Summary
Objectives
The goal of this study was to use protein array analysis to investigate temporal regulation of stimulated cytokine expression in trigeminal ganglia and spinal trigeminal nuclei in response to cotreatment of sumatriptan and naproxen sodium or individual drug.
Background
Activation of neurons and glia in trigeminal ganglia and spinal trigeminal nuclei leads to increased levels of cytokines that promote peripheral and central sensitization, which are key events in migraine pathology. While recent clinical studies have provided evidence that a combination of sumatriptan and naproxen sodium is more efficacious in treating migraine than either drug alone, it is not well understood why the combination therapy is superior to monotherapy.
Methods
Male Sprague Dawley rats were left untreated (control), injected with capsaicin, or pre-treated with sumatriptan/naproxen, sumatriptan, or naproxen for 1 hour prior to capsaicin. Trigeminal ganglia and spinal trigeminal nuclei were isolated 2 and 24 hours after capsaicin or drug treatment and levels of 90 proteins were determined using a RayBio® Label-Based Rat Antibody Array.
Results
Capsaicin stimulated a >3-fold increase in expression of the majority of cytokines in trigeminal ganglia at 2 hours that was sustained at 24 hours. Significantly, treatment with sumatriptan/naproxen almost completely abolished the stimulatory effects of capsaicin at 2 and 24 hours. Capsaicin stimulated >3-fold expression of more proteins in spinal trigeminal nuclei at 24 hours when compared to 2 hours. Similarly, sumatriptan/naproxen abolished capsaicin stimulation of proteins in spinal trigeminal nuclei at 2 hours and greatly suppressed protein expression 24 hours post capsaicin injection. Interestingly, treatment with sumatriptan alone suppressed expression of different cytokines in trigeminal ganglia and spinal trigeminal nuclei than repressed by naproxen sodium.
Conclusion
We found that the combination of sumatriptan/naproxen was effective in blocking capsaicin stimulation of pro-inflammatory proteins implicated in the development of peripheral and central sensitization in response to capsaicin activation of trigeminal neurons. Based on our findings that sumatriptan and naproxen regulate expression of different proteins in trigeminal ganglia and spinal trigeminal nuclei, we propose that these drugs function on therapeutically distinct cellular targets to suppress inflammation and pain associated with migraine.
Keywords: trigeminal ganglion, spinal trigeminal nuclei, sumatriptan, naproxen sodium, array
Introduction
Migraine is a prevalent neurological disease that is characterized by intense head pain and often accompanied by sensitivity to light and sound as well as other autonomic changes.1, 2 The pain associated with migraine attacks is thought to be mediated via activation of trigeminal nerves that provide sensory innervation to the dura and major cerebral blood vessels and thus, are responsible for conduction of nociceptive signals from peripheral tissues to the central nervous system.3 Based on preclinical and clinical data, migraine pathology involves both peripheral and central sensitization.4 Peripheral sensitization, which occurs at the beginning of a migraine attack and is thought to be of short duration, lowers that activation threshold of primary nociceptive neurons to inflammatory or noxious stimuli. In contrast, central sensitization, which has a later onset and is of much longer duration, involves lowering the activation threshold of second and third order neurons that contributes to hyperalgesia and allodynia. Development and maintenance of peripheral and central sensitization is now known to be promoted by increased expression and release of pro-inflammatory proteins including cytokines.5
Cytokines are a large family of structurally diverse proteins that promote inflammation and participate in almost all aspects of both innate and adaptive immune responses.6 The cytokines, which include interleukins, lymphokines, chemokines, and cell signal molecules, function by mediating cellular processes such as hematopoiesis, apoptosis, cell migration, and cell proliferation, and activation of pro-inflammatory signaling pathways.5, 6 While known to play an important role in immune system function, increased expression of cytokines is now implicated in the development and maintenance of sensitization of peripheral nociceptors and second order nociceptive neurons.5–7 Cytokines are synthesized and released by glial cells associated with peripheral and central neurons.8–11 The importance of glial cells in the underlying pathology of many neurological diseases is now recognized given their central role in regulating the extracellular environment around neurons and hence, neuronal excitability.12, 13 In particular, satellite glial cells are known to modulate the environment around neuronal cell bodies within trigeminal ganglia while a similar function is performed by astrocytes and microglia within the spinal cord and brain. Importantly, increased cytokine release from glial cells is thought to promote prolonged inflammation and a pathological pain state by increasing the number and function of ion channels, receptors, and signaling proteins that leads to a chronic hyperexcitable state in nociceptive neurons.14–16 Elevated levels of several pro-inflammatory cytokines and chemokines have been reported during migraine, menstrual migraine, as well as chronic migraine.17, 18
The two most popular classes of drugs used as abortive treatment for migraine are the triptans and non-steroidal anti-inflammatory drugs (NSAIDs). Although both drug classes are known to be effective migraine therapies, the mechanisms by which they function are thought to involve different cellular targets. Triptans are a class of selective serotonergic drugs that inhibit the release of neurotransmitters from activated trigeminal neurons.19, 20 While repression of neurotransmitter release in peripheral tissues, such as the dura, would block inflammation and peripheral sensitization, suppression of neurotransmitter secretion in the spinal cord and brainstem would prevent activation of second order neurons and spinal glial cells, and hence the development of central sensitization. On the other hand, the primary targets of NSAIDs such as naproxen are the COX-1 and COX-2 enzymes that are responsible for the production of prostaglandins, lipid compounds that promote inflammation and pain.21 Significantly, results from several studies have provided evidence that a single tablet of sumatriptan succinate and naproxen sodium (sumatriptan/naproxen) is superior to monotherapy for the treatment of migraine 22–24. While it has been proposed that sumatriptan and naproxen are likely to function in different ways to treat the diverse symptoms of migraine, the relative contributions of each drug within the trigeminal system have not been extensively investigated. Therefore, the goal of our study was to determine the effects of sumatriptan/naproxen or either drug alone on expression of multiple cytokines and signaling proteins within trigeminal ganglia and spinal trigeminal nucleus in response to stimulation of trigeminal neurons by the noxious agent capsaicin.
