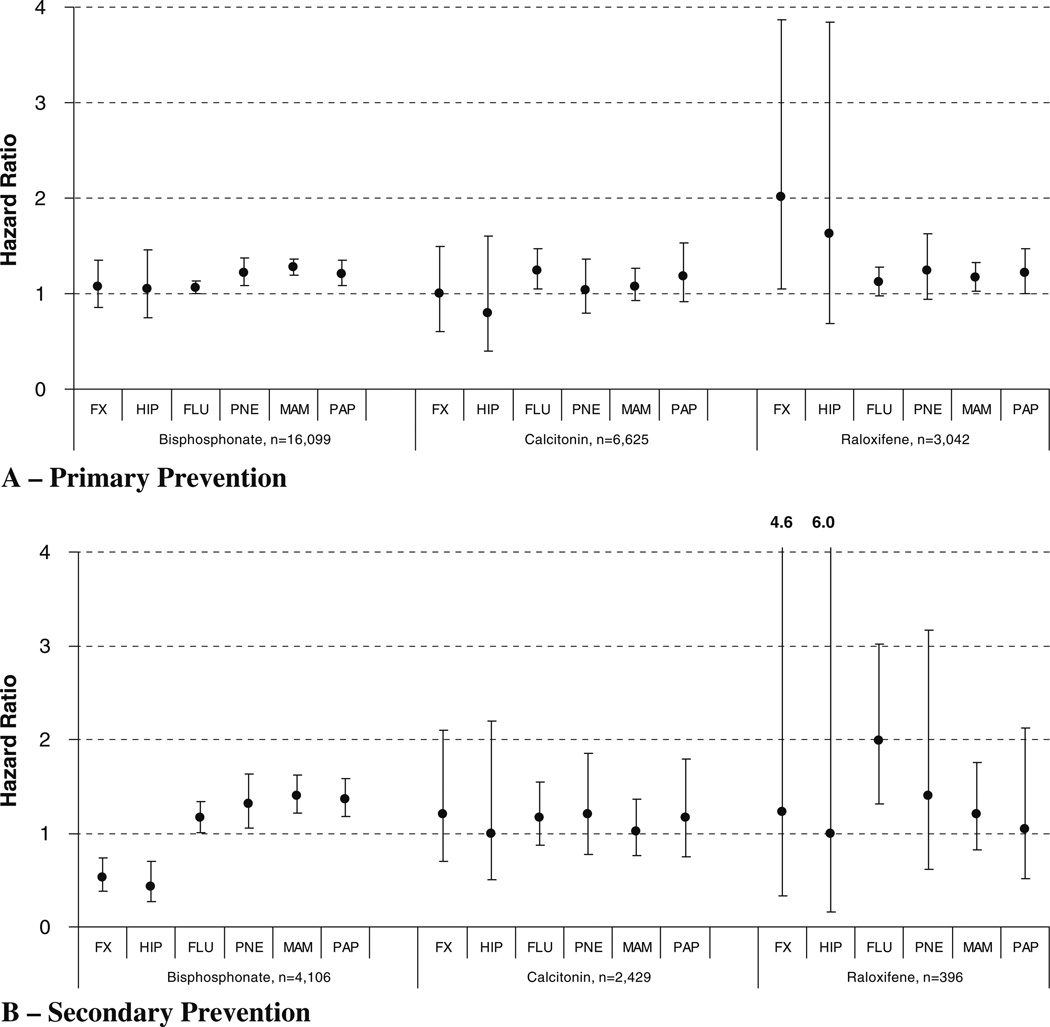
When considering rates of vaccination and preventive health screening, we generally document higher rates among high compared to low adherent users (Fig. 2). In each case, high adherent users were more likely to have vaccinations for influenza and pneumonia and were also more likely to have mammography and PAP testing, with hazard ratios consistently >1 compared to patients with low adherence. However, differences were only statistically higher among bisphosphonate users—for whom we had more patients for comparison (Fig. 2).
Fig. 2.
