Abstract
The prognostic significance of identifying lymph node (LN) metastases following surgical resection for colon and rectal cancer is well recognized and is reflected in accurate staging of the disease. An established body of evidence exists, demonstrating an association between a higher total LN count and improved survival, particularly for node negative colon cancer. In node positive disease, however, the lymph node ratios may represent a better prognostic indicator, although the impact of this on clinical treatment has yet to be universally established. By extension, strategies to increase surgical node harvest and/or laboratory methods to increase LN yield seem logical and might improve cancer staging. However, debate prevails as to whether or not these extrapolations are clinically relevant, particularly when very high LN counts are sought. Current guidelines recommend a minimum of 12 nodes harvested as the standard of care, yet the evidence for such is questionable as it is unclear whether an increasing the LN count results in improved survival. Findings from modern treatments, including down-staging in rectal cancer using pre-operative chemoradiotherapy, paradoxically suggest that lower LN count, or indeed complete absence of LNs, are associated with improved survival; implying that using a specific number of LNs harvested as a measure of surgical quality is not always appropriate. The pursuit of a sufficient LN harvest represents good clinical practice; however, recent evidence shows that the exhaustive searching for very high LN yields may be unnecessary and has little influence on modern approaches to treatment.
Keywords: Colon cancer, Rectal cancer, Lymph node, Quality indicator, Survival
INTRODUCTION
The presence of lymph node (LN) metastases in colo-rectal cancer is well recognized as one of the most important prognostic factors for long-term outcome[1,2]. In clinical practice, the presence of LN metastasis determines those patients most likely to benefit from adjuvant chemotherapy[3,4]. Debate exists regarding the importance of increased LN harvests with a rationale to “improve” staging - the conventional hypothesis is that by examining more LNs, the accuracy of staging will be improved and more patients will receive adjuvant chemotherapy. By extension, strategies to increase surgical node harvest and/or laboratory methods to increase LN yield seem logical pursuits. The detection of LN micrometastases, using methods such as immunohistochemistry and reverse transcriptase-polymerase chain reaction (RT-PCR) assay, has received recent publicity; however, the specific methodologies will not be explored in this review. Here, we examine the evidence relating to the importance of accurate demonstration of LNs, explore the evidence for current thinking regarding LNs as a marker of prognosis and assess the use of LNs as a quality indicator. This review provides a current prospective and updates previous work from the authors[5,6].
Systematic searches of the MEDLINE and EMBASE (until December 2010) were performed using keywords relevant to each section of this review. For example, the key words: “Lymph node ratio”, “colorectal cancer” and “survival” were used for the section “Percentage lymph node harvested and prognosis”. For pragmatic reasons, searches were limited predominantly to English languages articles. Additional publications were retrieved from the references cited in articles identified from the primary search of the literature. We also contacted authors directly[7] for clarifications and additional data.
LYMPH NODE POSITIVITY AND PROGNOSIS
LN assessment is fundamental in practically all pathological staging systems for colorectal cancer, including the American Joint Committee on Cancer (AJCC)[8,9], modified Dukes[10] and Astler and Coller[11]. The most commonly used staging system internationally is the AJCC TNM system, which describes stagesIto IV based on depth of tumor invasion (T), status of localized positive lymph nodes (N) and distant metastasis (M). This has been recently updated as its seventh edition and is detailed elsewhere[12,13]. In brief, stageIshows tumor invading into (but not through) the muscularis propria, stage II shows deeper tumor invasion without spread to the LNs, stage III shows lymphatic spread and stage IV shows distant metastases[8,9,12,13]. Data from the United States Surveillance, Epidemiology and End Results (SEER) cancer registry database shows that for each T stage, 5-year crude overall survival decreases with increasing LN involvement (Table 1).
Table 1.
Observed overall 5-year survival rates by stage for colonic and rectal cancer
| Rectum | Colon | ||
| TN | SEER- observed 5 year overall survival % | TN | SEER- observed 5 year overall survival % |
| T1 | |||
| N0 | 81.4 | N0 | 78.7 |
| N1 | 72.1 | N1a | 73.7 |
| N2a | 73.8 | N1b | 67.2 |
| N2b | 53.2 | N2a | 64.7 |
| N2b | 51.8 | ||
| T2 | |||
| N0 | 75.7 | N0 | 74.3 |
| N1 | 72.1 | N1a | 73.7 |
| N2a | 58.2 | N1b | 67.2 |
| N2b | 41.7 | N2a | 64.7 |
| N2b | 51.8 | ||
| T3 | |||
| N0 | 64.0 | N0 | 66.7 |
| N1a | 55.4 | N1a | 58.2 |
| N1b | 49.7 | N1b | 51.7 |
| N2a | 42.5 | N2a | 42.8 |
| N2b | 32.0 | N2b | 30.4 |
| T4a | |||
| N0 | 55.7 | N0 | 60.6 |
| N1a | 53.2 | N1a | 52.2 |
| N1b | 43.9 | N1b | 42.1 |
| N2a | 44.3 | N2a | 32.5 |
| N2b | 24.5 | N2b | 17.5 |
| T4b | |||
| N0 | 44.7 | N0 | 45.7 |
| N1 | 24.3 | N1a | 30.6 |
| N2a | 18.5 | N1b | 25.4 |
| N2b | 12.3 | N2a | 18.3 |
| N2b | 12.9 | ||
Taken from American Joint Committee on Cancer cancer staging manual 2009, Gunderson et al[12,13]. Nodal involvement: N0 - no involvement; N1 - 1-3 positive lymph nodes (LNs); N1a - 1 positive LN; N1b - 2-3 positive LN; N1c - tumor deposit(s) in the subserosa, mesentery or non-peritonealized pericolic or perirectal tissues without regional nodal metastases; N2- 4 or more positive LN; N2a- 4-6 positive LN; N2b- seven or more positive LN. SEER: Surveillance, Epidemiology and End Results.
LYMPH NODE HARVEST IS RELATED TO SURVIVAL
Evidence suggests that patients, particularly in stage II disease, with a reduced LN harvest have a worse prognosis[8,9,14-31]. Whether stage II colorectal cancer is considered together, or split separately into colon and rectal cancers, 5-year overall survival (OS) and disease-free survival (DFS) are significantly reduced in patients with low LN harvest. However, the cut-off points in these studies are invariably data-driven and vary from as low as 6 to a high of 21[14,16,17,20,22,23,28,30,31] (Table 2).
Table 2.