METHODS
Animals
All animal experimental procedures were conducted in accordance with institutional and National Institutes of Health guidelines. Male Sprague–Dawley rats (Charles River, Wilmington, MA) were housed in plastic cages on a 12 h light/dark cycle with unrestricted access to food and water. Every effort was made to minimize animal suffering and reduce the number of animals used.
In Vivo Treatments and Tissue Isolation
Male rats (250–300 g) were anesthetized by isoflurane inhalation (2.5–3%). Assessment of proper anesthesia was performed by monitoring writhing and tail flick reflexes. For induction of acute inflammation, rats were injected bilaterally in the eyebrow regions (25 µL total) with 1 µM capsaicin (diluted in 100% dimethylsulfoxide; Sigma-Aldrich, St. Louis, MO). To test the effect of different drug treatments, animals (n = 3 for each condition) were treated with systemic sumatriptan/naproxen (0.3 mg/kg; GlaxoSmithKline, Research Triangle Park, NC /2.8 mg/kg i.p.; Sigma-Aldrich) or pre-treated with sumatriptan/naproxen, naproxen (2.8 mg/kg i.p.), or sumatriptan (300 mg/kg i.p.) for 1 hour prior to capsaicin injection to allow time for absorption of drug. As controls, some rats (n = 3) did not receive injections. Both trigeminal ganglia and sections of the upper spinal cord containing the spinomedulary junction (Vc/C1) transition zone were isolated 2 and 24 hours after capsaicin injections following CO2 asphyxiation.
Array Analysis
The intracellular levels of 90 proteins were determined using the L Series 90: RayBio® Label-Based Rat Antibody Array 1 per manufacturer’s instructions (RayBiotech, Norcross, GA). Briefly, one trigeminal ganglion containing all three regions and sections of upper spinal cord containing spinal trigeminal nuclei (4–5 mm posterior to the obex) from each animal were homogenized in lysis buffer and dialyzed in phosphate buffered saline (PBS; pH 8.0) for a minimum of 9 hours with 3 PBS changes. Protein levels were determined by Bradford assay and diluted to 2 mg/mL in PBS (pH 8.0). Samples were incubated with labeling reagent and washed per manufacturer’s instructions. Samples were diluted 1:50 in blocking buffer and incubated on pre-blocked arrays overnight at 4°C. Membranes were then incubated in streptavadin-conjugated peroxidase for 2 hours and exposed to a peroxidase substrate for 5 min prior to developing on X-ray film. Densiometric analysis was performed on a Kodak ImageStation 4000M (Eastman Kodak Company, Rochester, NY) with background subtraction from spot edges. Spot data were normalized to a positive control spot on each array.
Statistics
Statistical analysis was performed with a non-parametric Mann–Whitney U-test. Differences were considered statistically significant at p < 0.05. All statistical tests were performed using SPSS Statistical Software, Release 16 (Chicago, IL).