Adjusted risk (hazard ratio and 95% confidence intervals) for non-vertebral fracture and preventive healthcare services for highly adherent versus low adherent users of oral bisphosphonates, calcitonin and raloxifene in primary prevention (a) and secondary prevention (b) cohorts. FX hip, humerus, radius, or ulna fracture (adjusts for fracture risk factors), HIP hip fracture (adjusts for fracture risk factors), FLU influenza vaccination (adjusts for age, hospitalization, comorbidity, number of drugs, and prior influenza vaccinations), PNE pneumoccocal vaccination (adjusts for age, hospitalization, comorbidity, number of drugs and prior pneumoccocal vaccination), MAM mammography (adjusts for age, hospitalization, comorbidity, number of drugs, and prior mammography), PAP Papanicolaou testing (adjusts for age, hospitalization, comorbidity, number of drugs, and prior PAP testing)
Discussion
We document that better adherence to bisphosphonates lowered non-vertebral fracture risk among those with prior fracture and observed no evidence of fracture reduction with better adherence to calcitonin or raloxifene (primary or secondary prevention). These data do not support our hypothesis that healthy adherer effects bias the association between better adherence to osteoporosis pharmacotherapy and fracture risk because our results are consistent with trial evidence. Rather, our result corroborate a recent meta-analysis that finds similar effect estimates between better adherence to bisphosphonates as documented in non-experimental studies to drug effects reported in clinical trials [5].
Contrary to expected, we document an increased risk for non-vertebral fracture with better adherence to raloxifene for primary prevention. We believe that this finding may be related to the heterogeneity of risk among patients who are prescribed osteoporosis pharmacotherapy for primary prevention. Osteoporosis guidelines recommend treatment to prevent osteoporosis; however, clinical trials document fracture prevention among those with osteoporosis or low BMD [2]. Therefore, treatment among those without low BMD may not be effective in reducing fracture risk. Prior evidence also suggests that raloxifene users are healthier and have lower risk for fracture compared with bisphosphonate users [22, 23]. Raloxifene users may therefore be prescribed more often at higher levels of BMD than patients treated with bisphosphonates. Patients with a better understanding of their clinical need for treatment based on BMD test results also adhere better to therapy [24]. Indeed, we document that osteoporosis diagnosis was more frequent among the high versus low adherence groups. Our observation that better adherence to raloxifene for primary prevention is associated with increased fracture risk is likely due to residual confounding, i.e., in primary prevention, patients with better adherence to raloxifene may have a higher background risk for fracture that is not adequately measured by healthcare utilization data.
Our findings of no benefit for primary prevention with bisphosphonatesmay seemin contrast to the FOSIT Study that identified non-vertebral fracture reduction with alendronate versus placebo among postmenopausal women with lumbar spine BMD T-score ≤ −2 SD, yet otherwise healthy [19]. However, as stated above, osteoporosis guidelines recommend treatment to prevent osteoporosis, yet clinical trials document fracture prevention among those with osteoporosis or low BMD [2]. Our finding may therefore suggest that bisphosphonate do not prevent fractures among those without low BMD. Alternatively, similar to our hypothesis that patients with better adherence to raloxifene have a higher background risk for fracture that is not adequately measured by healthcare utilization data, our results may reflect residual confounding. Unfortunately, we do not have BMD data to test our hypothesis that patients with high adherence to osteoporosis pharmacotherapy for primary prevention have lower BMD compared to patients with low adherence. We therefore encourage caution in the interpretation of our results that examine the association between level of adherence to osteoporosis pharmacotherapy and non-vertebral fracture risk for primary prevention. Better understanding of fracture reduction among bisphosphonates for primary prevention, as well as characteristics of people with high versus low levels of adherence to osteoporosis medications, is needed.