Survival in stage II colorectal cancer with increasing lymph nodes
| Authors | Study/country | Tumor location | No. of patients | LN cut-off parameters | 5-year survival % | Significance |
| Observational studies | ||||||
| Caplin et al[14] | Switzerland | Colorectal | 222 | ≤ 6 | 492 | P = 0.0014 |
| ≥ 7 | 682 | |||||
| Tepper et al[16] | National intergroup trial (United States) | Rectum | 1664 | < 5 | 642 | P = 0.0081 |
| ≥ 5 and < 9 | 632 | P = 0.041 | ||||
| ≥ 9 and < 14 | 612 | P = 0.021 | ||||
| ≥ 14 | 672 | |||||
| Cianchi et al[17] | Italy | Colorectal | 140 | ≤ 8 | 54.92 | P < 0.001 |
| > 9 | 79.92 | |||||
| Prandi et al[20] | National intergroup for Adjuvant Therapy on Colon Cancer (INTACC, Italy) | Colon | 3648 | 0-7 | 81; 663 | P trend = 0.00092 |
| 8-12 | 812; 743 | P trend < 0.00013 | ||||
| 13-17 | 872; 773 | |||||
| ≥ 18 | 892; 833 | |||||
| Law et al[22] | Canada | Colon | 115 | ≤ 6 | 622 | P = 0.03 |
| ≥ 7 | 862 | |||||
| Le Voyer et al[23] | Intergroup Trial INT-0089 (United States) | Colon | 3411 | ≤ 10 | 732; 804; 723 | P < 0.00012 |
| 11-20 | 802; 854;793 | P = 0.154 | ||||
| > 20 | 872; 924; 823 | P = 0.10953 | ||||
| Tsai et al[28] | Taiwan | Colorectal | 180 | < 18 | Percentage survival not stated | P = 0.015 |
| ≥ 18 | Percentage survival not stated | |||||
| Vather et al[30] | New Zealand Cancer Registry (NZCR) | Colon | 1945 | Decreasing mortality with increasing lymph nodes. Greatest statistical difference between ≤ 12 and >12 nodes | P = 0.0001 | |
| Choi et al[31] | Hong Kong | Colorectal | 664 | < 21 | 603 | P = 0.001 |
| ≥ 21 | 803 | |||||
| Multicenter observational study | ||||||
| Swanson et al[24] | National cancer database (NCD, United States) | Colon | 31 515 | 1-7 | 49.82 | P < 0.0001 |
| 8-12 | 56.22 | |||||
| ≥ 13 | 63.42 | |||||
Compared with patients with ≥ 14 lymph nodes (LNs) examined;
Overall survival;
Disease free survival;
Cause specific survival.
Although several studies fail to demonstrate the similar association between survival and LN harvest in stage III disease[14,16,20,25], others have shown this, as follows. Le Voyer et al[23] showed that for colon cancer patients with 1-3 positive LNs, there was an absolute 23% improvement in 5-year OS if > 40 LNs were analyzed vs ≤ 10 (P < 0.0001); and in patients with more than 4 positive LNs, 5-year OS following analysis of > 35 vs < 35 LNs were 71% and 51%, respectively (P = 0.002). Chen et al[27] showed that the median survival in colon cancer when 1-7, 8-14 and ≥ 15 LNs were examined was 46, 52 and 67 mo respectively (P < 0.001). Vather et al[30] reported that the mean number of LNs examined in stage III who die within 5 years was 13.1 vs 14.8 in those who remained alive (P < 0.0001).
OPTIMAL NUMBER OF LYMPH NODES FOR STAGING
There is debate regarding the optimal number of LNs required for adequate staging. The evaluation of at least 12 LNs following colorectal resection is widely cited in clinical guidelines[1,32-35]. This number was first proposed in 1990 by the Working Party Report to the World Congress of Gastroenterology in Sydney[32]. This is not, however, a scientific biological figure[36] and is a grade C recommendation based on level III-IV evidence[34,37-40]. Guidelines for colon and rectal cancer surgery published in 2001[34] again recommended 12 LNs as adequate sampling, basing their recommendation on data from a non-randomized observational United Kingdom one-center study (n = 103)[38]. In the latter paper, Scott et al[38] initially examined colorectal specimens using conventional LN counting before using fat clearance (xylene and alcohol) and re-examining. The mean number of LNs identified before and after fat clearance was 6.1 and 18.2 respectively. In LN positive cases (Dukes C; n = 50), they showed that 26/50 cases revealed a positive node after examining 6 LNs vs 47/50 (94%) when 13 nodes were examined. The total number of Dukes C cases pre and post-fat clearance was 43.7% and 48.5% respectively (non-significant). Using these data, Nelson et al[34] extrapolated that if one examines 12 LNs, node positivity is correctly identified 90% of the time (although Scott et al[38] recommended examining 13 LNs allowing a detection rate of 94%).
Following the publication of this guideline in 2001, some pathologists initially failed to classify specimens that did not contain 12 LNs since they were considered to be incompletely resected; however, within the same year, this practice was already being questioned[33]. Furthermore, in 2004, a notable Canadian study showed that only 58% of pathologists were aware of current guidelines and that only 25% recognized that a minimum of 12 LNs was necessary for accurate designation of node negativity[41]. Most recently in the United States, Medicare required only 4 LNs to be identified in a colonic resection specimen in order to claim full evaluation and subsequent reimbursement[42].
Arguing against the minimum number of 12 LNs required for adequate staging, several authors have suggested other cut-off values ranging from 6 to 21[14-17,24,27,30,31,38,43-45]. Arguments for any minimal LN cut off value, however, fail to recognize that the number of LNs across individuals varies and is dependent on several factors. Mean LN number decreases with increasing age[20,27,46-48] and with progression from the proximal to the distal colon/rectum[2,14,17,27,29,49,50]. Shen et al[48] demonstrated that in 434 consecutive colorectal patients at a single tertiary referral center, the mean number of LNs harvested was significantly less in patients aged ≤ 60 years vs > 60 years (P = 0.002) and significantly less in the sigmoid colon (P < 0.001) and the rectum (P = 0.001) when compared to the cecum/ascending colon (Table 3). LN harvest increases with increasing T stage[2,31,50,51] and is subject to natural anatomical variation[52,53]. LN harvest is also influenced by additional tumor factors (e.g. interleukin-10, transforming growth factor-β and vascular endothelial growth factor)[54,55] and by the use of neo-adjuvant chemoradiotherapy (CRT)[7,46,56-62]. The presence of microsatellite instability (MSI)[63] is also associated with increased LN harvest[64]. Søreide et al[64] found that MSI is an independent factor for harvesting LN ≥ 12 in stage II/III colon cancer (P = 0.026), however, the authors also showed that this was the case for proximal location (P = 0.003) which (as discussed above) is well documented. The evidence implies that the variation in LN numbers detected in post resection specimens is largely attributed to these biological factors. Therefore, setting an arbitrary cut off value for LN harvest to be used as a prognostic indicator is unfounded and, in clinical practice, the reliance on such guidelines should be re-examined.
Table 3.
Mean number of lymph nodes associated with age and tumor location
| No. of cases | No. of lymph nodes (mean ± SD) | |
| Age (yr) | ||
| ≤ 50 | 40 | 18.2 ± 12.6 |
| > 50 and ≤ 60 | 82 | 17.8 ± 9.5 |
| > 60 and ≤ 70 | 109 | 14.4 ± 8.0a |
| > 70 and ≤ 80 | 117 | 15.1 ± 7.2a |
| > 80 | 82 | 14.9 ± 7.4a |
| Location | ||
| Cecum/ ascending colon | 161 | 17.8 ± 7.9c |
| Transverse colon | 22 | 14.5 ± 7.0 |
| Descending colon | 15 | 19.0 ± 12.5 |
| Sigmoid colon | 167 | 14.3 ± 9.4 |
| Rectum | 69 | 13.7 ± 6.6 |
P = 0.002 vs age ≤ 60 years;
P < 0.001 and P = 0.001 vs tumors from sigmoid colon and rectum, respectively.
It is not surprising that internationally there is considerable variation in the actual number of LNs assessed following colorectal surgery. Baxter et al[2] reported 116 995 United States patients undergoing resection for colorectal cancer (without neo-adjuvant chemotherapy). The median number of LNs examined was 9. Only 37% of patients had ≥ 12 LNs examined, although this increased over time [1988, 32%; 2001, 44%; P (trend) < 0.001]. Bilimoria et al[65] showed that the proportion of United States hospitals achieving the guideline of 12 LNs in ≥ 75% of patients rose from 15% in 1996 -1997 to 38% in 2004-2005. However, data from the United Kingdom National Bowel Cancer Audit (2009)[35] showed that a median number of 15.1 LNs were examined in resection specimens for the period 2006/8, with 78.6% of United Kingdom providers achieving the guideline of 12. Single institution experiences in the United States also reveal a median LN harvest of 15[48].