RESULTS
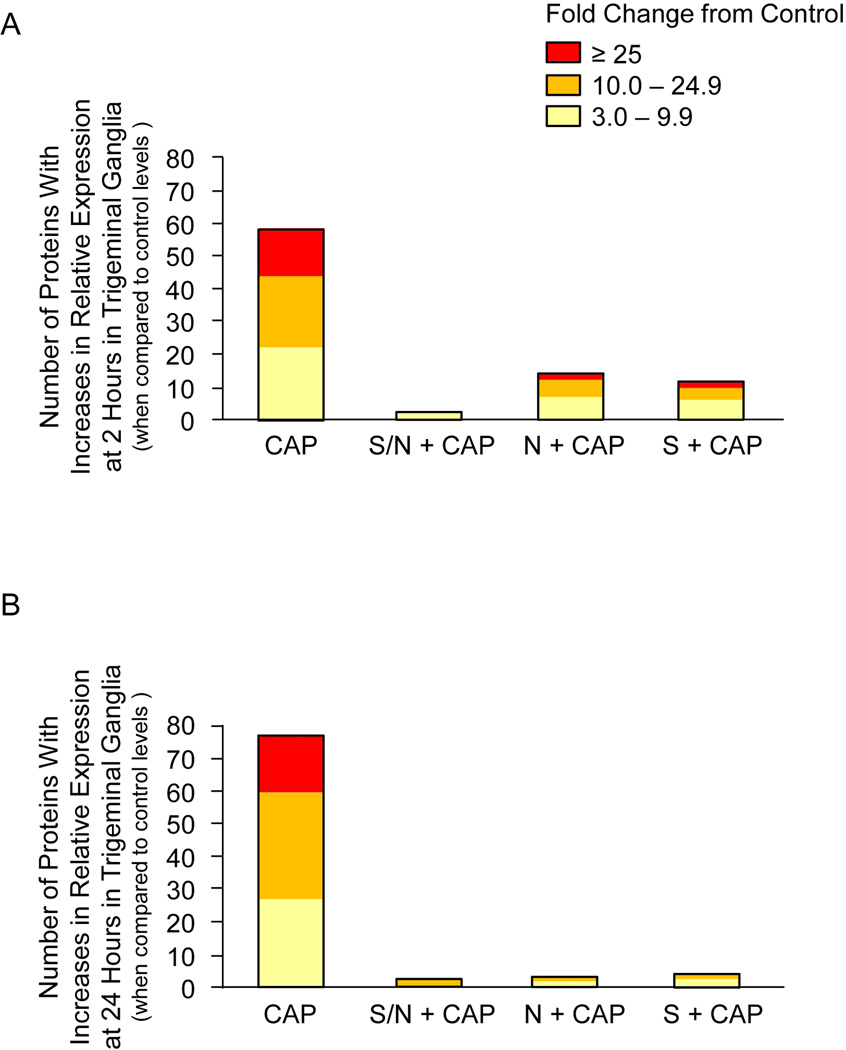
To investigate the temporal and tissue specific effects of sumatriptan/naproxen treatment, trigeminal ganglia and spinal trigeminal nucleus tissues obtained from male rats were homogenized and the expression of 90 cytokines and signaling proteins analyzed using RayBio® Label-Based Rat Antibody Arrays. The average fold change ± SEM from control, whose mean was made equal to one, for all proteins under each experimental condition and time point is reported in Excel formatted tables (Supplementary Materials). Only proteins whose mean was >3 fold above control levels and were significantly different from control (p < 0.05) were included in the figures. As seen in Figure 1A, capsaicin injections in the V1 region stimulated a >3-fold increase in expression of 57 proteins in the trigeminal ganglion at 2 hours. While 20 proteins were stimulated between 3.0–9.9 fold, 23 proteins were increased 10–24.9 fold and an additional 14 proteins more than 25 fold when compared to control levels. Importantly, treatment with sumatriptan/naproxen almost completely abolished the stimulatory effects of capsaicin at 2 hours by reducing both the number of stimulated proteins as well as the relative amount of stimulation (3–9.9 fold range). Treatment with naproxen or sumatriptan alone was not as effective in repressing capsaicin mediated increases in protein expression when compared to animals that received both drugs. In response to naproxen, the levels of 14 proteins were still elevated >3 fold above control while only 11 proteins were still increased following sumatriptan treatment. Interestingly, only 5 capsaicin stimulated proteins in the trigeminal ganglia were similarly repressed by treatment with naproxen or sumatriptan alone at the 2 hour time point (Table 1). In contrast, twenty-five proteins were differentially regulated by naproxen or sumatriptan at 2 hours post capsaicin injection (Table 2). The effects of sumatriptan/naproxen or either drug alone on protein expression in trigeminal ganglia 24 hours after capsaicin stimulation of V1 trigeminal neurons is shown in Figure 1B. The levels of an even greater number of proteins were increased >3 fold above control levels 24 hours after capsaicin treatment when compared to 2 hours post injection. While the levels of 26 proteins were elevated 3.0–9.9 fold, 34 proteins were increased 10.0–24.9 fold, and 15 were stimulated greater than 25.0 fold. Similar to the 2 hour time point, sumatriptan/naproxen treatment greatly repressed the stimulatory effects of capsaicin on cytokine expression with only 2 proteins having levels >3 fold than control. Treatment with only naproxen or sumatriptan was also very effective in suppressing capsaicin stimulation at 24 hours with levels of only 2 proteins elevated in response to naproxen and 3 elevated following treatment with sumatriptan. While the level of only one protein was repressed in response to treatment with naproxen or sumatriptan at the 24 hour time point after capsaicin stimulation (Table 1), five proteins were differentially regulated by each treatment alone (Table 2). Taken together, these data provide evidence that sumatriptan/naproxen treatment is more effective at repressing capsaicin stimulated cytokine and signaling protein expression at 2 hours than either drug alone, and that naproxen and sumatriptan regulate the expression of different proteins in trigeminal ganglia.