We recently identified a positive association between adherence to statin therapy and future vaccination (influenza or pneumonia) rates as well as breast, prostate, or colon cancer screening rates [25]. This finding provides some evidence that patients who adhere to statins are also more likely to use preventive health services. Another recent study identified that adherent statin users had lower rates of outcomes that should not be causally affected by statin exposure, such as workplace and motor vehicle accidents [26]. In our current analysis, we also document that highly adherent patients were more likely to use preventive healthcare (vaccinations and preventive screening tests) than patients with low adherence. Our data therefore support the notion that patients who adhere to preventive pharmacotherapy for asymptomatic conditions are different in many ways from patients who do not adhere to treatment. We are limited to healthcare utilization data and therefore cannot comment on other potential differences between high versus low adherence groups, such as differences in exercise, diet, calcium/vitamin D supplementation, cigarette smoking, or alcohol intake.
Nonetheless, we document little evidence of bias due to healthy adherer effects on fractures, the clinically relevant outcome in osteoporosis. Our data therefore suggest that fracture risk among frail seniors may be an outcome that is not strongly related to healthy behaviors. Alternatively, our data may add to literature suggesting that there is little evidence to support healthy adherer bias in secondary prevention of fractures. Prior evidence suggests that patients fail to associate fracture with underlying osteoporosis or future fracture risk [27, 28]. Data also identify that over half of women treated with osteoporosis pharmacotherapy have vitamin D deficiency [29]. However, the same study found that lack of exercise, absence of discussion with a physician regarding the importance of vitamin D to bone health, and education less than grade 12 were independently associated with vitamin D inadequacy [29]. If exercise, better communication with a physician regarding the importance of osteoporosis pharmacotherapy, and higher education are also associated with better adherence, then the association between better adherence to osteoporosis pharmacotherapy and fracture reduction may be partly attributed to residual confounding related to healthy adherer bias. It has also become clearer that calcium and vitamin D supplementation may reduce fracture risk through improvements in BMD and reduced risk of falls [30]. Therefore, further study is needed to clarify our findings that suggest little evidence of healthy adherer bias when studying the association between better adherence to osteoporosis pharmacotherapy and fracture risk reduction.
In addition to those already mentioned, three study limitations are worth noting. First, recent evidence identifies that many patients who discontinue osteoporosis pharmacotherapy reinitiate treatment after an extended gap in therapy [31, 32]. However, these studies defined a minimum gap length of 60 or 105 days, and we had complete drug data that documented no use within the previous 365 days. Evidence also suggests that the longer the period of time without therapy, the less likely a patient is to return to treatment [18]. Therefore, although it is possible that some patients included in our study had previously used osteoporosis drugs, we estimate that there would be few.
Second, we may have misclassified some women when dividing our sample into cohorts of primary and secondary prevention. We defined secondary prevention as any fracture diagnosis within the year prior to treatment initiation. We used this definition because we were most interested in studying the concept of healthy adherer bias and hypothesized that patients who had experienced a fracture within the year prior to treatment initiation would generally be more motivated to adhere to osteoporosis pharmacotherapy compared to an asymptomatic cohort. However, some women that we categorized in the primary prevention cohort may be more correctly classified as having being treated for secondary prevention with prior fracture occurring before the year prior to treatment initiation. This misclassification may have contributed to residual confounding among the primary prevention cohort, with women having had a fracture and misclassified as being treated for primary prevention, also being more likely to adhere to pharmacotherapy. Unfortunately, we are limited to a 1-year baseline period to identify fracture history and thus cannot examine potential misclassification based on fracture history occurring more than a year prior to treatment initiation.
Third, although we identify no evidence of healthy user bias when studying the impact of adherence to osteoporosis medication on fracture risk reduction, our results may not generalize to other populations. We studied a cohort of older frail women in whom there may be less room for healthy adherer bias because the range of fracture risk may be smaller than in a general population of younger new users (spectrum effect). We were also limited to a small number of raloxifene users, particularly for secondary prevention—that translated into few fracture and thus limited ability to identify differences in fracture risk between levels of adherence for secondary prevention.
