Abstract
Insomnia is prevalent, causing severe distress and impairment. This review focuses on illuminating the puzzling finding that many insomnia patients misperceive their sleep. They overestimate their sleep onset latency (SOL) and underestimate their total sleep time (TST), relative to objective measures. This tendency is ubiquitous (although not universal). Resolving this puzzle has clinical, theoretical, and public health importance. There are implications for assessment, definition, and treatment. Moreover, solving the puzzle creates an opportunity for "real world" applications of theories from clinical, perceptual, and social psychology as well as neuroscience. Herein we evaluate thirteen possible resolutions to the puzzle. Specifically, we consider the possible contribution, to misperception, of: (1) features inherent to the context of sleep (e.g., darkness); (2) the definition of sleep onset which may lack sensitivity for insomnia patients; (3) insomnia being an exaggerated sleep complaint; (4) psychological distress causing magnification; (5) a deficit in time estimation ability; (6) sleep being misperceived as wake; (7) worry and selective attention toward sleep-related threats; (8) a memory bias influenced by current symptoms and emotions, a confirmation bias/belief bias or a recall bias linked to the intensity/recency of symptoms; (9) heightened physiological arousal; (10) elevated cortical arousal; (11) the presence of brief awakenings; (12) a fault in neuronal circuitry; and (13) there being two insomnia subtypes (one with and one without misperception). The best supported resolutions were misperception of sleep as wake, worry, and brief awakenings. A deficit in time estimation ability was not supported. We conclude by proposing several integrative solutions.
Keywords: sleep, insomnia, perception, measurement, polysomnography, comorbidity
Insomnia is an ongoing difficulty initiating sleep, maintaining sleep, waking up too early, or experiencing chronically non-restorative sleep, despite adequate opportunity to sleep (Edinger et al., 2004). Approximately 6% of the adult population meet diagnostic criteria for insomnia (Ohayon, 2002). Insomnia can occur as the sole presenting problem and it commonly co-occurs with a wide range of other psychiatric disorders (Benca, Obermeyer, Thisted, & Gillin, 1992) and medical disorders (Smith, Huang, & Manber, 2005; Taylor et al., 2007).
Much progress has been made toward understanding insomnia. The adverse consequences for the individual are well documented and include negative psychosocial, occupational, health, and economic repercussions including more frequent sick leave and greater use of health care resources relative to good sleepers (Chilcott & Shapiro, 1996). Insomnia significantly heightens the risk of accident (Ohayon, Caulet, Philip, Guilleminault, & Priest, 1997) and longitudinal studies indicate that insomnia increases the risk of developing depression, anxiety, and substance-related problems (Breslau, Roth, Rosenthal, & Andreski, 1996; Ford & Kamerow, 1989). Moreover, insomnia is associated with a range of adverse health consequences (Buysse et al., 2007; Kyle, Morgan, & Colin, 2010).
Evidence relating to mechanisms continues to burgeon across levels of explanation. Behavioral contributors to insomnia include spending too much time in bed trying to sleep, erratic sleep-wake schedules as well as lifestyle choices that interfere with sleep (e.g., caffeine or alcohol use too close to bedtime; Bootzin & Perlis, 1992; Morin, 1993; Stepanski & Wyatt, 2003). Cognitive contributors include unhelpful beliefs about sleep, worry/rumination, and selective attention to threat (Espie, 2002; Harvey, 2002; Morin, 1993). The biological contributors include abnormalities in sleep EEG (beta EEG during sleep; Perlis, Giles, Mendelson, Bootzin, & Wyatt, 1997), neuroendocrine functioning (increased plasma level of adrenocorticotropic hormone and cortisl;Vgontzas et al., 2001), brain metabolism (greater global glucose metabolism during sleep; Nofzinger et al., 2004), and brain activity (an abnormality in the balance between the limbic system and the ventrolateral preoptic nucleus; Cano, Mochizuki, & Saper, 2008).
Research on treatments has been particularly prolific. Three decades of research has documented the efficacy and effectiveness of cognitive behavior therapy (CBT) as a treatment for insomnia (Morin et al., 2006). CBT-I is a multi-component treatment typically comprised of a set of surprisingly powerful, but relatively simple, procedures. The components typically include stimulus control, sleep restriction, sleep hygiene education, relaxation, and cognitive therapy (Morin et al., 2006). While there is evidence for medication approaches (Krystal, 2009; Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008), hypnotic medications carry risks of daytime residual effects as well as risks of tolerance, dependence, and rebound insomnia. Also, the durability of the improvement to sleep associated with pharmacological treatment is not well documented and there are questions about whether the sleep obtained confers the full benefit of "natural" sleep or sleep in the absence of medications (Seibt et al., 2008).
Taken together, this evidence documenting adverse consequences, mechanisms, and effective treatments is quite impressive. However, the field continues to puzzle over one robust finding; that many patients with insomnia overestimate their sleep onset latency (SOL) and underestimate their total sleep time (TST), relative to objective measures of sleep such as polysomnography (PSG; Bixler, Kales, Leo, & Slye, 1973; Carskadon et al., 1976; Coates et al., 1982; Frankel, Buchbinder, Coursey, & Snyder, 1973; Hauri & Fisher, 1986; Hoddes, Carskadon, Phillips, Zarcone, & Dement, 1972; Means, Edinger, Glenn, & Fins, 2003; Perlis, Smith, Andrews, Orff, & Giles, 2001; Roth, Lutz, Kramer, & Tietz, 1977; Schneider-Helmert & Kumar, 1995; Vanable, Aikens, Tadimeti, Caruana-Montaldo, & Mendelson, 2000) and actigraphy (Tang & Harvey, 2004a, 2006; Van den Berg et al., 2008; Wicklow & Espie, 2000). Table 1 includes a definition of, and further information about, PSG and actigraphy. This puzzling tendency toward misperception of sleep appears to be common among patients for whom insomnia is the only disorder as well as patients whose insomnia is comorbid with another psychiatric/medical disorder. Indeed, a discrepancy between subjective and objective measures of sleep have been documented in patients with posttraumatic stress disorder (Dagan, Zinger, & Lavie, 1997; Klein, Koren, Arnon, & Lavie, 2002, 2003), bipolar disorder (Harvey, Schmidt, Scarna, Semler, & Goodwin, 2005), unipolar depression (Rotenberg, Indursky, Kayumov, Sirota, & Melamed, 2000), alcohol problems (Currie, Malhotra, & Clark, 2004), chronic pain (Wilson, Watson, & Currie, 1998), chronic fatigue syndrome (Neu et al., 2007), irritable bowel syndrome (Elsenbruch, Harnish, & Orr, 1999), and rheumatoid arthritis (Hirsch et al., 1994), although we also note occasional non-replications (e.g., Armitage, Trivedi, Hoffmann, & Rush, 1997; Rotenberg et al., 2000). The tendency to misperceive has also been reported among youth with attention-deficit/hyperactivity disorder (Kaplan, McNicol, Conte, & Moghadam, 1987) and depressed youth, although not youth with anxiety disorders (Bertocci et al., 2005; Forbes et al., 2008). The tendency toward misperception is also evident regardless of whether sleep is monitored in the patient’s own home (Mercer, Bootzin, & Lack, 2002; Tang & Harvey, 2004a, 2006; Wicklow & Espie, 2000) or in the sleep lab (Bixler et al., 1973; Schneider-Helmert & Kumar, 1995). Moreover, if a person exhibits misperception on one night they tend to misperceive over multiple nights (Means et al., 2003). In stark contrast, the majority of normal sleepers are able to accurately perceive their sleep (Baekeland & Hoy, 1971; Manconi et al., 2010; Mendelson, 1995).
Table 1.
Description of ‘objective’ measures of sleep
| Technology | Description | Pros | Cons |
|---|---|---|---|
| Polysomnography (PSG) | Based on electrophysiological recordings of brain activity that radiates from the scalp (EEG; electroencephalogram) along with muscle activity (EMG; electromyogram) and eye movement (EOG; electrooculogram). The electrical output is scored and categorized into different stages of sleep/wakefulness (Wakefulness, REM sleep, Stages 1, 2, 3 & 4 sleep) to provide information about the pattern and architecture of sleep. |
|
|
| Actigraphy | Based on an estimate of sleep/wakefulness by monitoring physical motion. Aided by validated scoring algorithms, actigraphy provides information about the sleep pattern (e.g., sleep onset latency, wake after sleep onset, total sleep time, sleep efficiency) and diurnal variations of physical activity. Continuous use of actigraphy over successive 24 hour periods can provide reliable information about a person’s sleep-wake cycle. |
|
|
Another set of findings that add to the puzzle is that during certain phases of sleep, patients with insomnia interpret sleep as wakefulness. Rechtschaffen and Monroe (1969) were among the first to report this phenomenon. They found that, when woken up 10 minutes after the first sleep spindle, poor sleepers were more likely to report that they had been awake the moment just before they were called by the experimenter, compared to good sleepers. Borkovec, Lane, and Van Oot (1981) further examined this phenomenon by waking 25 patients with insomnia and 10 good sleepers five minutes after the first sleep spindle. Patients with insomnia were more likely, relative to good sleepers, to report that they had been awake the moment just before they were woken. This finding occurred regardless of whether the sleep was obtained at night or during an afternoon nap.
Taken together, this overall pattern of findings is very perplexing; many patients with insomnia perceive sleep as wake, systematically overestimate the time they take to get to sleep (SOL) and underestimate the time they sleep in total (TST). It is noteworthy that the diagnostic nomenclatures include a diagnosis that captures extreme cases of this tendency. For example, the Research Diagnostic Criteria (RDC; Edinger et al., 2004) defines "paradoxical insomnia" as a subtype of insomnia disorder and requires that PSG shows TST greater than 6.5 hours, sleep efficiency (SE; TST/Time in bed × 100) greater than 85% and daytime impairment that is less severe than would be typical from the subjective report of the extent of the sleep disturbance. The core of the International Classification of Sleep Disorders (American Academy of Sleep Medicine, 2005) definition of paradoxical insomnia involves the patient meeting criteria for insomnia for the past month as well as at least one of the following: a chronic pattern of reporting little or no sleep or a mismatch between objective and subjective findings.
The utility of such diagnoses has been questioned on the basis that misperception of sleep is so ubiquitous among people with insomnia (Edinger & Krystal, 2003; Reynolds, Kupfer, Buysse, Coble, & Yeager, 1991; Salin-Pascual, Roehrs, Merlotti, Zorick, & Roth, 1992). Indeed, there is evidence that misperception of sleep occurs to varying degrees across different insomnia diagnoses and subtypes including psychophysiologic insomnia, insomnia associated with depression and insomnia associated with inadequate sleep hygiene (Edinger & Fins, 1995; Hauri & Olmstead, 1983). It seems plausible that individuals who meet diagnostic criteria for paradoxical insomnia represent only one extreme of a continuum and that other individuals with insomnia fall somewhere along the continuum (Bonnet & Arand, 1997; Reynolds et al., 1991; Salin-Pascual et al., 1992; Trinder, 1988).
It is important to note that this tendency to misperceive is not universal among insomnia patients. In fact, a small proportion of patients display the opposite tendency; they perceive they are asleep when they are not. Or, put another way, they are exhibiting worse sleep than they are reporting (Attarian, Duntley, & Brown, 2004; Edinger et al., 2000; Schneider-Helmert, 2007; Trajanovic, Radivojevic, Kaushansky, & Shapiro, 2007). So while the present review focuses on the overall group tendency for patients with insomnia to overestimate SOL and underestimate TST, we emphasize that misperception of sleep is not characteristic of all insomnia sufferers (Means et al., 2003).
The aim of this paper is twofold. First, we outline why resolving the puzzle matters. Second, we propose and evaluate thirteen potential resolutions to the puzzle; drawing on the clinical and social psychology literatures as well as the neuroscience and perceptual psychology literatures. Throughout we will refer to "patients with insomnia" as encompassing those who have a sole diagnosis of insomnia as well as those whose insomnia is comorbid with another psychiatric/medical disorder (e.g., depression, anxiety disorder, pain disorder). To the best of our knowledge, there is currently no evidence that the misperception of sleep exhibited by patients who do and do not have a comorbid disorder is different.
Why Does Resolving this Puzzle Matter?
Clinical Implications
Trivialization of insomnia
We posit that the data indicating a discrepancy between subjectively perceived and objectively measured sleep may contribute to the tendency to trivialize, under treat, and under research insomnia and its associated distress (Leger, 2000; Nutt & Wilson, 1999). In addition, at various levels of resource allocation, including the allocation of clinical services and grants for research, objective verification may well be a seriously unfair standard. Indeed, objective verification is not used as a standard for resource allocation for other psychiatric disorders. For example, to meet diagnostic criteria, people with depression do not need to "objectively" verify their feelings or their negative view of themselves, the world and other people. A sceptic may critique this comparison by saying “but insomnia is different because if a person tells me he/she has insomnia but the sleep measures say he/she is sleeping normally, then I doubt the presence of insomnia”. In response, as will become evident in the discussion that follows (e.g., Resolutions #10 and #11), it is possible that the gold standard measures of sleep are not adequately capturing the sleep deficits that insomnia patients perceive. As such, the depression metaphor seems relevant. In sum, it is possible that an improved understanding of the discrepancy between subjectively perceived and objectively documented sleep deficits will have important implications for the allocation of treatment and research resources for insomnia.
Adverse consequences
We emphasize that a tendency to misperceive sleep does not preclude the presence of a real sleep deficit. It is not uncommon for a patient with insomnia to report sleeping only two hours a night. After completing an objective assessment, it often becomes evident that the patient is actually sleeping four hours. In this example, the patient is misperceiving their sleep and suffering from a serious "real" sleep deficit.
In fact, it has been suggested that patients who think they are sleeping less than they are actually sleeping but, in reality, are sleeping sufficiently, are at grave risk for getting trapped into becoming progressively more absorbed by and anxious about their sleep problem (Harvey, 2002). These are the patients at high risk for developing a serious real sleep deficit. The mechanisms for the latter have been proposed to include that misperception of a sleep deficit (a) fuels worry and excessive preoccupation, concern, and distress about sleep and (b) contributes to an escalating cycle of increasing anxiety and arousal that renders optimal sleep onset and maintenance even more difficult to achieve. This is consistent with the view that the tendency among insomnia patients to misperceive their sleep may be considered as a “prodromic or transitional state” in the development of insomnia that is characterized by a serious objective sleep deficit (e.g., Salin-Pascual et al., 1992).
Also consistent is the accruing evidence documenting adverse consequences of misperception of sleep. Sugerman, Stern, and Walsh (1985) compared two groups of insomnia patients; individuals whose report of sleep disturbance could be verified (the "objective insomnia" group) and individuals whose report of sleep disturbance could not be verified (the "subjective insomnia" group). Intriguingly, the subjective insomnia group exhibited impaired performance on vigilance tasks relative to the objective insomnia group. Vanable et al. (2000) reported that misperception of sleep among a diverse sample of patients was associated with longer periods of wakefulness following sleep onset and greater self-perceived sleep impairment. Means et al. (2003) found that insomnia patients who misperceived their sleep scored higher on the Stanford Sleepiness Scale than did groups of insomnia patients who did not overestimate their sleep. Van den Berg et al. (2008) found that more misperception was associated with poorer cognitive function and greater functional disability.
Treatment
As already noted, while there is no doubt that effective treatments have been developed for insomnia (e.g., Morin et al., 2006), the field is not yet at a point where patients can be offered a maximally effective treatment. This is indicated by the significant proportion of patients who do not improve following treatment and by the average overall improvement among those who do respond being 50–60% (e.g., Morin, Culbert, & Schwartz, 1994; Murtagh & Greenwood, 1995). Although this degree of change is statistically significant, it is not enough to convincingly move the average patient into a state where we would call them, and they would call themselves, good sleepers (Harvey & Tang, 2003). If the tendency to misperceive sleep turns out to be a mechanism that contributes to the escalation of insomnia, then reversing misperception - as part of a multi-component treatment for insomnia - may contribute to improving treatment outcomes. We return to this possibility later.