In clinical practice, there is clearly a need to strive to harvest as many LNs as possible[23]. However, a “ceiling effect” may be reached. Thus, in a recent large population study of 11 044 patients with pT3 colon cancer identified through the SEER cancer registry, Baxter et al[49] reported a dramatic increase in the odds ratio of node positivity with increasing node count up to 6 LNs. However, between the mid-point of the range 7 to 11 nodes and the mid-point of the range 12 to 17 nodes there is only a marginal increase; and when >17 nodes were evaluated, the odds of finding a positive LN actually declines (Figure 1). The authors concluded that staging of pT3 cancer is improved by increasing LN harvest but only when the yield is low and that at higher counts there is a marginal effect on staging.
Figure 1.
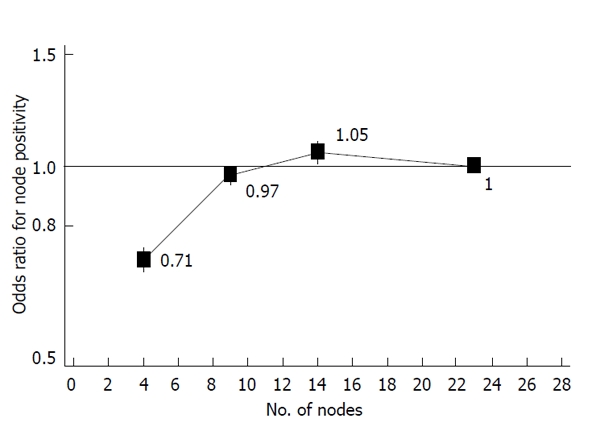
Odds ratio of node positivity in pT3 colonic cancer (Surveillance, Epidemiology and End Results 1986-2003; Baxter et al[49]).
Therefore, in summary we predict that the variation in LN harvest is influenced largely by factors related to patient demographics, tumor location and tumor biology and it is therefore a mistake to set an arbitrary cut off value for an adequate LN harvest as this will not improve the outcome for the individual. In practice, the aim should be to collect as many LNs as possible, although the exhaustive pursuit of very high numbers may be unjustifiable.
PERCENTAGE POSITIVE LYMPH NODES HARVESTED AND PROGNOSIS
The presence of positive LNs defines stage III disease. However, due to the recognized variability of LNs across individuals, it may be more appropriate to investigate the percentage of positive nodes rather than just the absolute number. The lymph node ratio (LNR), defined as the quotient between positive LNs and total number of LNs harvested, was first proposed for colorectal cancer by Berger et al[66] and subsequently adopted by others[30,67-76]. Debate remains concerning the exact figures for the LNR in order to predict outcome but the data suggest that a higher LNR equates to worsening survival (Table 4).
Table 4.
Survival by lymph node ratio in stage III colorectal cancer
| Authors | Study/country | Tumor location | No. of patients | Lower cut off LNR | 5-year survival % | Significance |
| Observational study | ||||||
| Lee et al[68] | South Korea | Colon | 201 | 0.01-0.11 | 83.64 | P < 0.0001 |
| 0.12-0.24 | 61.14 | |||||
| 0.25-0.92 | 204 | |||||
| Schumacher et al[69] | United States | Colon | 75 | < 0.18 | 8541 | P < 0.005 |
| ≥ 0.18 | 5041 | |||||
| Peschaud et al[73] | France | Rectum | 307 | 0.01-0.07 | 794; 9232 | P = 0.0064 |
| > 0.2 | 424; 6632 | P = 0.00033 | ||||
| Park et al[71] | South Korea | Colon | 318 | < 0.059 | 83.642 | P = 0.002 |
| 0.059-0.23 | 71.142 | |||||
| > 0.23 | 5542 | |||||
| Rosenberg et al[74] | Germany | Colorectal | 3026 | 0.01-0.17 | 60.63 | P < 0.001 |
| 0.18-0.41 | 34.43 | |||||
| 0.42-0.69 | 17.63 | |||||
| > 0.70 | 5.33 | |||||
| Multicenter observational study | ||||||
| De Ridder et al[67] | SEER cancer registry (Belgium) | Colon | 26 181 | ≤ 0.4 | 565 | P < 0.0001 |
| ≥ 0.4 | 255 | |||||
| Wang et al[70] | SEER cancer registry (United States) | Colon (stage IIIB) | 14 644 | < 1/14 | 63.53 | P < 0.0001 |
| 1/14-0.25 | 54.73 | |||||
| 0.25-0.50 | 44.43 | |||||
| 0.50-1.0 | 34.23 | |||||
| SEER cancer registry (United States) | Colon (stage IIIC) | 7658 | < 1/14 | Not estimated | P < 0.0001 | |
| 1/14-0.25 | 49.63 | |||||
| 0.25-0.50 | 41.73 | |||||
| 0.50-1.0 | 25.23 | |||||
| Rosenberg et al[75] | Munich Cancer Registry (MCR, Germany) | Colorectal | 27 803 | 0 | 71.43 | P < 0.001 |
| 0.01-0.17 | 52.43 | |||||
| 0.18-0.41 | 33.33 | |||||
| 0.42-0.69 | 19.83 | |||||
| > 0.70 | 8.33 | |||||
Approximate figures taken from graph in report. Exact 5-yar survival by lymph node ratio (LNR) not stated in report;
3-year survival, Surveillance, Epidemiology, and End Results (SEER);
Overall survival;
Disease free survival;
Cause specific survival.
Vather et al[30] split the LNR of 2364 stage III colon cancers into deciles, showing a 5-year mortality of 40% to 45% in the lowest decile (LNR 0-0.10) and increasing to 80% to 90% in the highest (LNR 0.91-1.0; P < 0.0001). Wang et al[72] presented the 5-year OS of 24 477 stage III colon carcinomas sub-grouped into 4 LNR groups < 0.07, 0.07-0.25, 0.25-0.50, and > 0.50 with 5-year OS of 64.8%, 56.2%, 45.1% and 29.6%, respectively (P < 0.0001).
Thus, LNR may be a superior prognostic indicator to total number of LNs and is justified if one considers the example proposed by Wang et al[70]. Using the AJCC staging system, the prognosis of a patient with 2 positive LNs is the same whether a total of 2 LNs or 40 LNs are examined, if all other conditions are matched. If one accepts the evidence presented above, one would predict that the survival in the former case (LNR 1.0) would be worse than that the latter (LNR 0.05). However, in this hypothetical example, the numerical value of the LNR will be disproportionally high if the total LN harvest is under-representative. Therefore, whilst many authors present evidence supporting the use of the LNR as a superior prognostic indicator to total LN harvest[30,66-76], calculation of an accurate LNR still relies on an adequate total LN harvest.
In clinical practice, in order to use LNR as a prognostic indicator of survival, it would need to be incorporated into a formal staging system such as a sub-classification of AJCC stage III or Dukes C. In the United Kingdom, LNR may, in the future, be incorporated into the minimum dataset for colorectal cancer pathological reporting[77]. Until these measures are taken, the relevance of LNR in influencing clinical treatment cannot be universally established.