Figure 1.
Sumatriptan/naproxen combination treatment suppresses capsaicin stimulated changes in the number and level of cytokine expression in trigeminal ganglia tissues at 2 (A) and 24 hours (B). Trigeminal ganglia were obtained from animals treated with capsaicin (CAP) alone, or cotreated with capsaicin and naproxen (N), sumatriptan (S), or the combination of both drugs (S/N). The height of each box corresponds to the number of proteins significantly (p < 0.05) elevated above untreated control levels, whose mean was made equal to one. The average fold change above control levels is illustrated by different colors. n = 3 independent experiments for each condition.
Table 1.
Capsaicin stimulated proteins similarly repressed by treatment with either naproxen sodium or sumatriptan in trigeminal ganglia.
| 2 Hr | 24 Hr |
|---|---|
| GM-CSF IFN-γ CSK CINC-2β MDC |
B7-1 |
Table 2.
Capsaicin stimulated proteins differentially regulated by treatment with either naproxen sodium or sumatriptan in trigeminal ganglia.
| 2 Hr | 24 Hr | ||
|---|---|---|---|
| N + CAP | S + CAP | N + CAP | S + CAP |
| β-NGF B7-1 CNTF Rα ICK IL-1α Osteopontin TIMP-1 TIMP-3 TNFα TRAIL Ubiquitin |
FADD Fas Fractalkine GHR ICAM-1 IL-13 IL-5 IL-6 Leptin MCP-1 RELMb Resistin TAL1A TGFβ2 |
Osteopontin | Hepassocin IL-5 Prolactin R TGFβ3 |
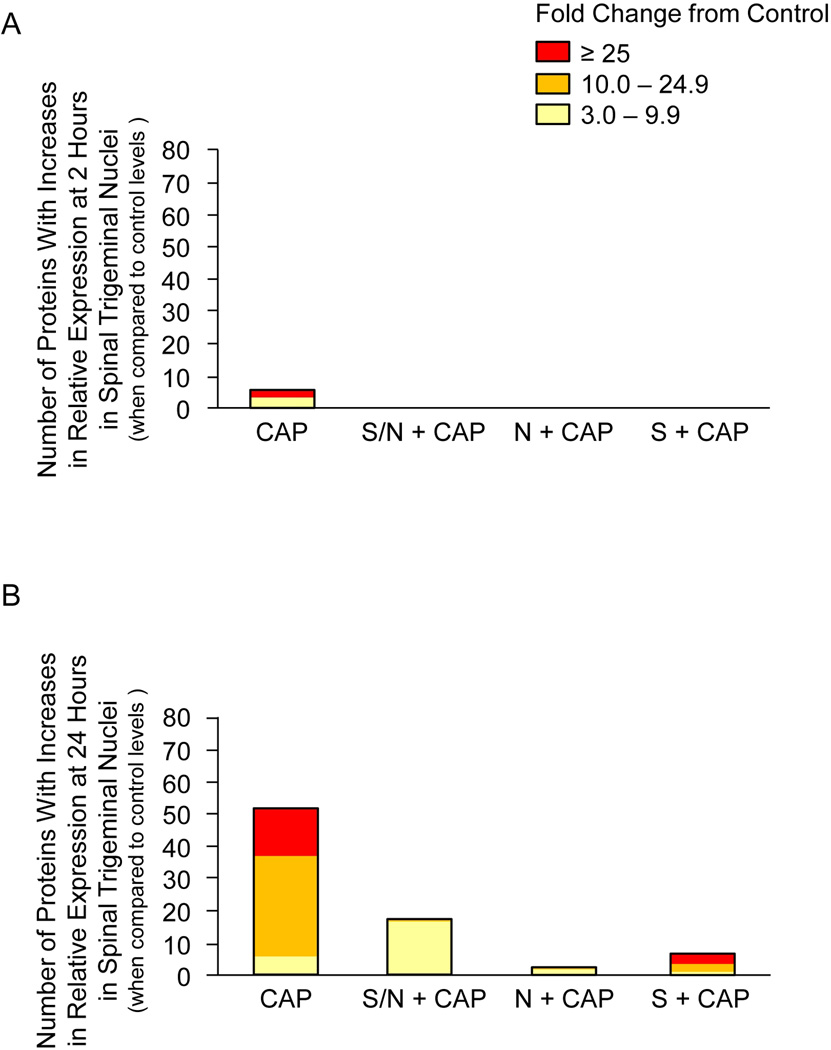
To determine changes in spinal cord tissue that contain centrally projecting fibers from trigeminal ganglia neurons, the same set of proteins was analyzed in tissues containing the spinal trigeminal nucleus. In contrast to the effect of capsaicin in trigeminal ganglia, capsaicin injections in the V1 region stimulated a >3 fold increase in expression of only 5 proteins in the spinal trigeminal nucleus at 2 hours (Figure 2A). However, each of the treatments was equally effective in repressing the stimulatory effects of capsaicin since none of the protein levels were significantly different than untreated control levels. At 24 hours, capsaicin stimulated >3 fold the expression of 53 different proteins in the spinal trigeminal nucleus (Figure 2B). While 6 proteins had levels elevated 3–9.9 fold above control, 31 proteins were increased 10–24.9 fold and another 16 proteins were stimulated >25 fold. Treatment with sumatriptan/naproxen greatly repressed the number and level of protein expression 24 hours after capsaicin injection with levels all in the range of 3–9.9 fold greater than control. Similarly, naproxen and sumatriptan treatment alone were effective in suppressing capsaicin mediated increases in cytokine and signaling protein levels. As seen in trigeminal ganglia tissue, naproxen and sumatriptan differentially regulate the expression of proteins in the spinal trigeminal nucleus at both the 2 and 24 time points (Table 3). In summary, capsaicin mediated changes in the number and level of cytokine and signaling protein expression in spinal trigeminal nuclei were greater at 24 hours than at 2 hours, and the stimulatory effects were greatly diminished by treatment with sumatriptan/naproxen or either drug alone.
Figure 2.