Definition and outcome measures
The resolution of the puzzle has implications for the definition of insomnia as well as the measurement of treatment outcome. At present, the field awkwardly straddles adopting a clinical definition of insomnia that relies solely on subjectively perceived sleep (Edinger et al., 2004) but yet recommending the research assessment of insomnia includes both subjective and objective measures (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006). The inclusion of objective measures before and after treatment can be expensive, time consuming and add considerable burden for patients and researchers. Hence, working to resolve which category of measures (objective or subjective) best captures insomnia, or whether both types are needed, is likely to be a worthwhile endeavour with implications for the definition of, and diagnostic criteria for, insomnia and for the selection of outcome measures.
Theoretical Implications
Lack of coherence
Over the past few decades there has been a sea change in the measurement methods available for psychological research. In particular, there has been a proliferation of measurement methods that do not rely on self-report. As a consequence, several literatures have noted a lack of coherence in the information obtained from the subjective and the objective measures. For example, in the emotion literature, lack of coherence has been observed between subjectively reported emotional experience and psychophysiological measures such as measures of heart rate, blood pressure and galvanic skin response (Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005). The consumers of such results face the challenge of valuing one measure over the other or of trying to reconcile the discrepancy.
With regard to the decision to value one level of measurement over another, we agree with Miller (1996) who warns that there “often seems to be an ideological war between psychologically and biologically inclined researchers … (and) intellectual atrocities (are) committed by both sides” (p. 619). Potential disadvantages of self-report include the assumption that all humans can/have equal ability to accurately introspect about and then accurately report on the phenomenon of interest. Potential disadvantages of so called "objective" methods are that they may not be valid. For example, Resolutions #10 and #11 raise the possibility that the gold standard measures of sleep are inadequate for capturing the sleep deficits that insomnia patients perceive. Also, potential biases in these measurements can be overlooked (Miller, 1996). Hence, a further reason for seeking resolution of the discrepancy between subjective and objective measures within the insomnia literature is that the process or outcome may contribute to reconciling discrepant findings within the broader psychological literature. It is compelling to consider ways to bring about resolution and depth of understanding, rather than fueling the so-called "ideological war".
One possibility is that the key may be within the incoherent state itself. For example, Mauss et al. (in press) have reported that greater coherence between subjective emotional experience and indirect measures of emotion (i.e., facial behavior and physiology) is related to increased positive affect and well-being. Moreover, a lack of coherence has been reported across a number of psychiatric disorders with adverse implications for functioning (e.g., Asendorpf & Scherer, 1983; Gruber, Johnson, Oveis, & Keltner, 2008; Kring & Neale, 1996).
Clinical/perceptual/social psychology and neuroscience
The puzzle at hand is also fascinating when taken as a "real world" application, and test of, theories within clinical, perceptual, and social psychology, as well as neuroscience. As will become evident later in this paper, empirical and theoretical findings within these literatures suggest several explanations for the puzzle that implicate contextual, psychological, and biological processes that may be operating to bias perception of sleep.
Resolutions to the Puzzle
In this section we offer, and critically evaluate, thirteen possible resolutions to the puzzle. As will become evident, these accounts are not necessarily mutually exclusive. Table 3 summarizes the key studies pertinent to each resolution.
Table 3.
Summary of Evidence for Each Resolution
| Authors | Groups N = Sample size |
Causal evidence? | Paradigm | Measure of Sleep | Findings |
|---|---|---|---|---|---|
| #1. The Challenge of the Context | |||||
| Tang et al., 2007 (Experiment 2) | Insomnia (n = 38) | Yes | Clock Monitoring vs. Digit Display Unit Monitoring | Actigraphy, Sleep diary | Clock monitoring resulted in greater misperception of sleep, relative to digit display unit monitoring |
| #2. An Artifact of Sleep Onset Definition | |||||
| Spielman et al., 1980 | Insomnia (n = 55) | Yes | Adjusted traditional definition of sleep onset | PSG, Sleep diary | Misperception decreased when sleep onset was defined as at least 10 minutes after the 1st stage of sleep |
| Hauri & Olmstead, 1983 | Insomnia (n= 56) Good sleepers (n = 10) |
Yes | As above | As above | Misperception decreased when sleep onset was defined as lights out to the beginning of the first 15 minutes of Stage 2 |
| #3 and 4. Exaggerated Complaint and Psychological Distress | |||||
| See Table 2 | See Table 2 | No | Administered MMPI | See Table 2 | See Table 2 |
| #5: A Deficit in Time Estimation | |||||
| Moore et al., 1982 | Insomnia (n = 12) Normal sleepers (n = 12) |
No | Time estimation tasks | N/A | Insomnia and normal sleeper groups differ on time estimate |
| Tang & Harvey, 2005 | Insomnia (n = 20) Normal sleepers (n = 20) |
No | As above | N/A | As above |
| Rioux et al., 2006 | Insomnia (n = 11) Good sleepers (n = 11) |
No | As above | N/A | As above |
| #6. Sleep as Wake | |||||
| Borkovec et al., 1981 | Insomnia (n = 25) Good sleeper (n = 10) |
Yes | Woken during Stage 2 sleep | PSG, Sleep report (awake vs. asleep) | Insomnia patients perceive sleep as wake |
| Mercer et al., 2002 | Insomnia (n = 14) Good sleepers (n = 8) |
Yes | As above | As above | As above plus this was correlated with extent of misperception of sleep |
| #7a. Worry | |||||
| Van Egeren et al., 1983 | Insomnia (n = 13) | No | Interview about presleep cognition, 5 nights in sleep laboratory. On the 5th night a presleep cognition sampling procedure was implemented | PSG, Sleep diary | Positive correlation between negative pre-sleep cognitive arousal and misperception of sleep |
| Tang & Harvey, 2005 | See #5 | No | See #5 | See #5 | Time overestimation correlated with cognitive arousal |
| Tang & Harvey, 2004 | Healthy good sleepers (n = 54) | Yes | Activated anxious cognitive arousal or neutral cognitive arousal before an afternoon nap | Actigraphy, Sleep diary | Presleep cognitive arousal contributes to misperception of sleep |
| Tang et al., 2007 | See #1 | Yes | See #1 | See #1 | Significant positive correlation between worry and misperception of sleep in clock monitoring group |
| #7b. Selective attention | |||||
| Rachman & Cuk, 1992 | Undergraduates 60 with a spider/snake phobia,60 with no phobia | Yes | Exposed to snake/spider | N/A | Participants with a snake/spider phobia reported misperceptions of the activity of the spider/snake |
| Ehlers, 1995 | 39 patients with panic disorder, 17 individuals whose panic disorder had remitted, 46 infrequent panicers, 22 simple phobics, 45 controls | Yes | Heart beat perception task. Assessed 1 year later. | N/A | Good heart beat perception at the first assessment predicted relapse 1 year later. |
| Tang et al., 2007 | See #1 | Yes | See #1 | See #1 | Clock monitoring was associated with greater misperception of sleep relative to digit display unit monitoring |
| #8a. Memory Bias due to Current Symptoms | |||||
| Eich et al., 1985 | 57 patients with history of chronic headaches | No | Rated pain each hour for one week. In clinic retrospectively rated intensity of pain, max/min/usual pain in the past week | N/A | Greater overestimation of pain among those with high levels of present pain |
| #8b. Memory Bias due to Emotions | |||||
| Schwarz & Clore, 1983 | Undergraduates Expt 1: 61 Expt 2: 93 |
Yes | Mood manipulation (happy, sad) Ratings of happiness and life satisfaction |
N/A | When in a bad mood, ratings of happiness and life satisfaction are lower. Impact of negative mood was eliminated when s’s induced to attribute present feelings to transient external sources. |
| Arntz et al., 1995 | 52 spider phobia patients 41 panic disorder patients 38 social phobia patients 31 other anxiety disorder patients 24 controls |
Yes | Rated scripts in which objective safety vs. danger and anxiety response vs. non-response varied | N/A | Anxiety patient ratings were influenced by anxiety response information (i.e., danger is inferred from anxiety response) |
| #8c. Memory bias due to Confirmation Bias/Belief Bias | |||||
| Evans et al., 1993 | 155 women with family history of breast cancer | No | Asked to identify population risk and own risk of breast cancer | N/A | Participants overestimated risk |
| Means et al., 2003 | 52 insomnia patients 49 normal sleepers |
No | Across 6 nights of PSG. Completed the Dysfunctional Beliefs and Attitudes Scale | PSG, Sleep diary | Insomnia patients who reported the most misperception also endorsed the most dysfunctional beliefs |
| #8d. Memory Bias due to the Intensity or Recency of Symptoms | |||||
| Redelmeier & Kahneman, 1996 | Patients undergoing colonoscopy (n = 154) and lithotripsy (n = 133) | No | Real time pain intensity ratings (during the procedure). After the procedure, retrospective ratings of pain intensity. | N/A | Retrospective evaluations were strongly correlated with peak pain and end pain. |
| #9. Physiological Arousal | |||||
| Bonnet & Arand, 1992 | Normal sleepers (n = 12, male) | Yes | 11 nights | 400 mgs of caffeine 3× a day for 7 nights/days | Caffeine increased metabolic rate. Sleep efficiency reduced. Participants with the greatest change in metabolic rate overestimated their sleep onset latency |
| Bonnet & Arand, 1994 | Several studies summarized, N not available | Yes | Naps and Overnight studies | Administer caffeine or benzodiazepines | Caffeine increased misperception; Dose response reduction in misperception with increase in dose of benzodiazepines |
| Bonnet & Arand, 1997 | Insomnia patients who misperceive their sleep n = 9, Controls n = 9 |
No | 2 nights of PSG, recording of metabolic rate | PSG, Sleep diary | The insomnia patients who misperceive their sleep exhibit a higher metabolic rate |
| Tang & Harvey, 2004 | 54 health good sleepers | Yes | Anxious cognitive arousal activated (speech threat) or physiological arousal activated (caffeine pill) during an afternoon nap | Actigraphy, Sleep diary | Both anxious cognitive arousal and physiological arousal contributed to misperception |
| #10. Cortical Arousal | |||||
| Perlis et al., 2001 | Primary insomnia (n = 9), Insomnia secondary to major depression (n = 9), Good sleepers (n = 9) |
No | 2 nights of PSG | PSG, Sleep diary | Primary insomnia exhibited more beta/gamma activity during NREM and this was negatively associated with misperception of sleep |
| Krystal et al., 2002 | Normal (n = 20), Subjective insomnia (n = 12), Objective insomnia (n = 18) |
No | 1 PSG night (at home) | PSG, Sleep diary | Lower delta and greater alpha, sigma and beta NREM EEG activity was found in ‘subjective’ insomnia but not ‘objective’ insomnia, compared to normal sleepers. |
| Buysse et al., 2008 | Primary insomnia (n = 48; 29 women), Good sleepers (n = 25; 15 women) | No | 1 PSG night | PSG, sleep diary | High frequency EEG in women, no correlated with misperception |
| #11. Transient Awakenings | |||||
| Knab & Engel, 1988 | Insomnia (n = 14), Controls (n = 14) |
No | PSG and microswitch taped to dominant hand. Participants were asked to press it 2×’s whenever they became aware of having just awakened | PSG, Sleep diary | Insomnia patients have difficulty perceiving short last sleep |
| Smith & Trinder, 2009 | Normal sleepers (n = 20) | Yes | Mask condition (wore a respiratory apparatus that induced brief arousals) vs. No mask condition | 2 nights of PSG and sleep diary | During the mask condition participants experienced more brief arousals and more misperception of sleep |
| Parrino et al., 2009 | Paradoxical insomnia (n = 20), Controls (n = 20) |
No | Scored CAP parameters | PSG, sleep diary | CAP rate was higher in insomnia patients who misperceived |
| #12. Fault in Neuronal Circuitry | |||||
| Cano et al., 2008 | Adult male rats (n = 59) | Yes | Psychological stress (cage exchange). Examined Fos expression. Fos = neural activation. | EEG sleep | Fos expression increased in cerebral cortex, limbic system and parts of arousal/autonomic system plus activation of sleep promotion system |
| #13. Subtypes/Phenotypes | |||||
| Fernandez-Mendoza et al., 2011 | 1,741 adults selected from central Pennsylvania | No | MMPI | PSG, Sleep diary | MMPI was characterized by a profile similar to patients with medical disorder among insomnia patients who don’t misperceive and by a profile indicating depression/rumination etc. among insomnia patients who do misperceive |
| Fernandez-Mendoza et al., 2010 | As above | No | Neuropsychological performance | PSG, Sleep diary | Performance was poorer among insomnia patients who don’t misperceive relative to patients who do misperceive |
Note. MMPI = Minnesota Multiphasic Personality Inventory. PSG = Polysomnography. NREM = Non rapid eye movement sleep, EEG = Electroencephalography.
# 1. The Challenge of the Context
The transition from wakefulness to sleep is marked by a progressive loss of consciousness, reduction of stimulus reception, cessation of behavioral response, and an absence of memory (Ogilvie, 2001). Accordingly, for the sleeper, the exact point of sleep onset is elusive and defined by the absence of memories. The period just prior to sleep onset is often just a vague memory and sleep onset typically occurs in darkness with few or no external time cues (Bonnet, 1990; Ogilvie & Harsh, 1994). Together, features inherent to the transition from wakefulness to sleep, as well as the context of sleep, constitute fertile ground for misperception.
Understandably, given this context, many patients with insomnia report checking the clock throughout the night in order to gauge how long they are taking to fall asleep or how long they have been awake. They then calculate how much sleep they will obtain (Semler & Harvey, 2004b). Perhaps paradoxically, clock checking appears to contribute to misperception. Tang, Schmidt, and Harvey (2007) randomly allocated insomnia patients to monitor either a clock or a digit display unit as they were trying to get to sleep. The digit display unit had the appearance of a digital clock (color, size of digits) but it was programmed to display only random digits. It was included as a control for eyes being open, for effort, and for cognitive processing. Compared to those who monitored the digit display unit, those who clock-monitored overestimated their SOL. In other words, using a clock to estimate time while falling asleep paradoxically contributed to more misperception of sleep. Two explanations have been offered for this finding. First, perceptual psychologists have established that time is perceived to be longer as the number of units of information processed per unit of time is increased (Thomas & Cantor, 1976). The clock-monitors had to process more information as a function of the clock-monitoring task and they also reported worrying more about how long it was taking them to fall asleep. Hence, this group is likely to have processed more information units, which may have contributed to time overestimation. Second, detailed analysis of the actigraphic data revealed that in the first 60 minutes after sleep onset clock-monitors experienced more awakenings relative to the digit display unit monitors. Perhaps the increased worry associated with clock monitoring made it more difficult for participants to move into the deeper stages of sleep and the lighter stages of sleep may be more likely to be perceived as wake (an explanation that is considered in #6). Also, it is possible that repeated awakenings may have been perceived as continuous wakefulness (Knab & Engel, 1988). In summary, it seems plausible that the context in which humans sleep may be an important contributor to the puzzle.
# 2. An Artifact of Sleep Onset Definition
A second suggested resolution is that the discrepancy between objective and subjective estimates of sleep onset, among patients with insomnia, is an artifact of the operational definition of sleep onset (Hauri & Olmstead, 1983; Olmstead, Hauri, Percy, & Hellekson, 1980; Spielman, Tannenbaum, Saskin, Adler, & Pollak, 1980). Until recently, sleep onset has been defined according to the original Rechtschaffen and Kales (1968) sleep scoring system. According to this system, sleep onset latency is defined as the period between "lights out" and the first three epochs of consecutive Stage 1 sleep or the first epoch of Stage 2 sleep. Although these are the most widely adopted scoring criteria currently in the literature, they may be less applicable to patients with insomnia. Indeed, Spielman, Tannenbaum, Adler, and Saskin (1980) evaluated 55 patients with chronic insomnia using PSG and a sleep diary completed soon after waking. The correlation between objectively-estimated SOL (EEG estimates) and subjectively-estimated SOL (sleep diary estimates) significantly improved when sleep onset was not defined as the first appearance of Stage 1 (r = .44) nor Stage 2 (r = .46) sleep, but as the beginning of “consolidated sleep” (r = .76). The latter was defined as the sleep obtained at least 10 minutes after the first sleep stage (Spielman, Tannenbaum, Adler, & Saskin, 1980).