INCREASED LYMPH NODE HARVEST: CLINICAL METHODS
Whilst there is considerable variation in LN counts across individuals, efforts to maximize the harvest in an individual patient seem an obvious measure of good clinical practice[23]. Considerations in the pre-operative, intra-operative and post-operative stages will increase the LN yield available for analysis.
Pre-operative clinical examination of LNs is of limited value in colorectal cancer since palpable nodes are uncommon and occur late in the disease[5]. Conventional trans-abdominal and endoluminal ultrasound, computed tomography and magnetic resonance (MR) imaging are all common place in initial disease staging but vary in their ability to accurately detect LN metastases[5]. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) scanning is becoming increasingly used in staging assessment. This has the advantage of being superior to diffusion weighted MR in the detection of primary lesions, but inferior with relation to LN metastases[78]. Comparison of preoperative FDG-PET to multi-detector row computerized tomography shows FDG-PET to be inferior for detection of primary tumor, LN involvement and liver metastases, but may have potential clinical value in patients with advanced colorectal cancer by detecting extra-hepatic distant metastases[79].
Intra-operatively, size alone cannot be used as an indicator of tumor involvement, since enlarged palpable LNs contain metastases in less than half of the cases[80,81] and up to 70% of LNs with metastases are < 5 mm in diameter[6]. Therefore the surgeon should aim to remove all macroscopic disease along with the draining LNs to ensure adequate staging[5]. In colon cancer, this is achieved by excising the segment of bowel with the tumor and associated mesentery. Ligation of the supplying blood vessels close to the originating feeding vessel will identify the apical node, required for differentiation of C1 and C2 in the modified Duke’s staging system[10]. In rectal cancer, adherence to the principles of total mesorectal excision (TME)[82] is required to ensure adequate removal of the mesorectal package. Failure to resect a wide enough LN basin draining the segment involved by cancer may result in an inadequate amount of nodes being assessed, irrespective of the diligence of the histopathological team[36]. Techniques such as lymphoscintigraphy, using technetium-99 m-phytate and patent blue, have been reported to identify LN metastases in 8.2% of individuals[83] but have yet to gain widespread application.
It is important to resect an adequate LN basin in order to achieve accurate staging[29], although some have argued that this is of limited therapeutic benefit[84]. When considering the practice of extended lymphadenectomy (EL) to treat lateral pelvic lymph nodes in low rectal cancer (<6cm from anal verge), the debate regarding the survival benefit continues[85]. Georgiou et al[85] presented a meta-analysis of the peri-operative outcomes, survival and recurrence rates of patients undergoing surgery for rectal cancer with either EL (n = 2577) or non-EL (n = 2925). The authors found that peri-operative mortality and morbidity were similar between the two groups. Data from individual studies within the meta-analysis showed that urinary dysfunction was more prevalent in the EL group (P = 0.0012). Male sexual dysfunction was significantly more common (P = 0.012) in the EL group (92.3%) vs the non-EL group (45.5%). There were no significant differences in 5-year OS or DFS and local or distant recurrence. The authors concluded that EL does not confer significant overall cancer survival but is associated with higher levels of morbidity. However, Yano et al[86] argued against these results, and contend that since the EL patients had more advanced tumors, the fact that there was no difference in survival can be interpreted as a survival benefit in the EL group. Due to concerns regarding increased rates of morbidity, EL is not routinely employed internationally, although in some countries (particularly Japan), EL techniques have improved[87]. By combining the treatment algorithms, for low rectal tumors, of countries that routinely utilize EL with those where EL is not employed, oncological results can be improved whilst preventing overtreatment and associated morbidity[88].
INCREASE LYMPH NODE HARVESTING: LABORATORY METHODS
Specimens need to be dissected by adequately trained personnel with enough time to perform a thorough LN harvest if accurate staging is to be achieved[6]. Manual palpation is the main technique utilized but this can miss small nodes[36]. Fat clearing solutions (xylene and alcohol) have been adopted in many centers to aid in node retrieval if counts by traditional methods are low[1,38,39]. Cawthorn et al[39] showed that the total number of LNs identified in the mesorectum of rectal resectates following fat clearance (mean, 23.1 ± 1.18) was significantly higher when compared with two patient groups that did not employ flat clearance techniques (group 1 mean, LN 13.1 ± 0.86; group 2 mean, LN 10.5 ± 0.6; P < 0.001). It is a common misconception that fat clearance is time consuming and/or expensive. In practice, when using fat clearance, the sample is prepared by a lab technician after the initial dissection by the pathologist. This actually saves the pathologist time, since the “hunt” for LNs is less arduous. Fat clearance also equilibrates the variable of the differing abilities of the pathologists in finding nodes in the “un-prepared” specimen. Therefore, it has significant implications for LN harvest with little bearing on overall total cost.
International standards of practice have yet to be developed for harvesting and processing of LNs[89]. The concern with rigidly fixing a guideline number (i.e., 12) is that once this figure is met, the search for LNs may end at that point regardless of how many (positive) nodes are left in the sample[49]. This adds further to the argument that the reliance on a specific LN cut off value should be questioned.
STAGE MIGRATION: THE WILL ROGERS PHENOMENON
The accepted theory is that by examining more nodes, one will increase the chances of finding a positive node and therefore upstage a patient from stage II (negative LNs) to stage III (positive LNs). The unanswered question, however, is what relevance does upstaging have for the individual patient? Population statistics show that upstaging a patient from stage II to III will increase the survival in both the group the patient is leaving and the one he/she is subsequently joining (the Will Rogers Phenomenon)[90]. However this results in misleading cancer survival statistics because regardless of the rise in survival rates in each population group, there is no resulting change in the outcome for the individual. In colorectal cancer, techniques used to upstage the patient may result in more patients being offered adjuvant chemotherapy[30]. However, studies have yet to be undertaken that evaluate the survival benefit of giving adjuvant therapy to those who would not ordinarily have been considered for treatment; therefore, improved prognostic discrimination does not necessarily mean improved treatment prediction[91].
PRE-OPERATIVE TREATMENT EFFECTS ON LYMPH NODE HARVEST
The intended purpose of neo-adjuvant (chemo) radiotherapy (CRT) is tumor down-staging by decreasing the primary tumor bulk and associated LN metastases[6]. It can also lead to fibrosis, which makes LNs more difficult to identify by palpation, and is associated with reduction in size of normal LNs, as well as nodes involved by tumor (Figure 2). In rectal cancer, whilst this is the desired effect of treatment, it contradicts the traditional goal of achieving a maximal LN harvest, since it has been shown to result in a significant decrease in the number of nodes found[46,56-60]. Morcos et al[57] studied 116 patients following TME for rectal adenocarcinoma, 59 of whom underwent neo-adjuvant CRT before surgery. A reduction in mean LN yield was found in patients who received neo-adjuvant therapy (from 19 to 16, P = 0.008). This reduction, however, did not affect survival. Rullier et al[7] reported on 495 patients who underwent rectal cancer treatments (neo-adjuvant radiotherapy n = 332; neo-adjuvant CRT n = 248; surgery alone n = 163). Neo-adjuvant therapy reduced both the mean LN harvest (from 17 in surgery alone to 13; P < 0.001) and mean positive LNs (from 2.3 in surgery alone to 1.2; P = 0.001). The authors found no statistical association with OS and DFS (Table 5). Doll et al[56] compared T3 patients following neo-adjuvant CRT (n = 102) with those who underwent primary surgery followed by adjuvant CRT (n = 114). Neo-adjuvant therapy reduced the mean LN harvest (from 21.4 to 12.9; P < 0.0001) and mean positive LNs (from 2.3 to 1.0, P = 0.014). Again there was no impact on OS, although in the neo-adjuvant group, OS was significantly influenced by the number of positive LNs (5-year OS: 88%, 63% and 39% for 0, 1-3, and > 3 positive LNs, respectively, P < 0.0001).