Sumatriptan/naproxen combination treatment suppresses capsaicin stimulated changes in the number and level of cytokine expression in spinal trigeminal nuclei at 2 (A) and 24 hours (B). Sections of the upper spinal cord containing the spinomedulary junction were obtained from animals treated with capsaicin (CAP) alone, or cotreated with capsaicin and naproxen (N), sumatriptan (S), or the combination of both drugs (S/N). The height of each box corresponds to the number of proteins significantly (p < 0.05) elevated above untreated control levels, whose mean was made equal to one. The average fold change above control levels is illustrated by different colors. n = 3 independent experiments for each condition.
Table 3.
Capsaicin stimulated proteins differentially regulated by treatment with either naproxen sodium or sumatriptan in spinal trigeminal nucleus.
| 2 Hr | 24 Hr | |||
|---|---|---|---|---|
| N + CAP | S + CAP | N + CAP | S + CAP | |
| CINC-2β IL-3 |
IFN-γ | CINC-2β Fas Ligand |
B7-1 CD106 GFRa1 GFRa2 IL-10 IL-1r Leptin MCP-1 |
Osteopontin TGFβ1 TGFβ3 Thrombospondin TNFα RALT Ubiquitin VEGF |
DISCUSSION
In our study, we found that injection of the noxious agent capsaicin in the eye brow region, which stimulates ophthalmic or V1 trigeminal neurons, resulted in a large increase in the number of cytokines and signaling proteins expressed in trigeminal ganglia 2 and 24 hours post injection. Capsaicin is known to cause activation of nociceptors, in particular unmyelinated C fibers and A-delta fibers, which are responsible for conducting pain signals from peripheral tissues to second order neurons in the spinal cord. We chose to excite V1 trigeminal neurons since activation of these neurons is implicated in the underlying pathology of migraine.2, 3, 25 In response to inflammatory or noxious stimuli, trigeminal neurons release the neuropeptide calcitonin gene-related peptide (CGRP), the neurotransmitter glutamate, and other pro-inflammatory and pro-nociceptive molecules that promote peripheral and central sensitization. Based on results from other studies, the marked increase in expression of cytokines and signaling proteins in trigeminal ganglia at 2 and 24 hours is likely the result of release of CGRP and other molecules from the cell body of trigeminal neurons, which leads to activation of satellite glial cells and subsequent release of cytokines and nitric oxide.26, 27 A somewhat surprising finding from our study was that significantly elevated protein levels were still observed 24 hours after a single capsaicin injection. While elevated levels of pro-inflammatory cytokines, such as IL-1β may play an important role in maintaining an inflammatory state since IL-1β has been shown to stimulate the release of CGRP from trigeminal neurons28, increased expression of IL-10 and IL-4 may function as anti-inflammatory proteins to modulate the response to capsaicin. Taken together, these results support the notion that activation of trigeminal neurons by a noxious stimulus leads to the establishment of an inflammatory loop that sustains sensitization of primary nociceptors at the level of the ganglion by facilitating increased neuron-glia communication via paracrine signaling.