Intriguingly though, this problem does not occur in good sleepers. Hauri and Olmstead (1983) evaluated the objective and subjective experience of sleep onset in 56 patients with insomnia and 10 good sleepers. The three different criteria used for scoring sleep-onset were: (a) the traditional criterion defined as from lights-out to the first sign of Stage 2 sleep; (b) the 15-minute criterion defined as from lights-out to the beginning of the first 15 minutes of Stage 2 sleep; (c) the 30-minute criterion defined as from lights-out to the beginning of the first 30 minutes of Stage 2 sleep. Using the traditional criterion, the insomnia group overestimated their SOL by an average of 29.1 minutes while the good sleeper group were found to estimate their SOL accurately to within 1 minute. When the 15-minute criterion was adopted, the insomnia group only overestimated their SOL by an average of 10.9 minutes, whereas the good sleeper group underestimated by an average of 15 minutes. Using the 30-minute criterion, both the insomnia group and the good sleeper group significantly underestimated their SOL by about an hour. Based on these findings, Hauri and Olmstead (1983) concluded that the 15-minute criterion might be a better marker for sleep onset in people with insomnia relative to the traditional criterion.
More recently the definition of sleep onset has changed to focus on the start of the first epoch scored as any stage other than Stage W (wake; Iber, Ancoli-Israel, Chesson, Quan, & Medicine, 2007). The implications of this change for patients with insomnia have not yet been empirically evaluated, although it seems likely that this definition will suffer from the same problems as the traditional criteria.
Taken together, the "artefact" hypothesis seeks to account for misperception of sleep by proposing that the subjective perception of sleep, among people with insomnia, occurs later in the EEG-defined transition from waking to sleep, relative to normal sleepers. We note that some studies are employing fMRI methods (although with good sleepers) to study the process of sleep onset with the advantage of gaining more detailed information about the biological underpinnings of the sleep onset process (Picchioni et al., 2008). Advances in knowledge in this domain likely holds promise for refining the "artifact hypothesis".
# 3. An Exaggerated Complaint?
Misperception of sleep among insomnia patients may occur because they simply exaggerate their subjective report of their sleep problems. For example, Freedman (1976) interpreted the discrepancy between subjective and objective sleep as a measure of the degree of "exaggeration" because of a positive correlation between the discrepancy values and the scores on two scales of the Minnesota Multiphasic Personality Inventory (MMPI); namely, the Conversion Hysteria (Hy; r = .52) and Psychopathic Deviate (Pd; r = .66) scales. Moreover, as reviewed in Table 2, there are a number studies reporting that individuals with insomnia had elevated F scores on the MMPI against the normative T-scores (Freedman, 1976) and higher F and K scores than those obtained by normal sleepers (Kales, Caldwell, Soldatos, Bixler, & Kales, 1983; Levin, Bertelson, & Lacks, 1984; Mendelson, Garnett, Gillin, & Weingartner, 1984; Monroe, 1967; Schneider-Helmert, 1987; Shealy, Lowe, & Ritzler, 1980). The F scale of the MMPI was originally developed to detect deviant or atypical responses to the test items. The K scale was developed as an index of attempts by examinees to exaggerate or conceal psychopathology. While elevated scores on the MMPI do not directly address the issue of misperception of sleep, the researchers suggested that elevated F and K scores question the validity of the sleep complaints made by patients with insomnia. Of course, it is also possible that the elevated scores may simply be capturing "real" insomnia and the associated distress.
Table 2.
MMPI findings for Insomnia Patients
| Method | MMPI Findings | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size |
Misperception examined? |
Measure of sleep |
Validity Scale |
Clinical Scale |
|||||||||||||
| PWI/PS | NC/GS | Y or N | L | F | K | Hs | D | Hy | Pd | Mf | Pa | Pt | Sc | Ma | Si | ||
| 1. Comparison between Patients with Insomnia and Normal Sleepers | |||||||||||||||||
| Adam et al., 1986 | 18 | 18 | Y | PSG / S | |||||||||||||
| Bonnet & Arand, 1995 | 10 | 10 | N | * | * | ||||||||||||
| Coursey, 1975 | 18 | 18 | N | * | * | * | * | ||||||||||
| Fernandez-Mendoza et al., 2011 # | 142 | 724 | Y | PSG / S | * | * | * | * | * | * | * | * | |||||
| Frankel et al., 1973, 1976 | 18 | 18 | Y | PSG / S | * | * | * | * | * | ||||||||
| Freedman & Sattler, 1982 | 12 | 12 | N | * | * | * | |||||||||||
| Hauri & Fisher, 1986 | 22 | 22 | Y | PSG / S | * | * | |||||||||||
| Johns et al., 1971 | 7 | 7 | N | * | * | * | |||||||||||
| Kales et al., 1983 | 279 | 97 | N | * | * | * | * | * | * | * | * | * | |||||
| Levin et al., 1984 | 74 | 26 | Y | PSG / S | * | * | * | * | * | ||||||||
| Mendelson et al., 1984 | 10 | 10 | N | * | * | * | * | ||||||||||
| Method | MMPI Findings | ||||||||||||||||
| Sample size |
Misperception examined? |
Measure of sleep |
Validity Scale |
Clinical Scale |
|||||||||||||
| PWI/PS | NC/GS | Y or N | L | F | K | Hs | D | Hy | Pd | Mf | Pa | Pt | Sc | Ma | Si | ||
| Monroe & Marks, 1977 | 53 | 53 | N | * | * | * | * | * | |||||||||
| Monroe, 1967 | 16 | 16 | N | * | * | * | * | * | * | * | * | * | |||||
| Rosa & Bonnet, 2000 | 121 | 56 | N | * | * | * | * | * | * | * | * | * | * | ||||
| Schneider-Helmert, 1987 | 16 | 16 | N | * | * | * | * | * | * | * | * | ||||||
| Seidel et al., 1984 | 52 | 45 | Y | PSG / S | |||||||||||||
| Shealy et al., 1980 | 40 | 40 | N | * | * | * | * | * | * | * | |||||||
| 2. Comparison between Patients with Sleep State Misperception and Normal Sleepers | |||||||||||||||||
| Bonnet & Arand, 1997 | 9 | 9 | Y | PSG / S | * | * | * | ||||||||||
| 3. Comparison of Patients with Insomnia Against Normative T-Scores | |||||||||||||||||
| Aikens et al., 1999 | 20 | N | |||||||||||||||
| Freedman, 1976 | 18 | Y | PSG / S | † | † | † | |||||||||||
| Kales et al., 1976 | 124 | N | † | † | |||||||||||||
| Method | MMPI Findings | ||||||||||||||||
| Sample size |
Misperception examined? |
Measure of sleep |
Validity Scale |
Clinical Scale |
|||||||||||||
| PWI/PS | NC/GS | Y or N | L | F | K | Hs | D | Hy | Pd | Mf | Pa | Pt | Sc | Ma | Si | ||
| Kalogjera-Sackellares & Cartwright, 1997 | 29 | N | † | ||||||||||||||
| Piccione et al., 1981 | 18 | 9 | N | † | † | † | † * | † | |||||||||
| Roth et al., 1974 | 30 | N | † | † | † | ||||||||||||
| Tan et al., 1984 | 100 | N | † | ||||||||||||||
| Tsushima & Ingolfsdottir, 2004 # | 104 | N | |||||||||||||||
Note. MMPI = Minnesota Multiphasic Personality Inventory. PWI/PS = People with insomnia or Poor sleepers. NC/GS = Normal controls or Good sleepers. Y = Yes. N = No. PSG = Polysomnography. S = Self-report. SSM = People with sleep state misperception. L = Lie; F = Infrequency; K = Correction; Hs = Hypochondriasis; D = Depression; Hy = Conversion Hysteria; Pd = Psychopathic Deviate; Mf = Masculinity-Feminity; Pa = Paranoia; Pt = Psychasthenia; Sc = Schizophrenia; Ma = Hypomania; Si = Social Introversion.
Significant difference between the insomnia group and the control group.
Elevated T-score (≥70) compared to the norm for adult.
used MMPI-2 for the assessment. See text for description of Vanable et al. (2000).
Evidence against this resolution includes that patients with insomnia do not exaggerate every aspect of their sleep difficulty. For example, there is evidence that patients with insomnia underestimate the number of awakenings throughout the night (Bixler et al., 1973; Carskadon et al., 1976; Coates et al., 1982). Also, the clear clinical impression is that patients with insomnia truly believe that they do not get sufficient sleep (Carskadon et al., 1976; Rechtschaffen & Monroe, 1969) and a range of adverse medical and psychiatric consequences have been documented as associated with insomnia (Smith et al., 2005; Taylor et al., 2007).
# 4. Psychological Distress?
A number of early studies examined the possibility that sleep misperception among patients with insomnia is a manifestation of more general psychological distress. The basis of this possible resolution is the finding by Aikens, Wagner, and Saelinger (1999) who documented that psychological distress is positively correlated with the magnification of symptoms, perhaps because psychological distress directs attention toward the domain of concern (Klinger, 1987).
This hypothesis has been pursued using validated scales developed to measure psychopathology and personality disturbances. Consistent with the hypothesis, individuals with insomnia score higher than normal sleepers on the Cornell Medical Index (Hauri & Fisher, 1986; Monroe, 1967; Roth, Kramer, & Schwartz, 1974; Shealy et al., 1980), the Neuroticism scale of the Eysenck Personality Questionnaire (Adam, Tomeny, & Oswald, 1986; Frankel et al., 1973; Shealy et al., 1980), and the MMPI (Kales et al., 1983). Furthermore, patients with insomnia and depression appear to be more prone to sleep misperception (Bliwise, Friedman, & Yesavage, 1993; Edinger & Fins, 1995).
To be more specific, Table 2 summarizes the results of 26 studies1 that compared (i) the MMPI scores of individuals with insomnia compared with the T-scores of a matched non-patient control group (n = 17 studies), (ii) the MMPI scores of patients with sleep state misperception with matched non-patient controls (n = 1 study), and (iii) the MMPI scores of individuals with insomnia with the normative T-scores (n = 8 studies). All studies used the original version of the MMPI, with the exception of the latest study by Tsushima and Ingolfsdottir (2004) and Fernandez-Mendoza et al. (2011) for which the MMPI-2 was employed. An asterisk in Table 2 represents a significant difference between the insomnia/sleep state misperception group and the non-patient control group and a cross symbol denotes a significantly elevated T-score (> 70). Apart from the study reported by Monroe and Mark (1977), in which the insomnia group scored lower on the Hypomania scale (Ma) compared to the control group and the study by Mendelson et al (1984), in which the insomnia group had a lower K score than normal controls, all other between-group differences were in the direction of a higher score in the insomnia group relative to the control group. Fourteen (82.4%) of the seventeen studies that compared the T-scores of the insomnia group with a control group indicated significant differences on at least two of the clinical scales. Six (75%) of the eight studies that compared the T-scores of the insomnia group with the normative data found that the insomnia group had elevated scores on several clinical scales, including the Hs (Hypochondriasis), D (Depression), and Hy (Conversion-Hysteria), and Pt (Psychasthenia) scales. These findings corroborate those reported by Vanable, Aikens, Tadimeti, Caruana-Montaldo, and Mendelson (2000). Vanable et al. (2000) compared MMPI scores among three insomnia groups: sleep overestimators, accurate sleep-estimators, and sleep underestimators. Sleep underestimators scored significantly higher than sleep overestimators on the Hs (Hypochondriasis), Hy (Conversion-Hysteria), and Pt (Psychasthenia) scales. These findings are also consistent with the study by Bonnet and Arand (1997) who found patients with sleep state misperception scored significantly higher on the Hs (Hypochondriasis), Pt (Psychasthenia) and Sc (Schizophrenia) scales. An elevated score on the Hs (Hypochondriasis) scale can be interpreted as a tendency to focus on somatic symptoms and an excessive concern over bodily functioning. The Pt (Psychasthenia) scale taps into fears, obsessive-compulsive features, self-criticism, difficulties in concentration, and guilt feelings. An elevated score on this scale suggests the presence of trait anxiety. We note that Vanable et al. (2000) is not included in Table 2 since it adopted a different design to those summarized.
We caution that this research evidence is correlational. Hence, we cannot conclude that a mildly disturbed psychological profile is the cause of misperception of sleep among individuals with insomnia (Carskadon et al., 1976). On the contrary, it is possible that mild psychological and personality disturbances are the result of misperception of sleep, given the accruing evidence for the impact of poor sleep on next day emotional functioning (Franzen, Siegle, & Buysse, 2008; Yoo, Gujar, Hu, Jolesz, & Walker, 2007).
# 5. A Deficit in Time Estimation Ability?
It has been proposed that patients with insomnia may simply be poor estimators of time (Bonnet, 1990; Borkovec & Hennings, 1978). We are aware of three published studies testing this hypothesis. First, Moore, Bonnet, and Warm (1982) compared the time estimation ability of 12 males with insomnia with 12 sex-matched normal sleepers. Participants were asked to estimate long and short temporal intervals (long interval: 19 minutes; short intervals: between 5 and 35 seconds) at different times of the day (7 a.m., 11 a.m., 3 p.m., 7 p.m., and 11 p.m.), and in different settings (half of the participants performed their tasks in a private bedroom in a hospital sleep laboratory and the remaining half in a normal testing room). The insomnia and normal sleeper groups did not differ in their estimation of both long and short intervals, regardless of the time and setting in which the time estimates were made.
In a second study, 20 individuals with insomnia and 20 normal sleepers – both males and females - were asked to perform two time estimation tasks; one in the laboratory during the day and one in the participants’ own bedroom during the night (Tang & Harvey, 2005). The two groups were compared for the accuracy of estimating unfilled temporal intervals of various lengths. Each time estimation task involved listening to, and estimating, the temporal length of six unfilled intervals, with five trial intervals preceded by one practice interval. The practice interval was 1 minute long and was not used for data analysis. The length of the five trial intervals were 5 seconds, 15 seconds, 35 seconds, 1 minute, and 15 minutes. Each of these intervals was followed by 10 seconds of silence. The participants were asked to use this gap between intervals to record their estimate on the log sheet. The insomnia group did not differ from the normal sleeper group, regardless of the context in which the time estimates were made.
Consistently, Rioux, Tremblay, and Bastien (2006) compared insomnia patients (n = 11) and good sleepers (n = 11) on a finger tapping time estimation task. They also reported no group differences and no relationship between the severity of the insomnia and the estimation of time.
In summary, all three of the published studies conducted to date do not provide support for the possibility that individuals with insomnia misperceive their sleep simply because they are poor estimators of time.
# 6. Sleep is Perceived as Wake
Consistent with the findings we described earlier (Borkovec et al., 1981; Rechtschaffen & Monroe, 1969), Mercer, Bootzin, and Lack (2002) compared the sleep perception of 14 patients with insomnia with 8 good sleepers. Sleep was monitored polysomnographically. When the participants were woken five minutes after the onset of Stage 2 sleep or during uninterrupted rapid eye movement (REM) sleep, patients with insomnia were more likely than the good sleepers to report experiencing wakefulness the moment just prior to being woken up. This finding applied to both the beginning and the end of the sleep period. Importantly, Mercer et al. (2002) also found that the laboratory measures of this tendency to interpret sleep as wakefulness correlated significantly with the discrepancy between subjective (sleep diary-defined) and objective (PSG-defined) sleep estimates based on 2 nights of home recordings (r = .35–.83). The latter finding suggests that the misperception of sleep exhibited by patients with insomnia may involve a tendency towards interpreting sleep as wakefulness. While the mechanisms are not known, we return to several possible mechanisms that may account for this finding.
# 7. Worry and Selective Attention/Monitoring
The tendency for "distortions in reality" has been long noted as characteristic of patients with psychiatric disorders (Beck, 1976). Indeed, people with anorexia nervosa think they are overweight when actually they are underweight and people with panic disorder think they are having a heart attack when actually they are experiencing symptoms of anxiety. Evidence has accrued to suggest that these "distortions in reality" arise, at least in part, from the action of a cascade of cognitive processes (Clark, 1999, 2004). These cognitive processes – namely, worry and selective attention and monitoring – have been evaluated for their contributions to misperception among insomnia patients.