Future study is warranted to more closely examine potential healthy adherer effects when studying the relationship between bisphosphonate adherence and fracture reduction in other populations. In fact, recent analysis of the placebo arm of the Women’s Health Initiative trial identifies an association between better adherence to placebo and hip fracture reduction (HR = 0.50, 95%CI = 0.32, 0.79) [10]. Therefore, although our results find little evidence of bias due to healthy adherer effects in a study of the effects of osteoporosis drugs on fracture risk, we cannot comment on potential healthy adherer effects on fracture risk attributed to better adherence to non-osteoporosis drugs. In addition, although relying on healthcare utilization data to study the effects of adherence to osteoporosis medications on fracture risk may not be confounded by healthy adherer bias in our study, this does not mean that healthy adherer bias may not be of concern when studying adherence to osteoporosis medications on other outcomes. Evidence from placebo arms of clinical trials identify that better adherence to placebo reduces mortality [8, 9] and fracture risk [10]. These data clearly support the concept of healthy adherer bias. A further study that considers different patient populations and different outcomes is important. We therefore suggest caution in the interpretation of our results in light of caveats highlighted and encourage more research to study potential healthy adherer bias.
Acknowledgments
The authors thank Raisa Levin for preparing the study data for analysis and kindly acknowledge helpful discussions with Drs. Jerry Avorn and Robert J. Glynn, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Harvard Medical School. Dr Brookhart is supported by the National Institute on Aging (K25 AG027400), Dr Cadarette is supported by the Canadian Institutes of Health Research (New Investigator Award in the Area of Aging and Osteoporosis, MSH95364), Dr Katz is supported by the National Institute of Arthritis and Muskuloskeletal and Skin Diseases (K24 AR02123 and P60 AR47782), and Dr Solomon is supported by the Arthritis Foundation and the National Institutes of Health (K24 AR055989, R21 AG027066 and P60 AR47782).
Footnotes
Conflicts of interest Co-authors have received salary support for research grants awarded to the Brigham and Women’s Hospital for unrelated work from: Amgen (Drs Brookhart and Solomon) and Novartis (Dr Solomon). There was no pharmaceutical industry support for this study.
Contributor Information
S. M. Cadarette, Leslie Dan Faculty of Pharmacy, University of Toronto, 144 College Street, Toronto, ON, Canada M5S 3M2, s.cadarette@utoronto.ca Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA.
D. H. Solomon, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Boston, MA, USA Harvard Medical School, Boston, MA, USA; Division of Rheumatology, Immunology and Allergy, Brigham and Women’s Hospital, Boston, MA, USA.
J. N. Katz, Harvard Medical School, Boston, MA, USA Division of Rheumatology, Immunology and Allergy, Brigham and Women’s Hospital, Boston, MA, USA; Department of Orthopedic Surgery, Brigham and Women’s Hospital, Boston, MA, USA.
A. R. Patrick, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Boston, MA, USA Harvard Medical School, Boston, MA, USA.
M. A. Brookhart, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Boston, MA, USA Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 3.Kothawala P, Badamgarav E, Ryu S, et al. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82:1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 4.Siris ES, Selby PL, Saag KG, et al. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009;122:S3–S13. doi: 10.1016/j.amjmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Wilkes MM, Navickis RJ, Chan WW, et al. Bisphosphonates and osteoporotic fractures: a cross-design synthesis of results among compliant/persistent postmenopausal women in clinical practice versus randomized controlled trials. Osteoporos Int. 2010;21:679–688. doi: 10.1007/s00198-009-0991-1. [DOI] [PubMed] [Google Scholar]
- 6.Rabenda V, Hiligsmann M, Reginster JY. Poor adherence to oral bisphosphonate treatment and its consequences: a review of the evidence. Expert Opin Pharmacother. 2009;10:2303–2315. doi: 10.1517/14656560903140533. [DOI] [PubMed] [Google Scholar]
- 7.Imaz I, Zegarra P, Gonzalez-Enriquez J, et al. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010 doi: 10.1007/s00198-009-1134-4. [DOI] [PubMed] [Google Scholar]