We also found that capsaicin stimulation of V1 trigeminal neurons led to a significant increase in cytokine and signaling protein expression within spinal trigeminal nuclei of male rats. While the levels of only a few proteins were elevated at the 2 hour time point, more than 50 proteins were significantly elevated 24 hours post injection with the majority being elevated >10 fold. Capsaicin binding to TRPV1 receptors on peripheral trigeminal sensory neurons results in the release of CGRP, glutamate, and other molecules from terminals of primary nociceptors within the spinal trigeminal nucleus leading to excitation of second order neurons and spinal trigeminal glia.29, 30 Excitation of the spinal glial cells, astrocytes and microglia, promote increased synthesis and the release of cytokines within the spinal cord.31–33 Based on numerous studies, cytokines are now known to play an important role in the initiation as well as maintenance of central sensitization by influencing the expression of inflammatory genes both in neurons and spinal glia.5, 34, 35 Importantly, elevated levels of pro-inflammatory cytokines within the spinal cord are reported to positively correlate with pain sensitivity.36 Cytokines function to modulate the excitability state of both neurons and glia by activation of signaling pathways that regulate the activity and expression of ion channels and membrane receptors. Although the levels of only a few cytokines and signaling proteins were elevated at 2 hours in response to capsaicin, the greater number associated with the 24 hour time point correlates with maintenance of central sensitization in response to noxious stimulation of trigeminal neurons, a phenomenon characteristic of migraine. In summary, capsaicin stimulation of trigeminal neurons leads to elevated levels of cytokines and signaling proteins both in trigeminal ganglia and spinal trigeminal nuclei that are implicated in peripheral and central sensitization, which are physiological processes that contribute to migraine pathology.
In agreement with findings from human clinical trials, we found that treatment with the combination of sumatriptan and naproxen was very effective at suppressing cytokine and signaling proteins levels in trigeminal ganglia at 2 and 24 hours following noxious stimulation of trigeminal neurons by capsaicin. We found that the combination therapy appeared to be somewhat superior to treatment with only naproxen or sumatriptan at 2 hours but the level of inhibition was comparable in response to each treatment at 24 hours. Similarly, while sumatriptan treatment at the 2 hour time point repressed a greater number of proteins when compared to only naproxen treatment, both drugs were equally effective at 24 hours. Within the spinal trigeminal nucleus, each of the treatments suppressed the capsaicin mediated increases in the levels of 5 proteins at 2 hours. However, while the combination of sumatriptan/naproxen greatly reduced the number and level of proteins at 24 hours, expression of a slightly larger number of cytokines and signaling proteins was repressed in response to treatment with only naproxen or sumatriptan. Based on clinical observations on the temporal response to these drugs on migraine, sumatriptan/naproxen is proposed to function primarily at the level of the ganglia to abort a migraine attack.37, 38 Our findings support such a notion. In this way, sumatriptan and naproxen repression of cytokines and signaling protein levels within the ganglia, as seen in our study, would suppress the development of peripheral sensitization of primary nociceptors, and hence limit the later development of central sensitization.
An important finding from our study was that sumatriptan and naproxen differentially regulate expression of cytokines and signaling proteins within trigeminal ganglia and spinal trigeminal nucleus in response to noxious activation of trigeminal neurons. Based on preclinical studies, sumatriptan and naproxen were proposed to target different pathophysiological events of migraine.24 For example, sumatriptan is thought to function at the level of the trigeminal ganglion and nerve fibers to block release of CGRP, glutamate, and other pro-inflammatory mediators in peripheral tissues and in the spinal trigeminal nucleus.39 In effect, inhibition of trigeminal nerves would block paracrine signaling in the ganglia and spinal cord and the subsequent activation of glial cells and hence, release of cytokines that would promote peripheral and central sensitization. On the other hand, naproxen is proposed to function as a migraine therapy by inhibiting the synthesis and release of prostaglandins from mast cells.40, 41 However, a somewhat surprising finding from our study was the large number of capsaicin stimulated cytokines and signaling proteins in trigeminal ganglia and spinal trigeminal nucleus that were negatively regulated by naproxen. Interestingly, there is emerging evidence of a co-regulatory loop in which prostaglandins can increase the synthesis of cytokines, and cytokines can also upregulate prostaglandin expression.42–44 Thus, it appears that naproxen functions to inhibit inflammatory events associated with peripheral and central sensitization by repressing the expression of specific prostaglandins and cytokines.