Figure 2.
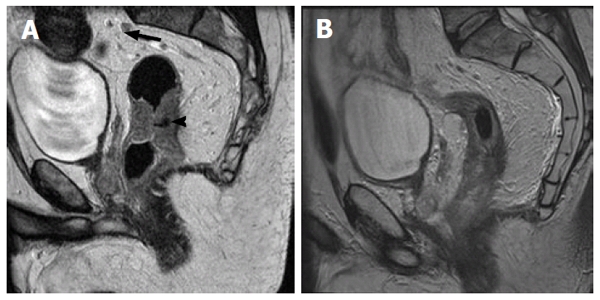
Sagital T2 weighted high resolution magnetic resonance image of the rectum. These demonstrate (A) circumferential mid-rectal tumor (arrow head). In addition there is a sub-centimeter pre-sacral node in close proximity to the superior rectal artery (arrow). (Although this is sub-centimeter it has the same signal intensity to the tumor); B demonstrates response to neo-adjuvant short course chemoradiotherapy (45 Gy over 25 fractions plus capcetabine 825 mg/m2 days 1 to 35). This demonstrates significant response of the tumor mass, with high signal intensity in the mucosa likely to be treatment related. The pre-sacral node has resolved.
Table 5.
Survival in patients with negative lymph nodes after pre-operative chemoradiotherapy
| Lymph nodesretrieved | No. of patients | Overall survival | Disease-free survival | ||
| % | P | % | P | ||
| < 4 | 22 | 95 | 76 | ||
| > 4 | 176 | 81 | 0.42 | 70 | 0.47 |
| < 5 | 31 | 85 | 67 | ||
| > 5 | 167 | 82 | 0.71 | 72 | 0.73 |
| < 6 | 45 | 85 | 66 | ||
| > 6 | 153 | 82 | 0.89 | 73 | 0.56 |
| < 7 | 58 | 84 | 64 | ||
| > 7 | 140 | 82 | 0.68 | 75 | 0.37 |
| < 8 | 79 | 83 | 66 | ||
| > 8 | 119 | 83 | 0.53 | 75 | 0.38 |
| < 9 | 88 | 84 | 69 | ||
| > 9 | 110 | 81 | 0.88 | 73 | 0.62 |
| < 10 | 101 | 86 | 72 | ||
| > 10 | 97 | 79 | 0.58 | 70 | 0.65 |
| < 11 | 109 | 87 | 74 | ||
| > 11 | 89 | 77 | 0.27 | 68 | 0.43 |
| < 12 | 117 | 87 | 74 | ||
| > 12 | 81 | 76 | 0.36 | 68 | 0.36 |
| < 13 | 129 | 87 | 74 | ||
| > 13 | 69 | 73 | 0.25 | 65 | 0.26 |
Showed no significant difference in 5-year overall survival and disease-free survival when increasing lymph nodes were harvested (Rullier et al[7]).
A complete absence of LNs in resection specimens following neo-adjuvant CRT for rectal cancer has also been reported. Habr-Gama et al[61] reported 281 patients who underwent chemoradiation prior to resection of rectal cancer. Patients were grouped as having no LNs (ypNx, n = 32, 11%), negative LNs (ypN0, n = 171, 61%) or positive LNs (ypN+, n = 87, 28%). The ypNx patients in this study were found to have better 5-year DFS than patients with ypN0 (74% vs 59%). Although this failed to reach statistical significance, the authors argue that this represents a clinically important survival benefit. Both groups were, however, found to have a significantly better 5-year DFS than patients with ypN+ disease (30%; P < 0.001). In contrast, Kim et al[62] reported on 258 rectal cancer patients, who received neo-adjuvant CRT and surgery. The authors found no significant difference in the 5-year OS among ypNx patients (n = 9; OS 88.9%) and a subset of ypN0 patients (n = 150) based on the number of nodes retrieved using three cut off values; 1-11 (n = 45; OS 80.4%), 12-25 (n = 60; OS 89.4%), and 25-65 (n = 45; OS 92.9%) nodes. These results suggest that ypNx leads to a better survival only when there is retrieval of up to 11 negative LNs and that those patients with 12 or greater negative LNs have a better outcome. The size of the ypNx sample (n = 9) may account for this finding. Both studies suggest that in rectal cancer, the absence of LNs following neo-adjuvant treatment does not represent an inferior resection and does not affect survival outcome. However, the patient numbers are small and statistical significance has never been shown. These results may represent a sub-group of patients with increased sensitivity to adjuvant CRT and the future challenge may be to identify these patients prior to treatment. Since the limited evidence paradoxically suggests that these “complete responders” have a better survival outcome, it may be necessary to establish whether current staging guidelines represent an appropriate indicator of prognosis in these patients, or whether an alternative staging regimen is required. In current practice, whilst adjuvant CRT for rectal cancer has been shown to reduce both the total number and positive LNs[46,56-60], the goal of the operating surgeon and pathologist should still be to ensure an adequate LN harvest.
SHOULD LYMPH NODE HARVEST BE USED AS A MEASURE OF QUALITY CONTROL?
There is much enthusiasm for using LN harvesting as a marker of quality control in colorectal cancer surgery, since it is easily measured and comparable between centers[92]. However, the routine use of total number of LNs as a measure of quality has been called into question[72], particularly since this does not indicate which part of the patient’s treatment is being evaluated; be it the surgeon, the pathologist, the institution or the tumor biology[92]. Baxter[92] presents a detailed review arguing against the reliance on LN harvesting as a quality indicator, principally because data showing that increased LN counts are associated with better survival are from observational studies[14-18,20,22,23,25,27,28,30,31,45] and are not repeated in large multicenter[24,49] or population-based[26,93] series. The mechanism underlying the association between survival and node count is unknown. Wong et al[93] reported on 30 625 non-metastatic colon cancer patients in the SEER cancer registry and found no significant difference between institution LN examination numbers and survival. As previously discussed, the number of LNs excised is influenced by the biology of the tumor and its response to adjuvant treatment rather than surgical technique and, therefore, its use as a surrogate marker of quality of surgery or the service provider should be re-considered.
CONCLUSION
In colorectal cancer, a primary focus of treatment over recent years has been to collect as many LNs as possible in order to “improve” staging and increase survival, with subsequent guidelines regulating LN harvests being developed. The scientific evidence for a minimum LN harvest, however, is questionable and the use of universal cut off values should be re-considered. This is particularly pertinent following neo-adjuvant therapy for rectal cancer, where, in a sub-group of patients, the finding of fewer LNs implies treatment response and may be oncologically more favorable. On balance, the aim of maximal LN retrieval, by both the operating surgeon and pathologist, represents good clinical practice; although the pursuit of very high numbers of nodes has not been met with equivalent benefit. The evidence for routine employment of LN harvest as a marker of service quality should be re-examined since tumor biology may account for differences in LN count rather than just the quality of surgical excision or pathological examination.