Worry
It is well established that many patients with insomnia report that they worry when they cannot get to sleep (see Harvey, 2005 for review). As already highlighted, the possibility that worry serves to trigger misperception of sleep is drawn from the robust finding in the time perception literature that time seems longer when the number of units of information processed per unit of time increases (Ornstein, 1969; Thomas & Cantor, 1975, 1976). In a similar way, worry while trying to get to sleep has been proposed to function to distort the perception of how long it takes to get to sleep (Borkovec, 1982). Several findings are consistent with this possibility. First, Van Egeren et al. (1983) studied sleep (across five nights of PSG in the sleep laboratory) and presleep cognition (in an interview and using a sampling procedure on the 5th night). Consistent with the proposed resolution, a positive correlation between negative pre-sleep cognitive arousal and misperception of sleep emerged. Second, the positive correlation between the MMPI scale 7 "Psychasthenia" and misperception of sleep suggests an association between misperception, rumination, and catastrophizing (Vanable et al., 2000). Third, in one of the time estimation studies discussed in #5, we included a self-report measure of cognition and physiological arousal adapting the Presleep Arousal Scale (Nicassio, Mendlowitz, Fussell, & Petras, 1985). The results indicated that time overestimation correlates positively with cognitive and physiological arousal experienced during the time estimation tasks (Tang & Harvey, 2005). Fourth, in an afternoon nap study insomnia patients were allocated to one of three conditions (n = 18 per condition; Tang & Harvey, 2004b). In the first, anxious pre-sleep cognitive arousal was activated by telling participants that they would need to give a speech to three psychologists and to a video camera on waking. In the second, neutral pre-sleep cognitive arousal was activated by telling participants that they would be asked to write an essay on waking. The topic was identical for the "speech task" and the "essay task"; both groups were asked to comment on the foot and mouth disease in Britain (at the time of the study this was an impending national crisis). The third group were simply told to take a nap (the no manipulation group). Both the anxious cognitive arousal group and the neutral cognitive arousal group exhibited a greater discrepancy between self-reported and actigraphy-defined sleep, relative to participants in the no manipulation group. Moreover, although the difference was not statistically significant, an inspection of the mean values indicated that the anxious cognitive arousal group exhibited a larger discrepancy score compared to the neutral cognitive arousal group. Finally, in the clock monitoring study already described in which clock monitoring and digit display unit monitoring were compared, we included a 0 "not at all" to 10 "very much" rating of how worried the participant felt about falling asleep. Consistent with this resolution, there was a significant positive correlation between worry and misperception of sleep onset in the group asked to monitor the clock (r = .54; Tang et al., 2007).
Selective attention and monitoring
A range of types of selective attention/monitoring have been identified as characteristic of insomnia. They can be broadly categorized into internal monitoring (e.g., body sensations) and external monitoring (e.g., the clock) or into monitoring during the sleep period (e.g., body sensations consistent/inconsistent with falling asleep, the environment for signs of not falling asleep, the clock to see how long it is taking to fall asleep, calculating how much sleep will be obtained), on waking (e.g., body sensations for signs of poor sleep, the clock to calculate how much sleep was obtained), and during the day (e.g., body sensations for signs of fatigue, mood for indications of tiredness/not coping, performance for indications that attention, memory and concentration are failing; Semler & Harvey, 2004a). This tendency toward selective attention among insomnia patients has been further documented in a series of studies using various cognitive tasks such as the emotional Stroop task (Taylor, Espie, & White, 2003), the flicker paradigm (Jones, Macphee, Broomfield, Jones, & Espie, 2005), and the dot probe (McMahon, Broomfield, & Espie, 2006).
This tendency toward selective attention and monitoring has been proposed to contribute to misperception of sleep. The rationale is that monitoring for sleep-related threat increases the chance of detecting random and meaningless cues that would otherwise pass unnoticed. When patients detect these cues they misinterpret them as indicative of threat (Clark, 1999; Salkovskis & Bass, 1997). Given that insomnia patients engage in various types of selective attention and monitoring, perhaps a similar mechanism may contribute to misperception of sleep (Harvey, 2002). For example, monitoring for and detecting body sensations on waking and detecting "sore head" and "heavy eyes", may result in a reappraisal of the amount of sleep obtained, contributing to misperception.
Two domains of evidence loan support to this possible resolution. First, experiments in patients with anxiety disorders show that manipulations that increase monitoring contribute to misperception (Arntz, Rauner, & Van den Hout, 1995; Clark et al., 1997; Rachman & Cuk, 1992). For example, patients with panic disorder who were asked to silently count their heart beat without taking their pulse (a manipulation that increases monitoring for a threat relevant to panic patients) increased anxiety and the probability of later panic attacks (Ehlers, 1995). Rachman and Cuk (1992) recruited 60 undergraduates who rated themselves as "extremely fearful" or "terrified" of spiders/snakes and fearful on approach of a spider/snake of more than 80 (on a 0–100 scale). Another 60 undergraduates who rated themselves as "not at all fearful" or a fear score of less than 10 were also recruited. These 120 participants engaged in a task in which they were asked to approach an exposed snake/spider (housed in a glass bowl) from 15 feet. Then the animal was covered and the participant was asked questions about the animal. The students who were fearful of snakes/spiders exhibited the greatest distortion in the amount of activity in which the snake/spider had engaged. For example, they were more likely to rate that the snake/spider had moved toward them and appeared to be coming out of its cage. Second, in the clock monitoring experiment already described, clock monitoring increased worry and was associated with greater misperception of sleep relative to monitoring the digit display unit (Tang et al., 2007).
# 8. Memory Bias
Another possible resolution is that misperception of sleep arises from a memory bias. This is argued to be plausible given that “personal recall involves an active, constructive process that is guided by people’s knowledge at the time of retrieval” (Ross, 1989). Subjective recall of sleep, in particular, relies on the ability to accurately recall the sleep state. As sleep is a context that is easily misperceived this is a difficult task. Four examples of processes that may contribute to memory bias will now be reviewed.
First, current symptom status is likely to influence retrieval. Eich, Reeves, Jaeger, and Graff-Radford (1985) asked patients with chronic headaches to keep a diary of hourly ratings of pain intensity. At the end of the week the participants attended the clinic. They were asked to rate (a) the intensity of pain and (b) to recall the maximum, minimum, and usual levels of pain in the past week. Those with high levels of present pain overestimated prior pain and those with low levels of present pain underestimated their earlier pain levels. In other words, memories were consistent with current state. In a similar way perhaps a current state of sleepiness, fatigue, and tiredness will bias retrieval of memory for recent sleep episodes by insomnia patients. This possibility is yet to be empirically evaluated.
Second, drawing on the emotional and ex-consequentia reasoning literature (Arntz et al., 1995), memory for sleep may be biased by judgments based on the emotional feelings present at the time of recall. It is clear from the social psychology literature that current mood impacts judgments of one’s current life situation (see Schwarz & Clore, 1983 for review). Also, Arntz et al. (1995) asked various groups of anxious patients to rate scripts describing an ambiguous situation. The scenario was manipulated so that the amount of objective safety versus objective danger information and the amount of information about anxiety response versus non-anxiety response was controlled. Consistent with the ex-consequentia reasoning literature, anxious participants made the judgment that there must be danger merely because they felt anxious. In a similar way, perhaps patients with insomnia draw faulty conclusions about the amount of sleep they have obtained because they base their judgment on how they feel. For example, when a patient with insomnia notices feeling anxious or sad when he or she wakes up, or throughout the day, the person may use this subjective feeling to conclude that they must not have slept well the previous night. Indeed, Semler and Harvey (2004) reported that monitoring mood throughout the day was common among insomnia patients.
Third, it is possible that beliefs influence the recall of sleep among patients with insomnia. This confirmation or belief bias is the tendency to agree with conclusions that fit prior beliefs (whatever their actual logical status). The social psychology literature demonstrates the human tendency to gather evidence that confirms our beliefs rather than evidence that challenges them (see Evans, Burnell, Hopwood, & Howell, 1993; Nickerson, 1998 for review). Confirmations of prior expectations are overvalued whereas disconfirmations of prior expectations are downplayed. In an interesting review, Ross (1989) convincingly argues that we all have implicit theories of stability and change with respect to the self and that these theories guide the construction process. For people suffering from insomnia, this implicit theory of stability (or belief) such as “I am a bad sleeper” may guide the reconstruction process when recalling the amount of sleep obtained. This process may alter the constructive process involved in remembering the amount of sleep obtained, thereby contributing to misperception. This possibility has not as yet been explicitly tested as an explanation for misperception of sleep among patients with insomnia. If this explanation turns out to be a contributor, it will add to the accruing evidence, following the work of Morin (1993), for the importance of unhelpful beliefs as important in insomnia. Interestingly, Means et al. (2003) report evidence that is consistent with this resolution. Over 6 nights sleeping in the lab, sleep was monitored with PSG and subjective estimates made on waking. The Dysfunctional Beliefs and Attitudes Scale (Morin, Stone, Trinkle, Mercer, & Remsberg, 1993) was also administered. Misperception of sleep and holding more dysfunctional beliefs about sleep were positively correlated. The insomnia patients who reported the most misperception of sleep also endorsed the most dysfunctional beliefs about sleep.
Finally, intensity and recency effects may bias recall of sleep. For example, Redelmeier and Kahneman (1996) investigated global evaluations of pain. They reported that despite substantial variation in the duration of painful procedures, lengthy procedures were not remembered as particularly aversive. However, memory for the procedures (i.e., retrospective evaluations) was strongly correlated with peak pain (most intensive period of pain) and end pain (most recent experience of pain). By implication, in the context of misperception of sleep in insomnia, perhaps memory for sleep may be biased by the worst night of sleep (peak/most intense) and the most recent night of sleep (end/most recent). The applicability of this hypothesis should be investigated in future research. By implication, in the context of misperception of sleep in insomnia, perhaps memory for sleep may be biased by the worst night of sleep (peak/most intense) and the most recent night of sleep (end/most recent). Note that this account would only be relevant to subjective/objective discrepancies made over a period of more than one night. For example, during an assessment for insomnia, patients are often asked for their subjective estimate of their sleep over the past week or past month. It seems likely that discrepancies in such estimates (with objective measures of sleep) may be, at least in part, accounted for by intensity and recency effects.
Four examples of processes that may adversely impact perception of sleep have been discussed; namely, the influence of current symptoms on recall of sleep, of emotion present around the time of recall, holding beliefs that hijack the memory reconstruction process and the influence of intensity and recency. While each account seems plausible they await empirical investigation in the context of insomnia and the puzzle at hand.
# 9. Physiological Arousal
Arousal is a concept that describes activation and/or agitation, which may be expressed in more than one interlinked domain (e.g., physiological, cortical, cognitive, and behavioural). Although it is clear that one form of arousal cannot always be used as a valid measure of another form of arousal, one possibility is that physiological arousal may aggravate sleep misperception through altering or increasing mentation during the pre-sleep period (Bonnet & Arand, 1992). This increase in pre-sleep mentation may blur the distinction between sleep and wakefulness, rendering the point of sleep onset more difficult to detect, increasing the chance of people interpreting sleep as wakefulness (Borkovec et al., 1981; Mercer et al., 2002; Rechtschaffen & Monroe, 1969).
Chronic physiological arousal is characteristic of insomnia. Evidence of accelerated heart rate, reduced heart beat interval and variability, heightened core body temperature, increased EMG, and even whole body metabolic rate has been found in patients with insomnia compared to normal controls (e.g., Monroe, 1967). Physiological arousal is thought to contribute to misperception of sleep (Bonnet & Arand, 1994, 1997). For example, Bonnet and Arand (1992) administered 400mg of caffeine three times a day for 7 nights and days to 12 normal young adults. These investigators managed to raise the metabolic rate of the volunteers [as estimated via the rate of carbon dioxide production (VCO2), oxygen consumption (VO2), and respiratory quotient] and successfully reproduced symptoms of insomnia (e.g., lower sleep efficiency). There was a significant negative correlation between metabolic rate at bedtime and sleep efficiency for the sleep period that followed. Most notable was the observation that participants with the greatest change in metabolic rate demonstrated a tendency to overestimate their sleep onset latency on nights when caffeine was administered, relative to the baseline nights. These findings raised the possibility that increased physiological arousal may contribute to misperception of sleep (Bonnet & Arand, 1992). Consistent with this possible resolution, in good sleepers, misperception of SOL decreases following the administration of benzodiazepines and increases following the administration of caffeine (Bonnet & Arand, 1994). Patients with insomnia, who misperceived their sleep, exhibited significantly increased 24-hour metabolic rate, relative to controls (Bonnet & Arand, 1997). Metabolic rate was estimated via the rate of carbon dioxide production (VCO2), oxygen consumption (VO2) and respiratory quotient and recorded 20 minutes before lights out on the second night of PSG as well as across the entire second night of PSG. Replication of this study with the inclusion of an insomnia group who do not display misperception of their sleep is needed. In another study, the impact of anxious cognitive arousal versus physiological arousal on sleep perception was evaluated in healthy good sleepers (Tang & Harvey, 2004b). The participants were experimentally induced to experience anxious cognitive arousal (via a request to give a speech – see #7 for further details) or physiological arousal (via caffeine administration), prior to an afternoon nap, both exhibited a greater discrepancy between the self-reported and actigraphy-defined sleep, relative to a non-manipulation control group. These two studies are consistent with the proposal that chronic physiological arousal may contribute to the tendency to misperceive among individuals with insomnia (Bonnet & Arand, 1994, 1997).
# 10. Cortical Arousal
The traditional analysis of PSG has been limited to phenomena visible to the naked eye with one judgment made for each 30-second epoch (Rechtschaffen, 1968). This method is the “gold standard” for analysis in sleep research; however it does not allow micro-information within the EEG to be examined. Tools have recently been developed which allow for a more detailed exploration of PSG. For example, power spectral analysis (PSA), is often used to quantify the power of the higher frequency EEGs in each 30 second epoch of sleep. PSA is a statistical technique for detecting periodicities within time series data. As employed within EEG, it is used to deconstruct complex waveform data into their constituent frequencies. The power of each waveform is defined as the area below the waveform, such that a waveform of the same frequency would have a greater power if the amplitude of the waveform was greater.
Perlis, Merica, Smith, and Giles (2001) presented a review of seven studies showing that people with insomnia exhibit more high frequency EEG [Beta (14–35Hz) to Gamma (35–45Hz) range] at or around sleep onset. This is surprising as EEG in the Beta to Gamma range is associated with a range of waking cognitive functions, such as attention, perception, problem-solving, and memory, in both animals and humans (Lazarev, 1998; Loring & Sheer, 1984; Rougeul, Bouyer, Dedet, & Debray, 1979; Spydell & Sheer, 1982; Tiitinen et al., 1993). Perlis et al. (2001) have proposed an account of these findings that includes that cortical arousal may be a contributor to misperception of sleep in individuals with insomnia by: (a) reducing the differentiation between sleep and wakefulness (Mendelson, 1993a) and (b) inhibiting the normal process of establishing sleep onset-related “mesograde amnesia” (Wyatt, Bootzin, Allen, & Anthony, 1997; Wyatt, Bootzin, Anthony, & Bazant, 1994). “Mesograde amnesia” refers to the decline in all aspects of memory function; namely, encoding, consolidation, and retrieval (Wyatt et al., 1997).
Two studies loan further support to this resolution and extend it by presenting evidence for the presence of beta/gamma EEG during NREM sleep. Perlis et al. (2001) reported a moderate correlation between NREM Beta activity (14–35Hz) and the discrepancy between subjective and objective estimates of TST (r = −.46). That is, those who exhibited more Beta activity during NREM sleep also exhibited greater underestimation of sleep duration. A similar trend was observed for SOL, although this correlation did not reach statistical significance (r = .33, p = .10). Krystal, Edinger, Wohlgemuth, and Marsh (2002) compared the EEG spectral characteristics of 30 patients with insomnia and 20 normal sleepers. The insomnia group comprised 12 patients with "subjective" insomnia (these patients met criteria for persistent primary insomnia but slept normally according to PSG) and 18 patients with "objective" insomnia. Elevated Alpha (8.5–12 Hz), Sigma (12.5–16 Hz) and Beta and diminished Delta EEG activity was found in patients with subjective insomnia, but not in normal sleepers nor in patients with objective insomnia. A third study by Buysee, Germain, Hall et al. (2008) suggests the picture may be more complicated. The results of this study indicated a sex difference; namely, women with insomnia, but not men, exhibited high frequency EEG activity during NREM. Moreover, high frequency EEG was not associated with misperception of sleep. In fact, in the first NREM period, beta EEG correlated with misperception of wakenings in the middle of the night, but only for men, and the direction of the correlation was the opposite to expectation. Specifically, greater misperception was associated with lower beta EEG.