As characteristic of migraine, protective pathological pain is proposed to result from the increased sensitivity of nociceptive primary afferent neurons (peripheral sensitization) and hyperexcitability and spontaneous firing of nociceptive neurons in the spinal trigeminal nucleus (central sensitization). Results from our study provide evidence that the reported benefit of co-treatment with sumatriptan and naproxen therapy is due in part to the ability of these drugs to significantly repress cytokine and signaling protein expression in trigeminal ganglia and spinal trigeminal nucleus, which would inhibit peripheral and central sensitization. Furthermore, our findings support the use of a combination of sumatriptan and naproxen to treat migraine patients that respond sub-optimally to monotherapy, since sumatriptan and naproxen were shown to spatially and temporally regulate the expression of different proteins in trigeminal ganglia and spinal cord. In addition, our finding that sumatriptan/naproxen repressed protein expression in spinal trigeminal nucleus may help to explain the reported benefit of combination therapy to suppress migraine recurrence 22 and treat patients with menstrual related migraine.45–47 In summary, results from our study provide a plausible explanation of the reported added benefits of combining sumatriptan with naproxen to treat migraine since these drugs repress different cytokines and signaling proteins in trigeminal ganglia and spinal trigeminal nuclei.
Supplementary Material
Acknowledgements
This project was supported by funding from GlaxoSmithKline and NIH grant DE017805.
Abbreviations
- CGRP
calcitonin gene-related peptide
- COX
cyclooxygenase
- NSAID
non-steroidal anti-inflammatory drugs
- STN
spinal trigeminal nucleus
- TG
trigeminal ganglion
Footnotes
Conflict of Interest: Paul L Durham has received grant funding from GlaxoSmithKline. Carrie V Vause has no conflicts of interest to report.
References
- 1.Goadsby P, Lipton R, Ferrari M. Migraine--current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 2.Hargreaves RJ, Shepheard SL. Pathophysiology of migraine--new insights. Can J Neurol Sci. 1999;26 Suppl 3:S12–S19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- 3.Pietrobon D. Migraine: new molecular mechanisms. Neuroscientist. 2005;11:373–386. doi: 10.1177/1073858405275554. [DOI] [PubMed] [Google Scholar]
- 4.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–110. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 5.Miller R, Jung H, Bhangoo S, White F. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol. 2009;194:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uceyler N, Schafers M, Sommer C. Mode of action of cytokines on nociceptive neurons. Exp Brain Res. 2009;196:67–78. doi: 10.1007/s00221-009-1755-z. [DOI] [PubMed] [Google Scholar]
- 7.White F, Jung H, Miller R. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci. 2007;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkins S, Rothwell N. Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci. 1995;18:83–88. 18:83-8. [PubMed] [Google Scholar]
- 9.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins L, Milligan E, Maier S. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 11.Wieseler-Frank J, Maier S, Watkins L. Glial activation and pathological pain. Neurochem Int. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 13.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeda M, Takahashi M, Matsumoto S. Contribution of the activation of satellite glia in sensory ganglia to pathological pain. Neurosci Biobehav Rev. 2009;33:784–792. doi: 10.1016/j.neubiorev.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Ren K. Neuron, Glia and Reciprocal Relationships in Pain Processing. Open Pain J. 2009;2:7–31. doi: 10.2174/1876386301003010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren K. Emerging role of astroglia in pain hypersensitivity. Jpn Dent Sci Rev. 2010;46:86. doi: 10.1016/j.jdsr.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarchielli P, Alberti A, Vaianella L, et al. Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache. 2004;44:961–968. doi: 10.1111/j.1526-4610.2004.04189.x. [DOI] [PubMed] [Google Scholar]
- 18.Kocer A, Memisogullari R, Domac FM, et al. IL-6 levels in migraine patients receiving topiramate. Pain Pract. 2009;9:375–379. doi: 10.1111/j.1533-2500.2009.00301.x. [DOI] [PubMed] [Google Scholar]
- 19.Goadsby PJ, Hargreaves RJ. Mechanisms of action of serotonin 5-HT1B/D agonists: insights into migraine pathophysiology using rizatriptan. Neurology. 2000;55:S8–S14. [PubMed] [Google Scholar]