Footnotes
Peer reviewer: Paolo Morgagni, MD, Departement of Sur-gical, GB Morgagni L Pierantoni General Hospital, Viale Forlanini 33, Forli 47100, Italy
S- Editor Wang JL L- Editor Roemmele A E- Editor Li JY
References
- 1.Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 2.Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst. 2005;97:219–225. doi: 10.1093/jnci/dji020. [DOI] [PubMed] [Google Scholar]
- 3.Comparison of flourouracil with additional levamisole, higher-dose folinic acid, or both, as adjuvant chemotherapy for colorectal cancer: a randomised trial. QUASAR Collaborative Group. Lancet. 2000;355:1588–1596. [PubMed] [Google Scholar]
- 4.Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 5.O'Dwyer ST, Haboubi NY, Johnson JS, Gardy R. Detection of lymph node metastases in colorectal carcinoma. Colorectal Dis. 2001;3:288–294. doi: 10.1046/j.1463-1318.2001.00251.x. [DOI] [PubMed] [Google Scholar]
- 6.Schofield JB, Mounter NA, Mallett R, Haboubi NY. The importance of accurate pathological assessment of lymph node involvement in colorectal cancer. Colorectal Dis. 2006;8:460–470. doi: 10.1111/j.1463-1318.2006.01044.x. [DOI] [PubMed] [Google Scholar]
- 7.Rullier A, Laurent C, Capdepont M, Vendrely V, Belleannée G, Bioulac-Sage P, Rullier E. Lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival. Am J Surg Pathol. 2008;32:45–50. doi: 10.1097/PAS.0b013e3180dc92ab. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti AL. AJCC Cancer Staging Manuel. New York: Springer; 2009. [Google Scholar]
- 9.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 10.Gabriel W, Dukes C, Bussey H. Lymphatic spread in cancer of the rectum. Br J Surg. 1935;23:395–413. [Google Scholar]
- 11.Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954;139:846–852. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart A. Revised tumor and node categorization for rectal cancer based on surveillance, epidemiology, and end results and rectal pooled analysis outcomes. J Clin Oncol. 2010;28:256–263. doi: 10.1200/JCO.2009.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–271. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplin S, Cerottini JP, Bosman FT, Constanda MT, Givel JC. For patients with Dukes' B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer. 1998;83:666–672. [PubMed] [Google Scholar]
- 15.Pocard M, Panis Y, Malassagne B, Nemeth J, Hautefeuillz P, Valleur P. Assessing the effectiveness of mesorectal excision in rectal cancer: prognostic value of the number of lymph nodes found in resected specimens. Dis Colon Rectum. 1998;41:839–845. doi: 10.1007/BF02235362. [DOI] [PubMed] [Google Scholar]
- 16.Tepper JE, O'Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB, Cummings B, Gunderson L, Macdonald JS, Mayer RJ. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157–163. doi: 10.1200/JCO.2001.19.1.157. [DOI] [PubMed] [Google Scholar]
- 17.Cianchi F, Palomba A, Boddi V, Messerini L, Pucciani F, Perigli G, Bechi P, Cortesini C. Lymph node recovery from colorectal tumor specimens: recommendation for a minimum number of lymph nodes to be examined. World J Surg. 2002;26:384–389. doi: 10.1007/s00268-001-0236-8. [DOI] [PubMed] [Google Scholar]
- 18.Cserni G, Vinh-Hung V, Burzykowski T. Is there a minimum number of lymph nodes that should be histologically assessed for a reliable nodal staging of T3N0M0 colorectal carcinomas? J Surg Oncol. 2002;81:63–69. doi: 10.1002/jso.10140. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. 2002;26:179–189. doi: 10.1097/00000478-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Prandi M, Lionetto R, Bini A, Francioni G, Accarpio G, Anfossi A, Ballario E, Becchi G, Bonilauri S, Carobbi A, et al. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: results of a secondary analysis of a large scale adjuvant trial. Ann Surg. 2002;235:458–463. doi: 10.1097/00000658-200204000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joseph NE, Sigurdson ER, Hanlon AL, Wang H, Mayer RJ, MacDonald JS, Catalano PJ, Haller DG. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol. 2003;10:213–218. doi: 10.1245/aso.2003.03.059. [DOI] [PubMed] [Google Scholar]
- 22.Law CH, Wright FC, Rapanos T, Alzahrani M, Hanna SS, Khalifa M, Smith AJ. Impact of lymph node retrieval and pathological ultra-staging on the prognosis of stage II colon cancer. J Surg Oncol. 2003;84:120–126. doi: 10.1002/jso.10309. [DOI] [PubMed] [Google Scholar]
- 23.Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–2919. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 24.Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Sarli L, Bader G, Iusco D, Salvemini C, Mauro DD, Mazzeo A, Regina G, Roncoroni L. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer. 2005;41:272–279. doi: 10.1016/j.ejca.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Bui L, Rempel E, Reeson D, Simunovic M. Lymph node counts, rates of positive lymph nodes, and patient survival for colon cancer surgery in Ontario, Canada: a population-based study. J Surg Oncol. 2006;93:439–445. doi: 10.1002/jso.20499. [DOI] [PubMed] [Google Scholar]
- 27.Chen SL, Bilchik AJ. More extensive nodal dissection improves survival for stages I to III of colon cancer: a population-based study. Ann Surg. 2006;244:602–610. doi: 10.1097/01.sla.0000237655.11717.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai HL, Lu CY, Hsieh JS, Wu DC, Jan CM, Chai CY, Chu KS, Chan HM, Wang JY. The prognostic significance of total lymph node harvest in patients with T2-4N0M0 colorectal cancer. J Gastrointest Surg. 2007;11:660–665. doi: 10.1007/s11605-007-0119-x. [DOI] [PubMed] [Google Scholar]
- 29.Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 30.Vather R, Sammour T, Zargar-Shoshtari K, Metcalf P, Connolly A, Hill A. Lymph node examination as a predictor of long-term outcome in Dukes B colon cancer. Int J Colorectal Dis. 2009;24:283–288. doi: 10.1007/s00384-008-0540-y. [DOI] [PubMed] [Google Scholar]
- 31.Choi HK, Law WL, Poon JT. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer. 2010;10:267. doi: 10.1186/1471-2407-10-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fielding LP, Arsenault PA, Chapuis PH, Dent O, Gathright B, Hardcastle JD, Hermanek P, Jass JR, Newland RC. Clinicopathological staging for colorectal cancer: an International Documentation System (IDS) and an International Comprehensive Anatomical Terminology (ICAT) J Gastroenterol Hepatol. 1991;6:325–344. doi: 10.1111/j.1440-1746.1991.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 33.Sobin LH, Greene FL. TNM classification: clarification of number of regional lymph nodes for pNo. Cancer. 2001;92:452. doi: 10.1002/1097-0142(20010715)92:2<452::aid-cncr1342>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 34.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 35. Available from: http: //www.acpgbi.org.uk/assets/documents/National_Bowel_Cancer_Audit_2009_Interactive_for_web_051109.pdf.