# 11. Transient Awakenings
It has been proposed that transient awakenings (between 3 and 30 seconds), too brief to be documented by the conventional sleep stage scoring, may account for the misperception of sleep among insomnia patients. For example, Knab and Engel (1988) compared patients with insomnia and controls over one night in the laboratory. A microswitch was taped to the dominant hand of the participants. They were asked to press the switch twice whenever they awoke (as an index of perceived awakenings). The authors concluded that, among insomnia patients, “perception seems to be biased in the direction of wakefulness unless sleep has been lengthy” (p. 271). Smith and Trinder (2000) empirically evaluated this hypothesis in 20 normal sleepers. An experimental manipulation involving wearing a respiratory apparatus (a mask) was used to induce brief awakenings. Half of the normal sleepers wore the mask. The remaining half went to sleep without wearing a mask, as an index of baseline responding. The participants were woken up from uninterrupted Stage 2 or a deeper stage of sleep to estimate their SOL. The experimental manipulation was successful in that the participants wearing the mask experienced more brief awakenings, compared to the no-mask group. In terms of the key hypothesis tested, relative to the no-mask group, the participants wearing the mask reported longer SOL despite there being no difference in PSG-defined SOL between the two groups. That is, the participants wearing a mask exhibited more misperception of sleep, compared to the no-mask group. Further, the participants wearing a mask were more likely to report that they had been awake the moment prior to being woken up from Stage 2 sleep. Based on these findings, Smith and Trinder (2000) concluded that an increase in the frequency of brief awakenings during the sleep onset period may be one of the factors contributing to an overestimation of SOL in patients with insomnia.
More recently, Parrino et al. compared individuals who met diagnostic criteria for paradoxical insomnia with healthy good sleepers. They scored cyclic alternating patterns (CAP) within the EEG. CAPs are indicative of unstable EEG and are defined as sequences of electrocortical events within non-REM sleep that are distinct from background EEG activity and recur every 20–40 seconds (Parrino, Milioli, De Paolis, Grassi, & Terzano, 2009). The paradoxical insomnia group exhibited a significantly higher arousal index and total CAP rate relative to the healthy controls. Moreover, in the period between the subjectively perceived sleep onset point and the objectively scored sleep onset point the CAP rate was significantly higher in the paradoxical insomnia group. While this study is consistent with the possibility that brief awakenings and other abnormalities in the EEG account for the tendency to misperceive sleep, without a comparison group of insomnia patients who accurately perceive their sleep we do not know if the findings are specific to the paradoxical insomnia group or to insomnia more generally.
We note that one study has reported no increased rate of awakenings in a group of individuals with insomnia who overestimated their sleep by at least two hours, relative to a group who correctly estimated their sleep (Trajanovic et al., 2007). However, few details as to the definition and scoring of awakenings were provided. As such, viewing this study as contrary to the possibility that misperception can be, at least in part, accounted for by transient awakenings does not seem warranted.
In sum, it seems plausible that this account may be one resolution to the puzzle. However, further research is needed to ascertain: (a) that patients with insomnia do experience more brief awakenings compared to normal sleepers, (b) that patients with insomnia who misperceive their sleep experience more brief awakenings relative to patients with insomnia who don’t misperceive their sleep, and (c) that the findings based on the analogue sample of good sleepers reported by Smith and Trinder (2000) are generalizable to individuals with chronic insomnia.
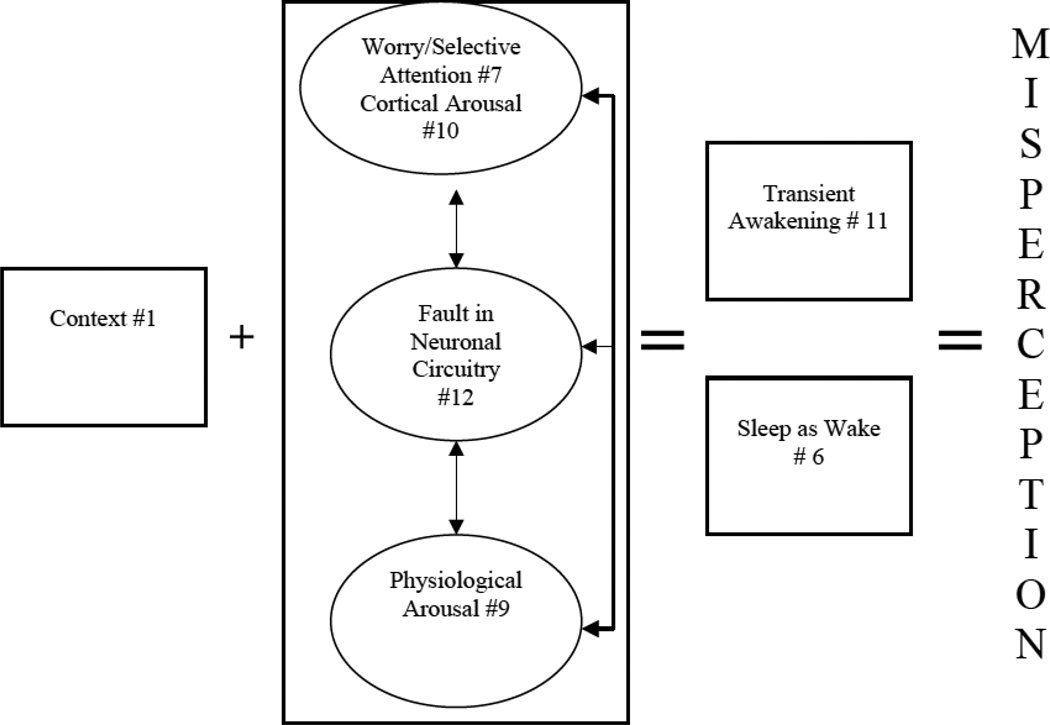
# 12. Fault in Neuronal Circuitry
Saper et al. (Cano et al., 2008; Saper, Chou, & Scammell, 2001) have proposed a "flip-flop switch" model of sleep that implicates the ventrolateral preoptic nucleus (VLPO; neurons in the VLPO are activated during sleep) and the arousal system. They also propose that insomnia is the result of the full activation of the VLPO coupled with it not being able to flip off the arousal system because it is "excited intensely" by the limbic system (Cano et al., 2008). Moreover, these authors suggest that this unique state of neuronal activation in the cortex promoting sleep (VLPO) coupled with the neuronal activation in the arousal system promoting wake (limbic system activation) might explain the tendency to misperceive sleep in individuals with insomnia. Intriguingly, Cano et al. (2008) present empirical evidence for this proposal in a stress-induced insomnia model in rats. Stress was induced via a cage exchange (the new cage had previously been occupied by a male rat, the odor is a species specific stressor). The rats exposed to the stress took longer to fall asleep and experienced more wakefulness during sleep. Fos expression was then analyzed as an indirect marker of neuronal activity and to identify brain circuitry activity. Fos expression increased in cerebral cortex, limbic system, and parts of arousal/autonomic system as well as activation of the sleep promotion system. Also intriguing, and consistent with Perlis et al.’s observation in humans with insomnia, Cano et al.’s stressed rats exhibited high frequency EEG activity alongside slow wave sleep.
#13. Subtypes/Phenotypes
The resolution proposed by Fernandez-Mendoza et al. (2011; 2010) is that we should divide insomnia into two distinct phenotypes. The first is insomnia that is associated with objective short sleep duration, no misperception or overestimation, an MMPI profile characteristic of patients with medical disorders (Fernandez-Mendoza, Calhoun, Bixler, Karataraki, Liao, Vela-Bueno, Ramos-Platon, et al., 2011) and poorer neuropsychological performance on measures of processing speed, attention, visual memory, and verbal fluency (Fernandez-Mendoza et al., 2010). The second is insomnia that is associated with objective normal sleep duration, clear misperception of sleep, an MMPI profile characterized by depressed mood, rumination, anxiety, intrusive thoughts, and poor resources for coping with stress (Fernandez-Mendoza, Calhoun, Bixler, Karataraki, Liao, Vela-Bueno, Ramos-Platon, et al., 2011) and no neuropsychological deficits (Fernandez-Mendoza et al., 2010). Research intended to replicate these findings is needed to determine whether misperception of sleep is on a continuum versus falls into these two distinct categories.
Integration
We have reviewed 13 possible resolutions to the puzzle of why patients with insomnia tend to misperceive their sleep. In Table 4 we indicate, for each resolution, the quality of the evidence that has accrued. The system we used to evaluate the quality of the research is adapted from previous work (Chambless et al., 1996; Harvey, Watkins, Mansell, & Shafran, 2004) and involves rating three quality levels of the studies presented in Table 3.
Table 4.
Summary of quality and direction of evidence for proposed resolutions
| Resolution | Quality of Evidence | Direction of Evidence |
|---|---|---|
| #1. The challenge of the context | ** | + |
| #2. An artifact of sleep onset definition | ** | + |
| #3. An exaggerated complaint | ** | +/− |
| #4. Psychological distress | ** | + |
| #5. A deficit in time estimation ability | ** | − |
| #6. Sleep as wake | *** | + |
| #7a. Worry | *** | + |
| #7b. Selective attention | ** | + |
| #8a. Memory bias due to current symptoms | * | + |
| #8b. Memory bias due to emotions | * | + |
| #8c. Memory bias due to a confirmation bias/belief bias | * | + |
| #8d. Memory bias due to the intensity or recency of symptoms | * | + |
| #9. Physiological arousal | ** | + |
| #10. Cortical arousal | ** | +/− |
| #11. Transient awakenings | *** | +/− |
| #12. Fault in neuronal circuitry | * | + |
| #13. Subtypes/Phenotypes | ** | + |
Note. The left hand column presents an indication of the quality of the evidence as follows:
good quality evidence,
moderate quality of evidence,
tentative quality of evidence (see text for definitions) and the right hand column presents an indication of the findings as follows:
positive findings,
negative findings,
mixed findings.
1. Good Quality Evidence
Good quality evidence is indicated by "***" and is declared if at least one study has been published that clarifies whether the proposed resolution has a causal influence on misperception (e.g., a study has been conducted that manipulated the possible resolution and showed an impact on misperception). In addition, two or more studies have been conducted that: (a) were based on diagnosed insomnia patients (as opposed to poor sleepers or good sleepers); (b) were methodologically strong (including consideration of sample size and appropriate comparisons groups); and (c) were conducted by at least two independent research groups.
2. Moderate Quality Evidence
Moderate quality evidence is indicated in the table by "**". Moderate quality evidence is declared if the criteria for good quality evidence are not met but the following criteria are met: one or more methodologically strong (see above) studies have been conducted including patients diagnosed with insomnia (see above).
3. Tentative Quality Evidence
Tentative quality evidence is indicated in the table by "*". This level of evidence is declared if the conditions for good and moderate quality evidence are not met but there is some evidence. Examples of scenarios when tentative quality evidence will be applied are: (a) the study employed non-patient (analogue) samples (i.e., poor sleepers), (b) the study was based on insomnia patients but there are methodological problems (i.e., small sample or absence of a comparison group), or (c) the study did not involve insomnia patients (e.g., the study involved good sleepers or patients with chronic pain or an anxiety disorder).
Within the table we have also included an indication of the direction of the evidence as follows.
Positive evidence: Positive evidence is indicated in the table by "+" and is declared when all of the studies reported positive findings (i.e., the resolution contributes to misperception).
Negative evidence: Negative evidence is indicated in the table by "−" and is declared when all of the studies reported negative findings (i.e., the resolution does not contribute to misperception).
Mixed evidence: Mixed evidence is indicated in the table by "+/−" and is declared when a mix of findings, some positive and some negative, are reported.
We checked to see if independent raters would come up with similar classifications. A total of six independent reviewers, of at least graduate-level of training, were invited to rate the quality and direction of the evidence based on the criteria specified above. Interrater reliability was calculated for the ratings using ReCal3 (Freelon, 2010). The average pairwise percent agreement for the ratings on the evidence quality was 73.8%. The interrater reliability was .60 as measured with Fleiss' Kappa (observed agreement = .74, expected agreement = .34; Fleiss, 1971) and .61 as measured with Krippendorff's Alpha (based on 96 decisions; Krippendorff, 2004). The average pairwise percent agreement for the ratings on the evidence direction was 92.5%. The interrater reliability was .80 as measured with Fleiss' Kappa (observed agreement = .93, expected agreement = .62), .80 as measured with Krippendorff's Alpha (based on 96 decisions). Hence, reliability is moderate for the quality ratings and strong for the direction ratings.
As already emphasized, the resolutions presented are not necessarily mutually exclusive and they will differ in the extent to which they explain the variance in misperception, a possibility that should be considered in future research. Several individual accounts may well stand-alone in offering a full account. In particular, it is plausible that future research may establish that either #2 "An artifact of sleep onset definition" or #13 "Subtypes/Phenotypes" provide a full account. Or, perhaps more likely, an account of misperception may require an integration across the proposed resolutions. To give just one example, as depicted in Figure 1, focusing on the best evidence emerging from this review, contextual factors (Resolution #1) may constitute fertile ground for misperception. Add to that a tendency for worry and selective attention (Resolution #7) that contributes a state of limbic activation as a result of a fault in neuronal circuitry (Resolution #12) that leads to chronic activation of the arousal system (Resolution #9), together preventing the sleeper from fully entering a sleep state. The bi-directional arrows in Figure 1 reflect the possibility that chronic activation of the arousal system may contribute to limbic activation and worry and that limbic activation may contribute to worry. Figure 1 also raises the possibility that cortical arousal (Resolution #10) may be the neural signature for worry and selective attention extending into sleep. This state of arousal may be a substrate in which the rate of transient awakenings increase (#11) and sleep is perceived as wake (#6). Together, these seven processes may constitute an account of misperception.
Figure 1.
An example of an integrative model implicating seven processes contributing to misperception of sleep. Focusing on the best evidence emerging from this review, contextual factors (Resolution #1) may constitute fertile ground for misperception. Add to that a tendency for worry and selective attention (Resolution #7) that contributes a state of limbic activation as a result of a fault in neuronal circuitry (Resolution #12) that leads to chronic activation of the arousal system (Resolution #9), together preventing the sleeper from fully entering a sleep state. This state of arousal may be a substrate for more transient awakenings increase (#11) and sleep being perceived as wake (#6).
We also note that some of the resolutions may represent different points along the spectrum of developing knowledge. For example, in a recent review, Bonnet and Arand (2010) conceptualized resolutions #9 (Physiological arousal), #10 (Cortical arousal), and #12 (Fault in neurocircuitry) as different points in the development of knowledge but all capture the same phenomenon. Another possible integration picks up on a theme that emerges across several resolutions (#2, #6, #10, #11); namely, that current measures of sleep are inadequate/flawed with respect to their ability to capture the sleep disturbance exhibited by insomnia patients.
Clinical Implications
Assessment
There are important implications of the discussion within this paper for the assessment of insomnia. Obviously research efforts focused on resolving the puzzle of misperception must continue to include multiple measures (Van den Berg et al., 2008), spanning self-reported sleep soon after waking, PSG and more detailed analyses of the sleep EEG (e.g., spectral analysis, CAP analysis). It seems essential to continue to pursue the latter, as well as other novel methods for scoring the complexities of sleep and improved technologies for measuring human sleep. Indeed, there are findings that signal that PSG may be far from an inadequate measure of the true nature of human sleep. For example, in a very interesting study, Zolpidem (Ambien) was shown to improve PSG measured sleep in kittens, but resulted in reduced development/cortical plasticity in the visual system by approximately 50%, relative to placebo (Seibt et al., 2008). This study raises the possibility that PSG is not picking up substantial and important aspects of the biological process of sleep and it marks out the critical importance of developing more subtle and complete measures of sleep, beyond PSG.
Into the future, Rosa and Bonnet (2000) raise the possibility that “we may need to reconsider our ideas about what constitutes insomnia and what the appropriate treatment end points might be” (p. 482). On the one hand, if future research continues to show subtle sleep abnormalities (as in #10), the field may need to deemphasize the use of PSG and continue to value subjective report or other methods that more accurately capture the problems insomnia patients are detecting within their sleep. On the other hand, and in line with the proposal by Fernandez-Mendoza et al. (2011; 2010) that there are two insomnia phenotypes (objective short sleep duration + no misperception or overestimation; objective normal sleep duration + clear misperception of sleep), the inclusion of an objective estimate of sleep may need to become part of the standard assessment for insomnia. At this point, neither PSG nor actigraphy are recommended for the standard assessment of insomnia (American Sleep Disorders Association, 1995; Reite, Buysse, Reynolds, & Mendelson, 1995).