- 8.Granger BB, Swedberg K, Ekman I, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 9.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis J, Larson J, Delzell E, et al. Does the benefit of medication adherence relate more to a drug effect or the behavior itself? Quantifying the effect of adherence behavior using data from the placebo arms of the WHI. Arthritis Rheum. 2009;60 (Suppl 10):613. (abstract) [Google Scholar]
- 11.American College of Physicians. Information on cost-effectiveness: an essential product of a national comparative effectiveness program. Ann Intern Med. 2008:956–961. doi: 10.7326/0003-4819-148-12-200806170-00222. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Jama. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 13.Janz NK, Champion VL, Strecher VJ. The health belief model. In: Glanz K, Rimer BK, Lewis FM, editors. Health behavior and health education: theory, research, and practice. San Francisco: Jossey-Bass; 2002. pp. 45–66. [Google Scholar]
- 14.DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care. 2007;45:521–528. doi: 10.1097/MLR.0b013e318032937e. [DOI] [PubMed] [Google Scholar]
- 15.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 16.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 17.Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 18.Cadarette SM, Burden AM. Measuring and improving adherence to osteoporosis pharmacotherapy. Curr Opin Rheumatol. 2010 doi: 10.1097/BOR.0b013e32833ac7fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pols HA, Felsenberg D, Hanley DA, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT Study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–468. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- 20.Suissa S. Immeasurable time bias in observational studies of drug effects on mortality. Am J Epidemiol. 2008;2008:329–335. doi: 10.1093/aje/kwn135. [DOI] [PubMed] [Google Scholar]
- 21.Ray WA, Griffin MR, Fought RL, et al. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 22.Cadarette SM, Katz JN, Brookhart MA, et al. Relative effectiveness of osteoporosis drugs for preventing nonvertebral fracture. Ann Intern Med. 2008;148:637–646. doi: 10.7326/0003-4819-148-9-200805060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster SA, Foley KA, Meadows ES, et al. Characteristics of patients initiating raloxifene compared to those initiating bisphosphonates. BMC Womens Health. 2008;8:24. doi: 10.1186/1472-6874-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickney CS, Arnason JA. Correlation between patient recall of bone densitometry results and subsequent treatment adherence. Osteoporos Int. 2005;16:1156–1160. doi: 10.1007/s00198-004-1818-8. [DOI] [PubMed] [Google Scholar]
- 25.Brookhart MA, Patrick AR, Avorn J, et al. Adherence to lipid-lowering therapy and the use of preventive health care services: an investigation of the healthy user effect. Am J Epidemiol. 2007;120:251–256. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 26.Dormuth CR, Patrick AR, Shrank WH, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119:2051–2057. doi: 10.1161/CIRCULATIONAHA.108.824151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giangregorio L, Dolovich L, Cranney A, et al. Osteoporosis risk perceptions among patients who have sustained a fragility fracture. Patient Educ Couns. 2009;74:213–220. doi: 10.1016/j.pec.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sale JEM, Beaton DE, Sujic R, et al. “If it was osteoporosis, I would have really hurt myself.” Ambiguity about osteoporosis and osteoporosis care despite a screening program to educate fragility fracture patients. J Eval Clin Pract. 2010;16:590–596. doi: 10.1111/j.1365-2753.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 29.Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 30.Miller PD, Derman RJ. What is the best balance of benefits and risks among anti-resorptive therapies for postmenopausal osteoporosis? Osteoporos Int. 2010 doi: 10.1007/s00198-010-1208-3. [DOI] [PubMed] [Google Scholar]
- 31.Brookhart MA, Avorn J, Katz JN, et al. Gaps in treatment among users of osteoporosis medications: the dynamics of noncompliance. Am J Med. 2007;120:251–256. doi: 10.1016/j.amjmed.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Roughead EE, Ramsay E, Priess K, et al. Medication adherence, first episode duration, overall duration and time without therapy: the example of bisphosphonates. Pharmacoepidemiol Drug Saf. 2009;18:69–75. doi: 10.1002/pds.1687. [DOI] [PubMed] [Google Scholar]