- 36.Wright FC, Law CH, Berry S, Smith AJ. Clinically important aspects of lymph node assessment in colon cancer. J Surg Oncol. 2009;99:248–255. doi: 10.1002/jso.21226. [DOI] [PubMed] [Google Scholar]
- 37.Pickren JW. Current concepts in cancer. Nodal clearance and detection. JAMA. 1975;231:969–971. doi: 10.1001/jama.231.9.969. [DOI] [PubMed] [Google Scholar]
- 38.Scott KW, Grace RH. Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg. 1989;76:1165–1167. doi: 10.1002/bjs.1800761118. [DOI] [PubMed] [Google Scholar]
- 39.Cawthorn SJ, Gibbs NM, Marks CG. Clearance technique for the detection of lymph nodes in colorectal cancer. Br J Surg. 1986;73:58–60. doi: 10.1002/bjs.1800730124. [DOI] [PubMed] [Google Scholar]
- 40.Jass JR, Miller K, Northover JM. Fat clearance method versus manual dissection of lymph nodes in specimens of rectal cancer. Int J Colorectal Dis. 1986;1:155–156. doi: 10.1007/BF01648442. [DOI] [PubMed] [Google Scholar]
- 41.Wright FC, Law CH, Last LD, Ritacco R, Kumar D, Hsieh E, Khalifa M, Smith AJ. Barriers to optimal assessment of lymph nodes in colorectal cancer specimens. Am J Clin Pathol. 2004;121:663–670. doi: 10.1309/17VK-M33B-FXF9-T8WD. [DOI] [PubMed] [Google Scholar]
- 42.Fleshman JW. Total number of lymph nodes as a quality of care measure for stage III colon cancer: is it reliable as a quality indicator? Ann Surg. 2009;249:564. doi: 10.1097/sla.0b013e3181a16d99. [DOI] [PubMed] [Google Scholar]
- 43.Maurel J, Launoy G, Grosclaude P, Gignoux M, Arveux P, Mathieu-Daude H, Raverdy N, Faivre J. Lymph node harvest reporting in patients with carcinoma of the large bowel: a French population-based study. Cancer. 1998;82:1482–1486. [PubMed] [Google Scholar]
- 44.Wong JH, Severino R, Honnebier MB, Tom P, Namiki TS. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol. 1999;17:2896–2900. doi: 10.1200/JCO.1999.17.9.2896. [DOI] [PubMed] [Google Scholar]
- 45.Goldstein NS, Sanford W, Coffey M, Layfield LJ. Lymph node recovery from colorectal resection specimens removed for adenocarcinoma. Trends over time and a recommendation for a minimum number of lymph nodes to be recovered. Am J Clin Pathol. 1996;106:209–216. doi: 10.1093/ajcp/106.2.209. [DOI] [PubMed] [Google Scholar]
- 46.Baxter NN, Morris AM, Rothenberger DA, Tepper JE. Impact of preoperative radiation for rectal cancer on subsequent lymph node evaluation: a population-based analysis. Int J Radiat Oncol Biol Phys. 2005;61:426–431. doi: 10.1016/j.ijrobp.2004.06.259. [DOI] [PubMed] [Google Scholar]
- 47.Wright FC, Law CH, Last L, Khalifa M, Arnaout A, Naseer Z, Klar N, Gallinger S, Smith AJ. Lymph node retrieval and assessment in stage II colorectal cancer: a population-based study. Ann Surg Oncol. 2003;10:903–909. doi: 10.1245/aso.2003.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Shen SS, Haupt BX, Ro JY, Zhu J, Bailey HR, Schwartz MR. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch Pathol Lab Med. 2009;133:781–786. doi: 10.5858/133.5.781. [DOI] [PubMed] [Google Scholar]
- 49.Baxter NN, Ricciardi R, Simunovic M, Urbach DR, Virnig BA. An evaluation of the relationship between lymph node number and staging in pT3 colon cancer using population-based data. Dis Colon Rectum. 2010;53:65–70. doi: 10.1007/DCR.0b013e3181c70425. [DOI] [PubMed] [Google Scholar]
- 50.Okuyama T, Oya M, Ishikawa H. Budding as a risk factor for lymph node metastasis in pT1 or pT2 well-differentiated colorectal adenocarcinoma. Dis Colon Rectum. 2002;45:628–634. doi: 10.1007/s10350-004-6259-0. [DOI] [PubMed] [Google Scholar]
- 51.Tekkis PP, Smith JJ, Heriot AG, Darzi AW, Thompson MR, Stamatakis JD. A national study on lymph node retrieval in resectional surgery for colorectal cancer. Dis Colon Rectum. 2006;49:1673–1683. doi: 10.1007/s10350-006-0691-2. [DOI] [PubMed] [Google Scholar]
- 52.Canessa CE, Badía F, Fierro S, Fiol V, Háyek G. Anatomic study of the lymph nodes of the mesorectum. Dis Colon Rectum. 2001;44:1333–1336. doi: 10.1007/BF02234794. [DOI] [PubMed] [Google Scholar]
- 53.Topor B, Acland R, Kolodko V, Galandiuk S. Mesorectal lymph nodes: their location and distribution within the mesorectum. Dis Colon Rectum. 2003;46:779–785. doi: 10.1007/s10350-004-6656-4. [DOI] [PubMed] [Google Scholar]
- 54.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 55.Galon J, Fridman WH, Pagès F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 56.Doll D, Gertler R, Maak M, Friederichs J, Becker K, Geinitz H, Kriner M, Nekarda H, Siewert JR, Rosenberg R. Reduced lymph node yield in rectal carcinoma specimen after neoadjuvant radiochemotherapy has no prognostic relevance. World J Surg. 2009;33:340–347. doi: 10.1007/s00268-008-9838-8. [DOI] [PubMed] [Google Scholar]
- 57.Morcos B, Baker B, Al Masri M, Haddad H, Hashem S. Lymph node yield in rectal cancer surgery: effect of preoperative chemoradiotherapy. Eur J Surg Oncol. 2010;36:345–349. doi: 10.1016/j.ejso.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Wijesuriya RE, Deen KI, Hewavisenthi J, Balawardana J, Perera M. Neoadjuvant therapy for rectal cancer down-stages the tumor but reduces lymph node harvest significantly. Surg Today. 2005;35:442–445. doi: 10.1007/s00595-004-2956-5. [DOI] [PubMed] [Google Scholar]
- 59.Wichmann MW, Müller C, Meyer G, Strauss T, Hornung HM, Lau-Werner U, Angele MK, Schildberg FW. Effect of preoperative radiochemotherapy on lymph node retrieval after resection of rectal cancer. Arch Surg. 2002;137:206–210. doi: 10.1001/archsurg.137.2.206. [DOI] [PubMed] [Google Scholar]
- 60.Nagtegaal ID, van Krieken JH. The role of pathologists in the quality control of diagnosis and treatment of rectal cancer-an overview. Eur J Cancer. 2002;38:964–972. doi: 10.1016/s0959-8049(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 61.Habr-Gama A, Perez RO, Proscurshim I, Rawet V, Pereira DD, Sousa AH, Kiss D, Cecconello I. Absence of lymph nodes in the resected specimen after radical surgery for distal rectal cancer and neoadjuvant chemoradiation therapy: what does it mean? Dis Colon Rectum. 2008;51:277–283. doi: 10.1007/s10350-007-9148-5. [DOI] [PubMed] [Google Scholar]
- 62.Kim YW, Kim NK, Min BS, Lee KY, Sohn SK, Cho CH, Kim H, Keum KC, Ahn JB. The prognostic impact of the number of lymph nodes retrieved after neoadjuvant chemoradiotherapy with mesorectal excision for rectal cancer. J Surg Oncol. 2009;100:1–7. doi: 10.1002/jso.21299. [DOI] [PubMed] [Google Scholar]