Manconi et al. (2010) have proposed, and demonstrated reliability for, a misperception index: objective total sleep time - subjective total sleep time / objective total sleep time. Positive values represent an underestimation and negative values represent an overestimation. It seems valuable to consider adding this parameter to the standard research assessment of insomnia (Buysse et al., 2006).
It’s also important to recognize that insomnia tends to occur sporadically. The quantitative criteria for insomnia define insomnia as occurring at least 3 nights per week (Lichstein, Durrence, Taylor, Bush, & Riedel, 2003). Hence, one night of PSG is likely to be insufficient to capture insomnia and more than one night is likely to be a significant personal burden to patients and cost burden to researchers and clinicians. So, yet again, this points to an urgent need to develop technologies that are easier to use, less expensive, and less intrusive to sleep.
Interestingly, dating back to a case study, published in the 1970s (Jason, 1975) and replicated many times since (e.g., Engle-Friedman, Bootzin, Hazlewood, & Tsao, 1992; Franklin, 1981) a "sleep diary-keeping effect" has been noted in which sleep appears to improve simply by keeping a sleep diary. Two possible accounts have been offered, both related to misperception. First, it is possible that the enhanced awareness of the sleep pattern may contribute to the alleviation of the patient’s subjective sleep complaints through a reduction of sleep-related anxiety (Morin, 1993). Second, keeping a sleep diary may, to some extent, overcome biases in retrospective memory (Schacter, 2002) and mistakes of averaging (Smith, Nowakowski, Soeffing, Orff, & Perlis, 2003).
Classification
Two of the major diagnostic nomenclatures base the diagnosis of insomnia on subtypes grouped according to presumed aetiology (American Academy of Sleep American Academy of Sleep Medicine, 2005; Edinger et al., 2004). If evidence continues to accrue for Fernandez-Mendoza et al.’s (2011; 2010) proposal for two phenotypes, then the tendency to misperceive may represent a more parsimonious method for defining subtypes.
Throughout this article we have referred to a categorical conceptualization of insomnia that may not reflect the clinical reality that insomnia likely occurs on a continuum. In contrast, a dimensional view assumes that “individuals assigned a DSM diagnosis differ from ‘normal’ persons only in the frequency and/or severity with which they experience the features that form the diagnostic criteria” (p. 22–23; Brown, 1996). As such, another topic for future research is to explore the possible contribution, to misperception, of the dimension that runs from good sleep to poor sleep to insomnia.
Intervention
As we highlighted at the outset, misperception of sleep is not characteristic of all patients with insomnia. Hence, to restate the wise words of Means et al. (2003), “clinicians should not assume that all insomnia sufferers are exaggerating their true sleep problem. Rather, they should consider the possibility that the patient is accurately perceiving or even minimizing her/his sleep difficulty” (p. 295).
In considering treatment implications, there are at least two possible scenarios that arise from the puzzle at hand. First, it may turn out that misperception of sleep is characteristic of many patients with insomnia. Second, it may turn out that misperception of sleep may be accounted for by an error of measurement and that the subjective report of patients with insomnia is accurate. In either case, the insomnia clearly warrants treatment given the associated adverse consequences outlined at the beginning of this paper. Assuming for a moment that further empirical work rules out the second scenario (i.e., that the misperception is simply an artifact of sleep definitions or problems with measurement), we move on to consider the treatment implications of the first scenario. In this scenario we suggest that there are at least two potential treatment targets. Before outlining these we note that, to date, none of the widely used treatments for insomnia have specifically targeted misperception of sleep (see Morin et al., 2006 for a review of current treatments).
1) Reversing misperception
Behavioral approach
To the best of our knowledge, three previous studies have attempted a behavioral approach to reversing misperception of sleep. At the outset we note that none of these studies included a follow-up assessment so we do not know if the gains were maintained. First, Downey and Bonnet (1992) attempted to correct misperception of sleep in ten individuals with subjective insomnia by teaching them to differentiate sleep from wakefulness. On the training night, the participants were woken up at different stages of their sleep (involving waking the patient 27 times on the training night). The experimenter gave the participant feedback regarding the sleep stage they were in before they were woken. The results of this study were promising - the participants demonstrated improvement in their perception of sleep onset, perception of sleep versus wake and ability to fall asleep.
Second, Mercer, Lack, and Bootzin (2005) gave retrospective feedback (based on two nights of PSG) and immediate feedback (based on four nights of PSG) to ten patients with insomnia. Feedback was given about whether the participant was awake or asleep and how long they had been sleeping since they had last been given feedback. In the immediate feedback condition, half of the feedbacks were given during wake and half were given following awakenings from Stage 2 and REM sleep. Both retrospective and immediate feedback were found to improve the sleep of the patients and reduce misperception of sleep, as measured by sleep diary and PSG.
Both of these studies are limited by their small samples and the procedure involved is not easily amenable to clinical practice and requires access to technically trained staff (overnight sleep technicians) and expensive equipment and procedures (PSG). A third study tried to overcome some of the above-mentioned limitations by developing a simple behavioral experiment designed to correct misperception of sleep (Tang & Harvey, 2004a). A behavioral experiment involves arranging real life experiences that provide a crystal clear and memorable demonstration to the patient that a perception or belief is not plausible or is unhelpful. It is designed so that the patient can derive salient corrective feedback (Beck, 1995; Clark, 1999; Salkovskis, 1999). The behavioral experiment to correct misperception of sleep was predicted to be associated with (a) more accurate subjective perception of sleep and (b) reduced sleep-related anxiety and preoccupation (Tang & Harvey, 2006). The behavioral experiment involved teaching patients with insomnia to download and read their actigraphy data and compare it to their sleep diary data, thereby discovering for themselves the tendency to overestimate SOL and underestimate TST. In an experiment to test the efficacy of this behavioral experiment, individuals with insomnia were asked to wear an actigraph and keep a sleep diary for 2 nights. On the following day, half were assisted to discover the discrepancy between the data recorded on the actigraph and their sleep diary via the behavioral experiment, the other half were told about the discrepancy verbally. Participants were then asked to wear the actigraph and keep a sleep diary for 2 further nights to index the effect of the instructions.
This experiment was purposively conducted over a short interval, without a substantial follow-up. The rationale was to test and refine a possibly effective treatment component via brief experimental studies, overcoming the disadvantages associated with basing treatment development on clinical judgment and devising complex multicomponent treatments for which the active ingredients are not known. The promise of this method – testing each new treatment component in a brief experiment before adding it to the multi-component treatment – is that it is likely to result in more effective and efficient treatments (Clark, 1999).
The behavioral experiment led to greater reduction in self-reported sleep-related anxiety and preoccupation, occurrence of insomnia symptoms, severity of sleep impairment, and distress about sleep, relative to when the patients were told about the discrepancy verbally. Effect sizes of the improvements shown by those who participated in the behavioral experiment were large, ranging from .70 to 1.06. Also, the behavioral experiment was regarded as a more beneficial and acceptable intervention strategy, compared to verbal feedback. Interestingly, both techniques were equally effective in reducing sleep misperception. The latter is good news as it suggests that when clinicians do not have access to actigraphy, they can contribute to improving perception of sleep by simple education approaches, even though there is a clear advantage to the behavioral experiment on a broad range of measures.
Medication approach
In a fascinating series of studies, Mendelson has raised the possibility that an important mode of action of some medications used to treat insomnia is to alter the perception of sleep quality. When insomnia patients were woken on a night they were taking a placebo, only 30% perceived they had been asleep. This increased to 97% when the patients were taking triazolam, 66% when taking flurazepam and 80% when taking zolpidem (Mendelson, 1993b, 1995). This effect was not observed in normal sleepers (Mendelson, 1995). Together these studies raise provocative questions about the mode of action for sleeping medications; namely, that one mode of action is that they may alter the perception of being awake or asleep, and this may be a more powerful influence over the perception of sleep quality than the actual sleep obtained (Mendelson, 1990). However, there is a need to rule out the possibility that the medications improve sleep and so misperception is simply less of an issue.
2) Reversing underlying factors
The discussion of resolutions to the puzzle of misperception suggest a number of other avenues for novel interventions. For example, one clinical implication of Sapar’s "flip-flop" switch model (Cano et al., 2008; Saper et al., 2001) is that interventions that reduce anxiety will reduce limbic system activation which, in turn, will prevent the VLPO from deactivating the arousal system and prevent the person from reaching a restful and continuous sleep state. The resolutions that implicate worry, selective attention and unhelpful beliefs also suggest that interventions targeting these potential contributors may be an effective means for reducing misperception of sleep. Such approaches are consistent with Rosa and Bonnet’s (2000) suggestion that “treatment for insomnia should be focused on tension/anxiety and hyperarousal rather then on sleep per se or at least on sleep as indexed by PSG” (p. 482). Although treatment approaches for these potential targets are being developed (e.g., Harvey, Sharpley, Ree, Stinson, & Clark, 2007; Morin & Espie, 2003) their potential to reduce misperception have not yet been empirically tested.
Conclusion
While the process of obtaining knowledge relating to the adverse consequences, mechanisms of insomnia, and effective treatments for insomnia is progressing well, the field continues to puzzle over one robust finding; that many patients with insomnia overestimate their sleep onset latency (SOL) and underestimate their total sleep time (TST), relative to objective measures of sleep such as polysomnography and actigraphy. This tendency is ubiquitous (although not universal). It is critical to note that the tendency to misperceive sleep by no means precludes the presence of a real sleep deficit.
We were motivated to take on the challenge of furthering understanding of this discrepancy between subjectively and objectively reported sleep because of the considerable clinical, theoretical, and public health importance of resolving the puzzle. For example, we are concerned that the discrepancy between subjective and objective measures may contribute to the trivialization of insomnia, which may have important implications for the allocation of treatment and research resources for insomnia. Importantly, if the tendency to misperceive sleep turns out to be a mechanism that contributes to the cause and/or maintenance and escalation of insomnia then reversing misperception may contribute to improving treatment outcomes for one of the most prevalent psychological health problems. We outline various forms that an intervention to correct misperception could take including adding a component to the current treatment of choice (CBT-I) that specifically targets misperception of sleep (e.g., Tang & Harvey, 2004b) or developing treatment components that reverse factors thought to underlie misperception of sleep, such as worry and selective attention and monitoring (e.g., Harvey et al., 2007).
We have identified and critically evaluated thirteen possible resolutions to the puzzle including: (1) features inherent to the context of sleep (e.g., darkness); (2) the definition of sleep onset which may lack sensitivity for insomnia patients; (3) insomnia being an exaggerated sleep complaint; (4) psychological distress causing magnification; (5) a deficit in time estimation ability; (6) sleep being misperceived as wake; (7) worry and selective attention toward sleep-related threats; (8) a memory bias influenced by current symptoms and emotions, a confirmation bias/belief bias, or a recall bias linked to the intensity/recency of symptoms; (9) heightened physiological arousal; (10) elevated cortical arousal; (11) the presence of brief awakenings; (12) a fault in neuronal circuitry; (13) there being two insomnia subtypes (one with and one without misperception). We conclude by proposing several integrative solutions. Given that only three of the resolutions met our criteria for good quality evidence, there are many opportunities to contribute to research in this important domain.
We recommend that research efforts focused on resolving the puzzle of misperception continue to include multiple measures, spanning self-reported sleep soon after waking, PSG and more detailed analyses of the sleep EEG (e.g., PSA, CAP analysis) and stay open to the suggestion that the field may need to reconsider what constitutes insomnia and that this may involve deemphasizing the use of PSG (Rosa & Bonnet, 2000).
Acknowledgments
This research was supported by an NIMH Grant R34MH080958 awarded to AGH. The authors are grateful to Matt Walker, Teresa Caffe, Ben Mullin, Lance Kriegsfeld, Michael Perlis, Jamie Rifkin, Adriane Soehner, and Daniel Taylor for helpful discussions with regard to this paper.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bul.
These studies were identified via a PsycINFO (1806-Oct 2009) and Medline (1950-Oct 2009) search, using keywords “insomnia” and “MMPI” simultaneously. A total of 115 papers were returned from the search. Twenty-five papers published in English were included because of their relevance and the availability of data needed for Table 2.
Contributor Information
Allison G. Harvey, Department of Psychology, University of California, Berkeley
Nicole Tang, Department of Psychology, Institute of Psychiatry, London, United Kingdom.