- 20.Goadsby P. Serotonin 5-HT1B/1D receptor agonistis in migraine. CNS Drugs. 1998;10:271–286. [Google Scholar]
- 21.Moyer S. Pharmacokinetics of naproxen sodium. Cephalalgia. 1986;6 Suppl 4:77–80. doi: 10.1177/03331024860060S409. [DOI] [PubMed] [Google Scholar]
- 22.Krymchantowski AV. Naproxen sodium decreases migraine recurrence when administered with sumatriptan. Arq Neuropsiquiatr. 2000;58:428–430. doi: 10.1590/s0004-282x2000000300006. [DOI] [PubMed] [Google Scholar]
- 23.Landy S, White J, Lener SE, McDonald SA. Fixed-dose Sumatriptan/Naproxen Sodium Compared with each Monotherapy Utilizing the Novel Composite Endpoint of Sustained Pain-free/no Adverse Events. Ther Adv Neurol Disord. 2009;2:135–141. doi: 10.1177/1756285609102769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathew NT, Landy S, Stark S, et al. Fixed-dose sumatriptan and naproxen in poor responders to triptans with a short half-life. Headache. 2009;49:971–982. doi: 10.1111/j.1526-4610.2009.01458.x. [DOI] [PubMed] [Google Scholar]
- 25.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Vause CV, Durham PL. Calcitonin gene-related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Res. 2008;1196:22–32. doi: 10.1016/j.brainres.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thalakoti S, Patil VV, Damodaram S, et al. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009;5:43. doi: 10.1186/1744-8069-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meents JE, Neeb L, Reuter U. TRPV1 in migraine pathophysiology. Trends Mol Med. 2010;16:153–159. doi: 10.1016/j.molmed.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J, Ji R. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo W, Zingg JM, Meydani M, Azzi A. Alpha-Tocopherol counteracts ritonavir-induced proinflammatory cytokines expression in differentiated THP-1 cells. Biofactors. 2007;31:171–179. doi: 10.1002/biof.5520310304. [DOI] [PubMed] [Google Scholar]
- 33.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010;16:519–531. doi: 10.1177/1073858409360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Khoury CK, Couch JR. Sumatriptan-naproxen fixed combination for acute treatment of migraine: a critical appraisal. Drug Des Devel Ther. 2010;4:9–17. doi: 10.2147/dddt.s8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silberstein SD, Mannix LK, Goldstein J, et al. Multimechanistic (sumatriptannaproxen) early intervention for the acute treatment of migraine. Neurology. 2008;71:114–121. doi: 10.1212/01.wnl.0000316800.22949.20. [DOI] [PubMed] [Google Scholar]
- 39.Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46 Suppl 1:S3–S8. doi: 10.1111/j.1526-4610.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT1B/1D receptor agonists. Proc Natl Acad Sci. 2004;101:4274–4279. doi: 10.1073/pnas.0306147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy D, Zhang XC, Jakubowski M, Burstein R. Sensitization of meningeal nociceptors: inhibition by naproxen. Eur J Neurosci. 2008;27:917–922. doi: 10.1111/j.1460-9568.2008.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.St-Jacques B, Ma W. Role of prostaglandin E2 in the synthesis of the proinflammatory cytokine interleukin-6 in primary sensory neurons: an in vivo and in vitro study. J Neurochem. 2011 doi: 10.1111/j.1471-4159.2011.07230.x. [DOI] [PubMed] [Google Scholar]
- 43.Waschbisch A, Fiebich BL, Akundi RS, et al. Interleukin-1 beta-induced expression of the prostaglandin E-receptor subtype EP3 in U373 astrocytoma cells depends on protein kinase C and nuclear factor-kappaB. J Neurochem. 2006;96:680–693. doi: 10.1111/j.1471-4159.2005.03599.x. [DOI] [PubMed] [Google Scholar]
- 44.Yang MS, Ji KA, Jeon SB, et al. Interleukin-13 enhances cyclooxygenase-2 expression in activated rat brain microglia: implications for death of activated microglia. J Immunol. 2006;177:1323–1329. doi: 10.4049/jimmunol.177.2.1323. [DOI] [PubMed] [Google Scholar]
- 45.Durham PL, Vause CV, Derosier F, McDonald S, Cady R, Martin V. Changes in salivary prostaglandin levels during menstrual migraine with associated dysmenorrhea. Headache. 2010;50:844–851. doi: 10.1111/j.1526-4610.2010.01657.x. [DOI] [PubMed] [Google Scholar]
- 46.Cady RK, Diamond ML, Diamond MP, et al. Sumatriptan-Naproxen Sodium for Menstrual Migraine and Dysmenorrhea: Satisfaction, Productivity, and Functional Disability Outcomes. Headache. 2011;51:664–673. doi: 10.1111/j.1526-4610.2011.01894.x. [DOI] [PubMed] [Google Scholar]
- 47.Mannix LK, Martin VT, Cady RK, et al. Combination treatment for menstrual migraine and dysmenorrhea using sumatriptan-naproxen: two randomized controlled trials. Obstet Gynecol. 2009;114:106–113. doi: 10.1097/AOG.0b013e3181a98e4d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.