- 63.Söreide K, Janssen EA, Söiland H, Körner H, Baak JP. Microsatellite instability in colorectal cancer. Br J Surg. 2006;93:395–406. doi: 10.1002/bjs.5328. [DOI] [PubMed] [Google Scholar]
- 64.Søreide K, Nedrebø BS, Søreide JA, Slewa A, Kørner H. Lymph node harvest in colon cancer: influence of microsatellite instability and proximal tumor location. World J Surg. 2009;33:2695–2703. doi: 10.1007/s00268-009-0255-4. [DOI] [PubMed] [Google Scholar]
- 65.Bilimoria KY, Stewart AK, Edge SB, Ko CY. Lymph node examination rate, survival rate, and quality of care in colon cancer. JAMA. 2008;299:896; author reply 897–898. doi: 10.1001/jama.299.8.896-a. [DOI] [PubMed] [Google Scholar]
- 66.Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 67.De Ridder M, Vinh-Hung V, Van Nieuwenhove Y, Hoorens A, Sermeus A, Storme G. Prognostic value of the lymph node ratio in node positive colon cancer. Gut. 2006;55:1681. doi: 10.1136/gut.2006.104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee HY, Choi HJ, Park KJ, Shin JS, Kwon HC, Roh MS, Kim C. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14:1712–1717. doi: 10.1245/s10434-006-9322-3. [DOI] [PubMed] [Google Scholar]
- 69.Schumacher P, Dineen S, Barnett C, Fleming J, Anthony T. The metastatic lymph node ratio predicts survival in colon cancer. Am J Surg. 2007;194:827–831; discussion 831-832. doi: 10.1016/j.amjsurg.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Hassett JM, Dayton MT, Kulaylat MN. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008;15:1600–1608. doi: 10.1245/s10434-007-9716-x. [DOI] [PubMed] [Google Scholar]
- 71.Park IJ, Choi GS, Jun SH. Nodal stage of stage III colon cancer: the impact of metastatic lymph node ratio. J Surg Oncol. 2009;100:240–243. doi: 10.1002/jso.21273. [DOI] [PubMed] [Google Scholar]
- 72.Wang J, Kulaylat M, Rockette H, Hassett J, Rajput A, Dunn KB, Dayton M. Should total number of lymph nodes be used as a quality of care measure for stage III colon cancer? Ann Surg. 2009;249:559–563. doi: 10.1097/SLA.0b013e318197f2c8. [DOI] [PubMed] [Google Scholar]
- 73.Peschaud F, Benoist S, Julié C, Beauchet A, Penna C, Rougier P, Nordlinger B. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg. 2008;248:1067–1073. doi: 10.1097/SLA.0b013e31818842ec. [DOI] [PubMed] [Google Scholar]
- 74.Rosenberg R, Friederichs J, Schuster T, Gertler R, Maak M, Becker K, Grebner A, Ulm K, Höfler H, Nekarda H, et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3,026 patients over a 25-year time period. Ann Surg. 2008;248:968–978. doi: 10.1097/SLA.0b013e318190eddc. [DOI] [PubMed] [Google Scholar]
- 75.Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch KW, Kopp R, Pütterich E, Ruppert R, Schuster T, et al. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. 2010;251:1070–1078. doi: 10.1097/SLA.0b013e3181d7789d. [DOI] [PubMed] [Google Scholar]
- 76.Telian SH, Bilchik AJ. Significance of the lymph node ratio in stage III colon cancer. Ann Surg Oncol. 2008;15:1557–1558. doi: 10.1245/s10434-008-9862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Available from: http: //www.acpgbi.org.uk/assets/documents/COLO_guides.pdf.
- 78.Ono K, Ochiai R, Yoshida T, Kitagawa M, Omagari J, Kobayashi H, Yamashita Y. Comparison of diffusion-weighted MRI and 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for detecting primary colorectal cancer and regional lymph node metastases. J Magn Reson Imaging. 2009;29:336–340. doi: 10.1002/jmri.21638. [DOI] [PubMed] [Google Scholar]
- 79.Akiyoshi T, Oya M, Fujimoto Y, Kuroyanagi H, Ueno M, Yamaguchi T, Koyama M, Tanaka H, Matsueda K, Muto T. Comparison of preoperative whole-body positron emission tomography with MDCT in patients with primary colorectal cancer. Colorectal Dis. 2009;11:464–469. doi: 10.1111/j.1463-1318.2008.01643.x. [DOI] [PubMed] [Google Scholar]
- 80.Herrera-Ornelas L, Justiniano J, Castillo N, Petrelli NJ, Stulc JP, Mittelman A. Metastases in small lymph nodes from colon cancer. Arch Surg. 1987;122:1253–1256. doi: 10.1001/archsurg.1987.01400230039006. [DOI] [PubMed] [Google Scholar]
- 81.Ratto C, Sofo L, Ippoliti M, Merico M, Doglietto GB, Crucitti F. Prognostic factors in colorectal cancer. Literature review for clinical application. Dis Colon Rectum. 1998;41:1033–1049. doi: 10.1007/BF02237397. [DOI] [PubMed] [Google Scholar]
- 82.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 83.Quadros CA, Lopes A, Araújo I. Retroperitoneal and lateral pelvic lymphadenectomy mapped by lymphoscintigraphy for rectal adenocarcinoma staging. Jpn J Clin Oncol. 2010;40:746–753. doi: 10.1093/jjco/hyq060. [DOI] [PubMed] [Google Scholar]
- 84.Chen SL, Bilchik AJ. Resecting lymph nodes in colon cancer: more than a staging operation? Ann Surg Oncol. 2007;14:2175–2176. doi: 10.1245/s10434-007-9430-8. [DOI] [PubMed] [Google Scholar]
- 85.Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJ, Tekkis P. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol. 2009;10:1053–1062. doi: 10.1016/S1470-2045(09)70224-4. [DOI] [PubMed] [Google Scholar]
- 86.Yano H, Moran BJ, Watanabe T, Sugihara K. Lateral pelvic lymph-node dissection: still an option for cure. Lancet Oncol. 2010;11:114; author reply 114–115. doi: 10.1016/S1470-2045(09)70361-4. [DOI] [PubMed] [Google Scholar]
- 87.Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka S, Shirouzu K, Muto T. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. 2006;49:1663–1672. doi: 10.1007/s10350-006-0714-z. [DOI] [PubMed] [Google Scholar]
- 88.Moriya Y. Differences in rectal cancer surgery: East versus West. Lancet Oncol. 2009;10:1026–1027. doi: 10.1016/S1470-2045(09)70321-3. [DOI] [PubMed] [Google Scholar]
- 89.Simunovic M, Baxter NN. Lymph node counts in colon cancer surgery: lessons for users of quality indicators. JAMA. 2007;298:2194–2195. doi: 10.1001/jama.298.18.2194. [DOI] [PubMed] [Google Scholar]
- 90. Available from: http: //en.wikipedia.org/wiki/Will_Rogers_phenomenon.
- 91.Buyse M, Sargent DJ, Grothey A, Matheson A, de Gramont A. Biomarkers and surrogate end points--the challenge of statistical validation. Nat Rev Clin Oncol. 2010;7:309–317. doi: 10.1038/nrclinonc.2010.43. [DOI] [PubMed] [Google Scholar]
- 92.Baxter NN. Is lymph node count an ideal quality indicator for cancer care? J Surg Oncol. 2009;99:265–268. doi: 10.1002/jso.21197. [DOI] [PubMed] [Google Scholar]
- 93.Wong SL, Ji H, Hollenbeck BK, Morris AM, Baser O, Birkmeyer JD. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA. 2007;298:2149–2154. doi: 10.1001/jama.298.18.2149. [DOI] [PubMed] [Google Scholar]