References
- Adam K, Tomeny M, Oswald I. Physiological and psychological differences between good and poor sleepers. Journal of Psychiatric Research. 1986;20:301–316. doi: 10.1016/0022-3956(86)90033-6. [DOI] [PubMed] [Google Scholar]
- Aikens JE, Wagner LI, Saelinger LJ. Reduced medical utilization and costs in anxious and depressed primary care patients receiving behavioral intervention. Advances in Medical Psychotherapeutic Psychodiagnostics. 1999;10:169–178. [Google Scholar]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd Ed. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- American Sleep Disorders Association. Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. Sleep. 1995;18:285–287. doi: 10.1093/sleep/18.4.285. [DOI] [PubMed] [Google Scholar]
- Armitage R, Trivedi M, Hoffmann R, Rush AJ. Relationship between objective and subjective sleep measures in depressed patients and healthy controls. Depression and Anxiety. 1997;5:97–102. doi: 10.1002/(sici)1520-6394(1997)5:2<97::aid-da6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Arntz A, Rauner M, Van den Hout M. "If I feel anxious, there must be danger": Ex-consequentia reasoning in inferring danger in anxiety disorders. Behaviour Research and Therapy. 1995;33:917–925. doi: 10.1016/0005-7967(95)00032-s. [DOI] [PubMed] [Google Scholar]
- Asendorpf JB, Scherer KR. The discrepant repressor: differentiation between low anxiety, high anxiety, and repression of anxiety by autonomic-facial-verbal patterns of behavior. Journal of Personality and Social Psychology. 1983;45:1334–1346. doi: 10.1037//0022-3514.45.6.1334. [DOI] [PubMed] [Google Scholar]
- Attarian HP, Duntley S, Brown KM. Reverse sleep state misperception. Sleep Medicine. 2004;5:269–272. doi: 10.1016/j.sleep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Baekeland F, Hoy P. Reported vs recorded sleep characteristics. Archives of General Psychiatry. 1971;24:548–551. doi: 10.1001/archpsyc.1971.01750120064011. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. Oxford, England: International Universities Press; 1976. [Google Scholar]
- Beck JS. Cognitive therapy: Basics and beyond. New York: Guilford Press; 1995. [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Archives of General Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669-670. [DOI] [PubMed] [Google Scholar]
- Bertocci MA, Dahl RE, Williamson DE, Iosif A, Birmaher B, Axelson D, Ryan ND. Subjective sleep complaints in pediatric depression: A controlled study and comparison with EEG measures of sleep and waking. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:1158–1166. doi: 10.1097/01.chi.0000179057.54419.17. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Kales A, Leo LA, Slye TA. A comparison of subjective estimates and objective sleep laboratory findings in insomnia patients [Abstract] Sleep Research. 1973;2:143. [Google Scholar]
- Bliwise DL, Friedman L, Yesavage JA. Depression as a confounding variable in the estimation of habitual sleep time. Journal of Clinical Psychology. 1993;49:471–477. doi: 10.1002/1097-4679(199307)49:4<471::aid-jclp2270490403>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. The perception of sleep onset in insomniacs and normal sleepers. In: Bootzin R, Kihlstrom JF, Schacter D, editors. Sleep and cognition. Washington D.C.: American Psychological Association; 1990. pp. 148–158. [Google Scholar]
- Bonnet MH, Arand DL. Caffeine use as a model of acute and chronic insomnia. Sleep. 1992;15:526–536. [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Impact of the level of physiological arousal on estimates of sleep latency. In: Ogilvie RD, Harsh JR, editors. Sleep onset: Normal and abnormal processes. Washington, D. C.: American Psychological Association; 1994. pp. 127–139. [Google Scholar]
- Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Physiological activation in patients with Sleep State Misperception. Psychosomatic Medicine. 1997;59:533–540. doi: 10.1097/00006842-199709000-00011. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Perlis ML. Nonpharmacologic treatments of insomnia. Journal of Clinical Psychiatry. 1992;53:37–41. [PubMed] [Google Scholar]
- Borkovec TD. Insomnia. Journal of Consulting and Clinical Psychology. 1982;50:880–895. doi: 10.1037//0022-006x.50.6.880. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Hennings BL. The role of physiological attention-focusing in the relaxation treatment of sleep disturbance, general tension, and specific stress reaction. Behaviour Research and Therapy. 1978;16:7–19. doi: 10.1016/0005-7967(78)90085-2. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Lane TW, VanOot PH. Phenomenology of sleep among insomniacs and good sleepers: Wakefulness experience when cortically asleep. Journal of Abnormal Psychology. 1981;90:607–609. doi: 10.1037//0021-843x.90.6.607. [DOI] [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biological Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Brown TA. Validity of the DSM-III-R and DSM-IV classification systems for anxiety disorders. In: Rapee RM, editor. Current controversies in the anxiety disorders. New York: Guilford Press; 1996. pp. 21–45. [Google Scholar]
- Buysse D, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Buysse D, Germain A, Hall M, Moul D, Nofzinger E, Begley A, Kupfer D. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–1682. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Thompson W, Scott J, Franzen PL, Germain A, Hall M, Kupfer DJ. Daytime symptoms in primary insomnia: A prospective analysis using ecological momentary assessment. Sleep Medicine. 2007;8:198–208. doi: 10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. The Journal of Neuroscience. 2008;28:10167–10184. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. American Journal of Psychiatry. 1976;133:1382–1388. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Sanderson WC, Shoham V, Bennett Johnson S, Pope KS, Crits-Christoph P. An update on empirically validated therapies. The Clinical Psychologist. 1996;49:5–18. [Google Scholar]
- Chilcott LA, Shapiro CM. The socioeconomic impact of insomnia. An overview. Pharmacoeconomics. 1996;10 Suppl 1:1–14. doi: 10.2165/00019053-199600101-00003. [DOI] [PubMed] [Google Scholar]
- Clark DM. Anxiety disorders: why they persist and how to treat them. Behaviour Research and Therapy. 1999;37 Suppl 1:S5–S27. doi: 10.1016/s0005-7967(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Clark DM. Developing new treatments: On the interplay between theories, experimental science and clinical innovation. Behaviour Research and Therapy. 2004;42:1089–1104. doi: 10.1016/j.brat.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Clark DM, Salkovskis PM, Ost LG, Breitholtz E, Koehler KA, Westling BE, Gelder M. Misinterpretation of body sensations in panic disorder. Journal of Consulting and Clinical Psychology. 1997;65:203–213. doi: 10.1037//0022-006x.65.2.203. [DOI] [PubMed] [Google Scholar]
- Coates TJ, Killen JD, George J, Marchini E, Silverman S, Thoresen C. Estimating sleep parameters: a multitrait--multimethod analysis. Journal of Consulting and Clinical Psychology. 1982;50:345–352. [PubMed] [Google Scholar]
- Currie SR, Malhotra S, Clark S. Agreement among subjective, objective, and collateral measures of insomnia in postwithdrawal recovering alcoholics. Behavioral Sleep Medicine. 2004;2:148–161. doi: 10.1207/s15402010bsm0203_4. [DOI] [PubMed] [Google Scholar]
- Dagan Y, Zinger Y, Lavie P. Actigraphic sleep monitoring in posttraumatic stress disorder (PTSD) patients. Journal of Psychosomatic Research. 1997;42:577–581. doi: 10.1016/s0022-3999(97)00013-5. [DOI] [PubMed] [Google Scholar]
- Downey R, Bonnet MH. Training subjective insomniacs to accurately perceive sleep onset. Sleep. 1992;15:58–63. doi: 10.1093/sleep/15.1.58. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Stepanski EJ. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Fins AI. The distribution and clinical significance of sleep time misperceptions among insomniacs. Sleep. 1995;18:232–239. doi: 10.1093/sleep/18.4.232. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Fins AI, Glenn DM, Sullivan RJ, Jr, Bastian LA, Marsh GR, Vasilas D. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? Journal of Consulting and Clinical Psychology. 2000;68:586–593. [PubMed] [Google Scholar]
- Edinger JD, Krystal AD. Subtyping primary insomnia: is sleep state misperception a distinct clinical entity? Sleep Medicine Reviews. 2003;7:203–214. doi: 10.1053/smrv.2002.0253. [DOI] [PubMed] [Google Scholar]
- Ehlers A. A 1-year prospective study of panic attacks: Clinical course and factors associated with maintenance. Journal of Abnormal Psychology. 1995;104:164–172. doi: 10.1037//0021-843x.104.1.164. [DOI] [PubMed] [Google Scholar]
- Eich E, Reeves JL, Jaeger B, Graff-Radford SB. Memory for pain: relation between past and present pain intensity. Pain. 1985;23:375–380. doi: 10.1016/0304-3959(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Elsenbruch S, Harnish MJ, Orr WC. Subjective and objective sleep quality in irritable bowel syndrome. American Journal of Gastroenterology. 1999;94:2447–2452. doi: 10.1111/j.1572-0241.1999.01374.x. [DOI] [PubMed] [Google Scholar]
- Engle-Friedman M, Bootzin RR, Hazlewood L, Tsao C. An evaluation of behavioral treatments for insomnia in the older adult. Journal of Clinical Psychology. 1992;48:77–90. doi: 10.1002/1097-4679(199201)48:1<77::aid-jclp2270480112>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Espie CA. Insomnia: Conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annual Review of Psychology. 2002;53:215–243. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- Evans DG, Burnell LD, Hopwood P, Howell A. Perception of risk in women with a family history of breast cancer. British Journal of Cancer. 1993;67:612–614. doi: 10.1038/bjc.1993.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun S, Bixler E, Karataraki M, Liao D, Vela-Bueno A, Vgontzas A. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosomatic medicine. 2011;73:88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fernandez-Mendoza J, Calhoun S, Bixler E, Karataraki M, Liao D, Vela-Bueno A, Vgontzas A. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosomatic Medicine. 2011;73:88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun S, Bixler E, Pejovic S, Karataraki M, Liao D, Vgontzas A. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–465. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL. Measuring nominal scale agreement among many raters. Psychological Bulletin. 1971;76:378–382. [Google Scholar]
- Forbes EE, Bertocci MA, Gregory AM, Ryan ND, Axelson DA, Birmaher B, Dahl RE. Objective sleep in pediatric anxiety disorders and major depressive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:148–155. doi: 10.1097/chi.0b013e31815cd9bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Journal of the American Medical Association. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Frankel BL, Buchbinder R, Coursey R, Snyder F. Sleep patterns and psychological test characteristics of chronic primary insomniacs [Abstract] Sleep Research. 1973;2:149. [Google Scholar]
- Frankel BL, Coursey RD, Buchbinder R, Snyder F. Recorded and reported sleep in chronic primary insomnia. Archives of General Psychiatry. 1976;33:615–623. doi: 10.1001/archpsyc.1976.01770050067011. [DOI] [PubMed] [Google Scholar]
- Franklin J. The measurement of sleep onset latency in insomnia. Behaviour Research and Therapy. 1981;19:547–549. doi: 10.1016/0005-7967(81)90082-6. [DOI] [PubMed] [Google Scholar]
- Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. Journal of Sleep Research. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR. Psychological and physiological characteristics of sleep-onset insomniacs [Abstract] Sleep Research. 1976;6:170. [Google Scholar]
- Freedman RR, Sattler HL. Physiological and psychological factors in sleep-onset insomnia. Journal of Abnormal Psychology. 1982;91:380–389. doi: 10.1037//0021-843x.91.5.380. [DOI] [PubMed] [Google Scholar]
- Freelon DG. Intercoder reliability calculation as a web service. International Journal of Internet Science. 2010;5:20–33. [Google Scholar]
- Gruber JL, Johnson SL, Oveis C, Keltner D. Mania vulnerability and heightened positive emotional responding across contexts. Emotion. 2008;8:23–33. doi: 10.1037/1528-3542.8.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG. A cognitive model of insomnia. Behaviour Research and Therapy. 2002;40:869–894. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- Harvey AG. Unwanted intrusive thoughts in insomnia. In: Clark DA, editor. Intrusive thoughts in clinical disorders: Theory, research, and treatment. New York: Guilford Press; 2005. pp. 86–118. [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. American Journal of Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Sharpley AL, Ree MJ, Stinson K, Clark DM. An open trial of cognitive therapy for chronic insomnia. Behaviour Research and Therapy. 2007;45:2491–2501. doi: 10.1016/j.brat.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Tang NKJ. Cognitive behavior therapy for insomnia: can we rest yet? Sleep Medicine Reviews. 2003;7:237–262. doi: 10.1053/smrv.2002.0266. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Watkins E, Mansell W, Shafran R. Cognitive behavioural processes across psychological disorders : A transdiagnostic approach to research and treatment. Oxford: Oxford University Press; 2004. [Google Scholar]
- Hauri PJ, Fisher J. Persistent psychophysiologic (learned) insomnia. Sleep. 1986;9:38–53. doi: 10.1093/sleep/9.1.38. [DOI] [PubMed] [Google Scholar]
- Hauri PJ, Olmstead EM. What is the moment of sleep onset for insomniacs? Neurophysiology. 1983;6:10–15. doi: 10.1093/sleep/6.1.10. [DOI] [PubMed] [Google Scholar]
- Hirsch M, Carlander B, Vergé M, Tafti M, Anaya J, Billiard M, Sany J. Objective and subjective sleep disturbances in patients with rheumatoid arthritis. Arthritis & Rheumatism. 1994;37:41–49. doi: 10.1002/art.1780370107. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Carskadon M, Phillips R, Zarcone V, Dement WC. Total sleep time in insomniacs [Abstract] Sleep Research. 1972;1:152. [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF, Medicine ftAAoS. The AASM Manual for the Scoring of Sleep and Associated Events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Jason L. Rapid improvement in insomnia following self-monitoring. Journal of Behavioral Therapy and Experimental Psychiatry. 1975;6:346–350. [Google Scholar]
- Johns MW, Gay TJ, Masterton JP, Bruce DW. Relationship between sleep habits, adrenocortical activity and personality. Psychosomatic Medicine. 1971;33:499–508. doi: 10.1097/00006842-197111000-00003. [DOI] [PubMed] [Google Scholar]
- Jones B, Macphee L, Broomfield N, Jones B, Espie C. Sleep-related attentional bias in good, moderate, and poor (primary insomnia) sleepers. Journal of Abnormal Psychology. 2005;114:249–258. doi: 10.1037/0021-843X.114.2.249. [DOI] [PubMed] [Google Scholar]
- Kales A, Caldwell AB, Soldatos CR, Bixler EO, Kales JD. Biopsychobehavioral correlates of insomnia. II. Pattern specificity and consistency with the Minnesota Multiphasic Personality Inventory. Psychosomatic Medicine. 1983;45:341–356. doi: 10.1097/00006842-198308000-00008. [DOI] [PubMed] [Google Scholar]
- Kales JD, Caldwell AB, Preston TA, Healey S, Kales JD. Personality patterns in insomnia. Archives of General Psychiatry. 1976;33:1128–1134. doi: 10.1001/archpsyc.1976.01770090118013. [DOI] [PubMed] [Google Scholar]
- Kalogjera-Sackellares D, Cartwright RD. Comparison of MMPI profiles in medically and psychologically based insomnias. Psychiatry Research. 1997;70:49–56. doi: 10.1016/s0165-1781(97)03078-3. [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, McNicol J, Conte RA, Moghadam HK. Sleep disturbance in preschool-aged hyperactive and nonhyperactive children. Pediatrics. 1987;80:839–844. [PubMed] [Google Scholar]
- Klein E, Koren D, Arnon I, Lavie P. No evidence of sleep disturbance in post-traumatic stress disorder: A polysomnographic study in injured victims of traffic accidents. Israel Journal of Psychiatry and Related Sciences. 2002;39:3–10. [PubMed] [Google Scholar]
- Klein E, Koren D, Arnon I, Lavie P. Sleep complaints are not corroborated by objective sleep measures in post-traumatic stress disorder: A 1-year prospective study in survivors of motor vehicle crashes. Journal of Sleep Research. 2003;12:35–41. doi: 10.1046/j.1365-2869.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- Klinger E. Current concerns and disengagement from incentives. In: Halisch F, Kuhl J, editors. Motivation, intention and violation. Berlin: Springer-Verlag; 1987. [Google Scholar]
- Knab B, Engel RR. Perception of waking and sleeping: Possible implications for the evaluation of insomnia. Sleep. 1988;11:265–272. doi: 10.1093/sleep/11.3.265. [DOI] [PubMed] [Google Scholar]
- Kring AM, Neale JM. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? Journal of Abnormal Psychology. 1996;105:249–257. doi: 10.1037//0021-843x.105.2.249. [DOI] [PubMed] [Google Scholar]
- Krippendorff K. Content analysis: An Introduction to its Methodology. Thousand Oaks, CA: Sage; 2004. [Google Scholar]
- Krystal AD. A compendium of placebo-controlled trials of the risks/benefits of pharmacological treatments for insomnia: the empirical basis for U.S. clinical practice. Sleep Medicine Reviews. 2009;13:265–274. doi: 10.1016/j.smrv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–640. [PubMed] [Google Scholar]
- Kyle SD, Morgan K, Colin AE. Insomnia and health-related quality of life. Sleep Medicine Reviews. 2010;14:69–82. doi: 10.1016/j.smrv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Lazarev VV. On the intercorrelation of some frequency and amplitude parameters of the human EEG and its functional significance. Communication I: Multidimensional neurodynamic organization of functional states of the brain during intellectual, perceptive and motor activity in normal subjects. International Journal of Psychophysiology. 1998;28:77–98. doi: 10.1016/s0167-8760(97)00068-8. [DOI] [PubMed] [Google Scholar]
- Leger D. Public health and insomnia: economic impact. Sleep. 2000;23 Suppl 3:S69–S76. [PubMed] [Google Scholar]
- Levin D, Bertelson AD, Lacks P. MMPI differences among mild and severe insomniacs and good sleepers. Journal of Personality Assessment. 1984;48:126–129. doi: 10.1207/s15327752jpa4802_3. [DOI] [PubMed] [Google Scholar]
- Lichstein K, Durrence H, Taylor D, Bush A, Riedel B. Quantitative criteria for insomnia. Behaviour Research and Therapy. 2003;41:427–445. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- Loring DW, Sheer DE. Laterality of 40 Hz EEG and EMG during cognitive performance. Psychophysiology. 1984;21:34–38. doi: 10.1111/j.1469-8986.1984.tb02313.x. [DOI] [PubMed] [Google Scholar]
- Manconi M, Ferri R, Sagrada C, Punjabi N, Tettamanzi E, Zucconi M, Ferini-Stambi Measuring the error in sleep estimation in normal subjects and in patients with insomnia. Journal of Sleep Research. 2010;19:478–486. doi: 10.1111/j.1365-2869.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Shallcross AJ, Troy AS, John OP, Ferrer E, Wilhelm FH, Gross JJ. Don't hide your happiness! Positive emotion dissociation, social connectedness, and psychological functioning. Journal of Personality and Social Psychology. doi: 10.1037/a0022410. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon K, Broomfield N, Espie C. Attentional bias for sleep-related stimuli in primary insomnia and delayed sleep phase syndrome using the dot-probe task. Sleep. 2006;29:1420–1427. doi: 10.1093/sleep/29.11.1420. [DOI] [PubMed] [Google Scholar]
- Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Medicine. 2003;4:285–296. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Mendelson WB. Clinical neuropharmacology of sleep. Neurology Clinics. 1990;8:153–160. [PubMed] [Google Scholar]
- Mendelson WB. Insomnia and related sleep disorders. Psychiatric Clinics of North America. 1993a;16:841–851. [PubMed] [Google Scholar]
- Mendelson WB. Pharmacologic alteration of the perception of being awake or asleep. Sleep. 1993b;16:641–646. [PubMed] [Google Scholar]
- Mendelson WB. Effects of flurazepam and zolpidem on the perception of sleep in normal volunteers. Sleep. 1995;18:88–91. doi: 10.1093/sleep/18.2.88. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Garnett D, Gillin JC, Weingartner H. The experience of insomnia and daytime and nighttime functioning. Psychiatry Research. 1984;12:235–250. doi: 10.1016/0165-1781(84)90029-5. [DOI] [PubMed] [Google Scholar]
- Mercer J, Lack L, Bootzin R. Feedback of sleep/wake state improves subjective and objective sleep of insomniacs. Sleep. 2005;28:A242–A243. [Google Scholar]
- Mercer JD, Bootzin RR, Lack LC. Insomniacs' perception of wake instead of sleep. Sleep. 2002;25:564–571. [PubMed] [Google Scholar]
- Miller GA. How we think about cognition, emotion, and biology in psychopathology. Psychophysiology. 1996;33:615–628. doi: 10.1111/j.1469-8986.1996.tb02356.x. [DOI] [PubMed] [Google Scholar]
- Monroe LJ. Psychological and physiological differences between good and poor sleepers. Journal of Abnormal Psychology. 1967;72:255–264. doi: 10.1037/h0024563. [DOI] [PubMed] [Google Scholar]
- Monroe LJ, Marks PA. MMPI differences between adolescent poor and good sleepers. Journal of Consulting and Clinical Psychology. 1977;45:151–152. doi: 10.1037//0022-006x.45.1.151. [DOI] [PubMed] [Google Scholar]
- Moore SE, Bonnet MH, Warm JS. Time estimation in insomniac and normal sleepers. Sleep Research. 1982;11:161. [Google Scholar]
- Morin C, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychology and Aging. 1993;8:463–467. doi: 10.1037//0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- Morin CM. Insomnia: Psychological Assessment and Management. New York: Guilford Press; 1993. [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: An update of recent evidence (1998–2004) Sleep. 2006;29:1396–1406. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- Morin CM, Culbert JP, Schwartz SM. Nonpharmacological Interventions for insomnia: a meta-analysis of treatment efficacy. American Journal of Psychiatry. 1994;151:1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- Morin CM, Espie CA. Insomnia : A clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. Journal of Consulting and Clinical Psychology. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- Neu D, Mairesse O, Hoffmann G, Dris A, Lambrecht LJ, Linkowski P, Le Bon O. Sleep quality perception in the chronic fatigue syndrome: correlations with sleep efficiency, affective symptoms and intensity of fatigue. Neuropsychobiology. 2007;56:40–46. doi: 10.1159/000110727. [DOI] [PubMed] [Google Scholar]
- Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: The development of the pre-sleep arousal scale. Behaviour Research and Therapy. 1985;23:263–271. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- Nickerson RS. Confirmation bias: A ubiquitous phenomenon in many guises. Review of General Psychology. 1998;2:175–220. [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. American Journal of Psychiatry. 2004;161:2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Wilson S. Evaluation of severe insomnia in the general population--implications for the management of insomnia: the UK perspective. Journal of Psychopharmacology. 1999;13:S33–S34. doi: 10.1177/026988119901304S09. [DOI] [PubMed] [Google Scholar]
- Ogilvie RD. The process of falling asleep. Sleep Medicine Reviews. 2001;5:247–270. doi: 10.1053/smrv.2001.0145. [DOI] [PubMed] [Google Scholar]
- Ogilvie RD, Harsh JR, editors. Sleep onset: Normal and abnormal processes. Washington, D. C.: American Psychological Association; 1994. [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Caulet M, Philip P, Guilleminault C, Priest RG. How sleep and mental disorders are related to complaints of daytime sleepiness. Archives of Internal Medicine. 1997;157:2645–2652. [PubMed] [Google Scholar]
- Olmstead EM, Hauri PJ, Percy L, Hellekson C. Subjective versus objective estimation of sleep onset in normal sleepers and in insomniacs [Abstract] Sleep Research. 1980;9:216. [Google Scholar]
- Ornstein RE. On the experience of time. Harmondsworth, U. K.: Penguin; 1969. [Google Scholar]
- Parrino L, Milioli G, De Paolis F, Grassi A, Terzano MG. Paradoxical insomnia: The role of CAP and arousals in sleep misperception. Sleep Medicine. 2009;10:1139–1145. doi: 10.1016/j.sleep.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. Journal of Sleep Research. 1997;6:179–188. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Medicine Reviews. 2001;5:363–374. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- Picchioni D, Fukunaga M, Carr WS, Braun AR, Balkin TJ, Duyn JH, et al. fMRI differences between early and late stage-1 sleep. Neuroscience Letters. 2008;441:81–85. doi: 10.1016/j.neulet.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccione P, Tallarigo R, Zorick F, Wittig R, Roth T. Personality differences between insomniac and non-insomniac psychiatry outpatients. Journal of Clinical Psychiatry. 1981;42:261–263. [PubMed] [Google Scholar]
- Rachman S, Cuk M. Fearful distortions. Behaviour Research and Therapy. 1992;30:583–589. doi: 10.1016/0005-7967(92)90003-y. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles (University of California): Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- Rechtschaffen A, Monroe LJ. Laboratory studies of insomnia. In: Kales A, editor. Sleep: physiology and pathology. Philadelphia: Lippincott; 1969. pp. 158–169. [Google Scholar]
- Redelmeier DA, Kahneman D. Patients' memories of painful medical treatments: real-time and retrospective evaluations of two minimally invasive procedures. Pain. 1996;66:3–8. doi: 10.1016/0304-3959(96)02994-6. [DOI] [PubMed] [Google Scholar]
- Reite M, Buysse D, Reynolds C, Mendelson W. The use of polysomnography in the evaluation of insomnia. Sleep. 1995;18:754–756. doi: 10.1093/sleep/18.1.58. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Kupfer D, Buysse D, Coble P, Yeager A. Subtyping DSM-III--R primary insomnia: A literature review by the DSM-IV work group on sleep disorders. American Journal of Psychiatry. 1991;148:432–438. doi: 10.1176/ajp.148.4.432. [DOI] [PubMed] [Google Scholar]
- Rioux I, Tremblay S, Bastien CH. Time estimation in chronic insomnia sufferers. Sleep. 2006;29:486–493. doi: 10.1093/sleep/29.4.486. [DOI] [PubMed] [Google Scholar]
- Rosa RR, Bonnet MH. Reported chronic insomnia is independent of poor sleep as measured by electroencephalography. Psychosomatic Medicine. 2000;62:474–482. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Ross M. Relation of implicit theories to the construction of personal histories. Psychological Review. 1989;96:341–357. [Google Scholar]
- Rotenberg VS, Indursky P, Kayumov L, Sirota P, Melamed Y. The relationship between subjective sleep estimation and objective sleep variables in depressed patients. International Journal of Psychophysiology. 2000;37:291–297. doi: 10.1016/s0167-8760(00)00110-0. [DOI] [PubMed] [Google Scholar]
- Roth T, Kramer M, Schwartz J. Some preliminary observations of the nature of insomniac patients [Abstract] Sleep Research. 1974;3:145. [Google Scholar]
- Roth T, Lutz T, Kramer M, Tietz E. The relationship between objective and subjective evaluation of sleep in isomniacs [Abstract] Sleep Research. 1977;6:178. [Google Scholar]
- Rougeul A, Bouyer JJ, Dedet L, Debray O. Fast somato-parietal rhythms during combined focal attention and immobility in baboon and squirrel monkey. Electroencephalogric and Clinical Neurophysiology. 1979;46:310–319. doi: 10.1016/0013-4694(79)90205-0. [DOI] [PubMed] [Google Scholar]
- Salin-Pascual RJ, Roehrs TA, Merlotti LA, Zorick F, Roth T. Long-term study of the sleep of insomnia patients with sleep state misperception and other insomnia patients. American Journal of Psychiatry. 1992;149:904–908. doi: 10.1176/ajp.149.7.904. [DOI] [PubMed] [Google Scholar]
- Salkovskis PM. Understanding and treating obsessive-compulsive disorder. Behaviour Research and Therapy. 1999;37 Suppl 1:S29–S52. [PubMed] [Google Scholar]
- Salkovskis PM, Bass C. Hypochondriasis. In: Clark DM, Fairburn CG, editors. Science and practice of cognitive behavioral therapy. Oxford: Oxford University Press; 1997. pp. 313–339. [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends in Neurosciences. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Forgotten ideas, neglected pioneers: Richard Semon and the story of memory. Neuropsychological Rehabilitation. 2002;12:477–478. [Google Scholar]
- Schneider-Helmert D. Twenty-four-hour sleep-wake function and personality patterns in chronic insomniacs and healthy controls. Sleep. 1987;10:452–462. doi: 10.1093/sleep/10.5.452. [DOI] [PubMed] [Google Scholar]
- Schneider-Helmert D. Asymptomatic insomnia. Sleep Medicine. 2007;8:107–110. doi: 10.1016/j.sleep.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Schneider-Helmert D, Kumar A. Sleep, its subjective perception, and daytime performance in insomniacs with a pattern of alpha sleep. Biological Psychiatry. 1995;37:99–105. doi: 10.1016/0006-3223(94)00162-V. [DOI] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of Clinical Sleep Medicine. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- Schwarz N, Clore GL. Mood, misattribution, and judgments of well-being: Informative and directive functions of affective states. Journal of Personality and Social Psychology. 1983;45:513–523. [Google Scholar]
- Seibt J, Anto SJ, Jha SK, Coleman T, Dumoulin MC, Frank MG. The non-benzodiazepine hypnotic zolpidem impairs sleep-dependent cortical plasticity. Sleep. 2008;31:1381–1391. [PMC free article] [PubMed] [Google Scholar]
- Seidel WF, S B, Cohen S, Patterson Y, Yost D, Dement WC. Daytime alertness in relation to mood, performance, and nocturnal sleep in chronic insomniacs and noncomplaining sleepers. Sleep. 1984;7:230–238. doi: 10.1093/sleep/7.3.230. [DOI] [PubMed] [Google Scholar]
- Semler C, Harvey AG. An investigation of monitoring for sleep-reated threat in primary insomnia. Behaviour Research and Therapy. 2004a;42:1403–1420. doi: 10.1016/j.brat.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Semler C, Harvey AG. Monitoring for sleep-related threat: A pilot study of the sleep associated monitoring index (SAMI) Psychosomatic Medicine. 2004b;66:242–250. doi: 10.1097/01.psy.0000114870.50968.90. [DOI] [PubMed] [Google Scholar]
- Shealy RC, Lowe JD, Ritzler BA. Sleep onset insomnia: personality characteristics and treatment outcome. Journal of Consulting and Clinical Psychology. 1980;48:659–661. [PubMed] [Google Scholar]
- Smith LJ, Nowakowski S, Soeffing JP, Orff HJ, Perlis ML. The measurement of sleep. In: Perlis ML, Lichstein KL, editors. Treating sleep disorders: principles and practice of behavioral sleep medicine. New York, NY: John Wiley & Sons, Inc.; 2003. pp. 29–73. [Google Scholar]
- Smith M, Huang M, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clinical Psychology Review. 2005;25:559–592. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Smith S, Trinder J. The effect of arousals during sleep onset on estimates of sleep onset latency. Journal of Sleep Research. 2000;9:129–135. doi: 10.1046/j.1365-2869.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- Spielman AJ, Tannenbaum R, Adler J, Saskin P. MMPI and recorded sleep latency in insomnia: A failure to support a corollary of the arousal hypothesis. Paper presented at the Paper presented at the Associated Professional Sleep Societies Meeting.1980. [Google Scholar]
- Spielman AJ, Tannenbaum R, Saskin P, Adler J, Pollak CP. A comparison between objective and subjective sleep latency in patients with chronic insomnia [Abstract] Sleep Research. 1980;9:225. [Google Scholar]
- Spydell JD, Sheer DE. Effect of problem solving on right and left hemisphere 40 hertz EEG activity. Psychophysiology. 1982;19:420–425. doi: 10.1111/j.1469-8986.1982.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Medicine Reviews. 2003;7:215–225. doi: 10.1053/smrv.2001.0246. [DOI] [PubMed] [Google Scholar]
- Sugerman JL, Stern JA, Walsh JK. Daytime alertness in subjective and objective insomnia: Some preliminary findings. Society of Biological Psychiatry. 1985;20:741–750. doi: 10.1016/0006-3223(85)90153-2. [DOI] [PubMed] [Google Scholar]
- Tang NKY, Harvey AG. Correcting distorted perception of sleep in insomnia: a novel behavioural experiment? Behaviour Research and Therapy. 2004a;42:27–39. doi: 10.1016/s0005-7967(03)00068-8. [DOI] [PubMed] [Google Scholar]
- Tang NKY, Harvey AG. Effects of cognitive arousal and physiological arousal on sleep perception. Sleep. 2004b;27:69–78. doi: 10.1093/sleep/27.1.69. [DOI] [PubMed] [Google Scholar]
- Tang NKY, Harvey AG. Time estimation ability and distorted perception of sleep in insomnia. Behavioral Sleep Medicine. 2005;3:134–150. doi: 10.1207/s15402010bsm0303_2. [DOI] [PubMed] [Google Scholar]
- Tang NKY, Harvey AG. Altering misperception of sleep in insomnia: behavioral experiment versus verbal feedback. Journal of Consulting and Clinical Psychology. 2006;74:767–776. doi: 10.1037/0022-006X.74.4.767. [DOI] [PubMed] [Google Scholar]
- Tang NKY, Schmidt DE, Harvey AG. Sleeping with the enemy: Clock monitoring in the maintenance of insomnia. Journal of Behaviour Therapy and Experimental Psychiatry. 2007;48:40–55. doi: 10.1016/j.jbtep.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Taylor D, Mallory L, Lichstein K, Durrence H, Riedel B, Bush A. Comorbidity of chronic insomnia with medical problems. Journal of Sleep and Sleep Disorders Research. 2007;30:213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- Taylor LM, Espie CA, White CA. Attentional bias in people with acute versus persistent insomnia secondary to cancer. Behavioral Sleep Medicine. 2003;1:200–212. doi: 10.1207/S15402010BSM0104_3. [DOI] [PubMed] [Google Scholar]
- Thomas EAC, Cantor NE. On the duality of simultaneous time and size perception. Perceptual Psychophysics. 1975;18:44–48. [Google Scholar]
- Thomas EAC, Cantor NE. Simultaneous time and size perception. Perceptual Psychophysics. 1976;19:353–360. [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Trajanovic NN, Radivojevic V, Kaushansky Y, Shapiro CM. Positive sleep state misperception: A new concept of sleep misperception. Sleep Medicine. 2007;8:111–118. doi: 10.1016/j.sleep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Trinder J. Subjective insomnia without objective findings: A pseudo diagnostic classification? Psychological Bulletin. 1988;103:87–94. doi: 10.1037/0033-2909.103.1.87. [DOI] [PubMed] [Google Scholar]
- Tsushima WT, Ingolfsdottir E. MMPI-2 scores of patients with insomnia. Psychology Reports. 2004;94:267–272. doi: 10.2466/pr0.94.1.267-272. [DOI] [PubMed] [Google Scholar]
- Van den Berg JF, Van Rooij FJA, Vos H, Tulen JHM, Hofman A, Miedema HME, Tiemeier H. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. Journal of Sleep Research. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- Van Egeren L, Haynes SN, Franzen M, Hamilton J. Presleep cognitions and attributions in sleep-onset insomnia. Journal of Behavioral Medicine. 1983;6:217–232. doi: 10.1007/BF00845382. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Aikens JE, Tadimeti L, Caruana-Montaldo B, Mendelson WB. Sleep latency and duration estimates among sleep disorder patients: variability as a function of sleep disorder diagnosis, sleep history, and psychological characteristics. Sleep. 2000;23:71–79. [PubMed] [Google Scholar]
- Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, Chrousos GP. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. Journal of Clinical Endocrinology and Metabolism. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: Towards a cognitive model of insomnia. Behaviour Research and Therapy. 2000;38:679–693. doi: 10.1016/s0005-7967(99)00136-9. [DOI] [PubMed] [Google Scholar]
- Wilson KG, Watson ST, Currie SR. Daily diary and ambulatory activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. 1998;75:75–84. doi: 10.1016/S0304-3959(97)00207-8. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Bootzin RR, Allen JJ, Anthony JL. Mesograde amnesia during the sleep onset transition: replication and electrophysiological correlates. Sleep. 1997;20:512–522. [PubMed] [Google Scholar]
- Wyatt JK, Bootzin RR, Anthony J, Bazant S. Sleep onset is associated with retrograde and anterograde amnesia. Sleep. 1994;17:502–511. doi: 10.1093/sleep/17.6.502. [DOI] [PubMed] [Google Scholar]
- Yoo S, Gujar N, Hu P, Jolesz F, Walker M. The human emotional brain without sleep: A prefrontal-amygdala disconnect? Current Biology. 2007;17:R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]