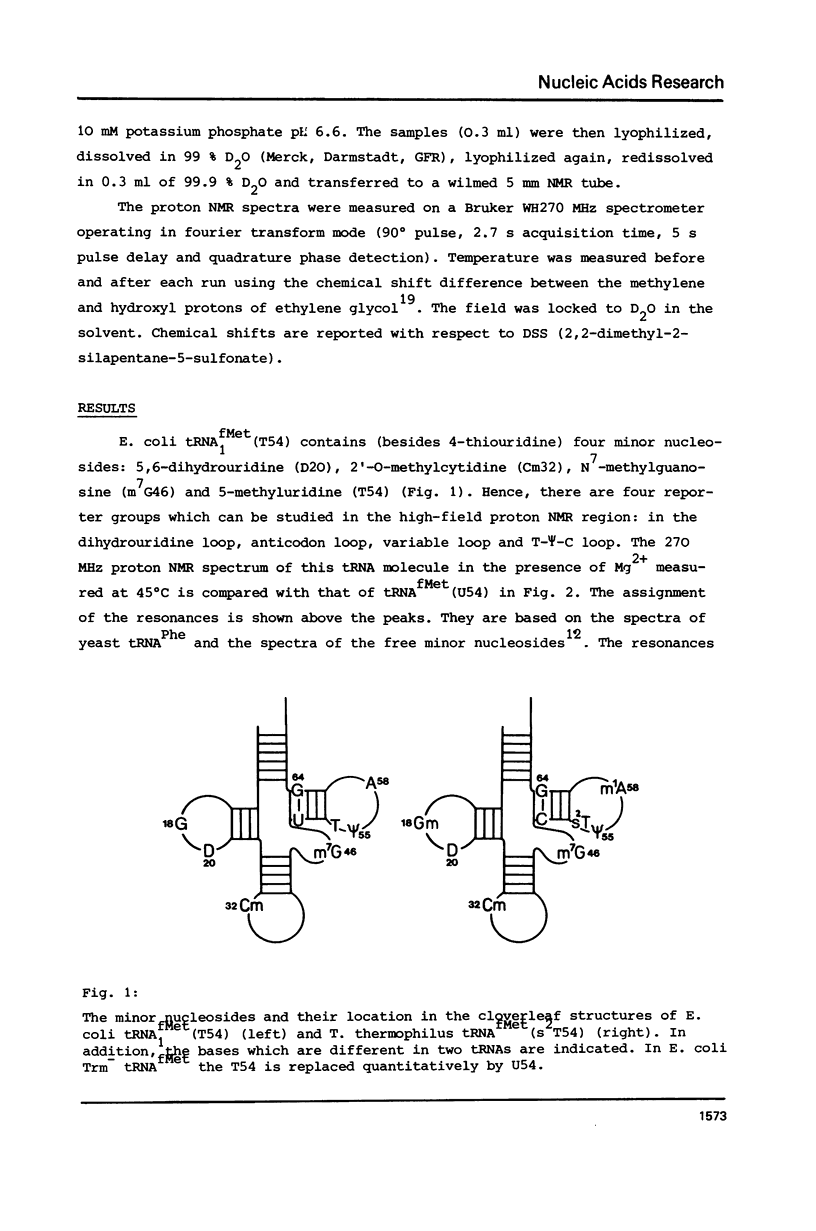
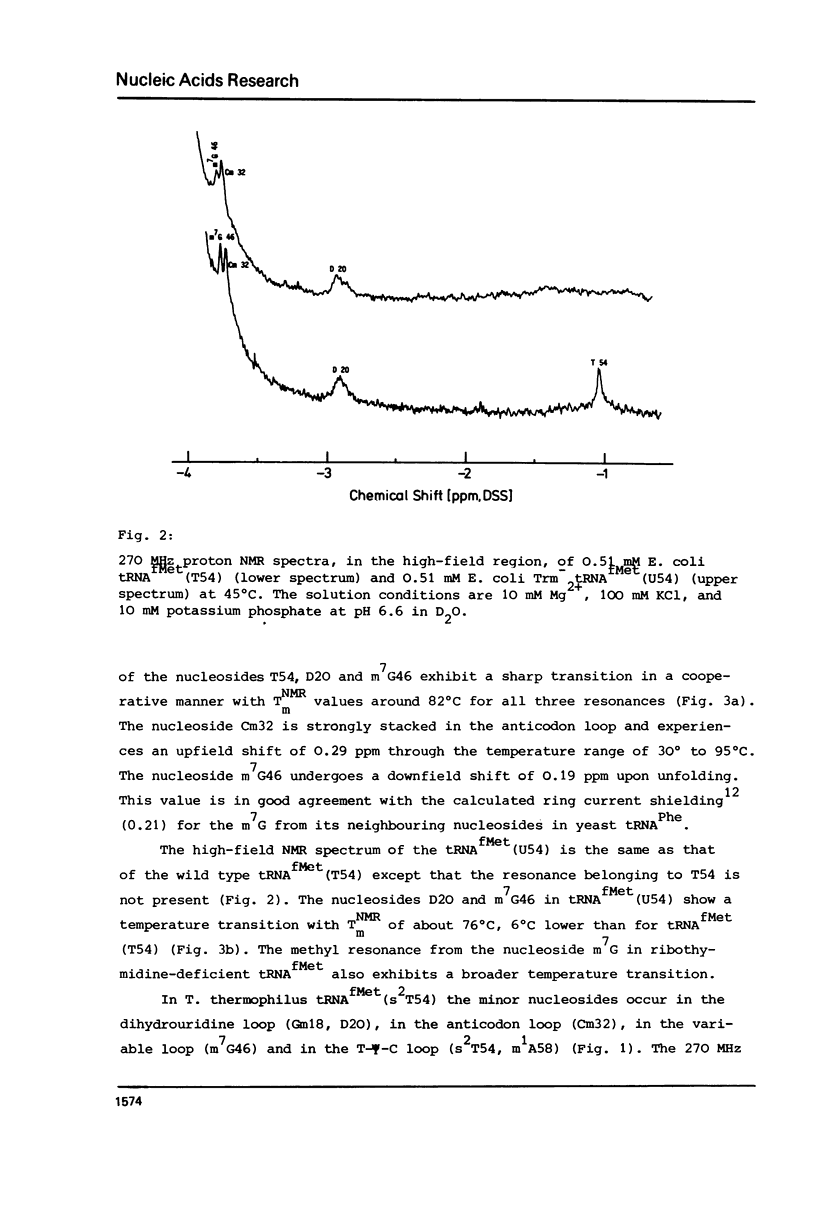
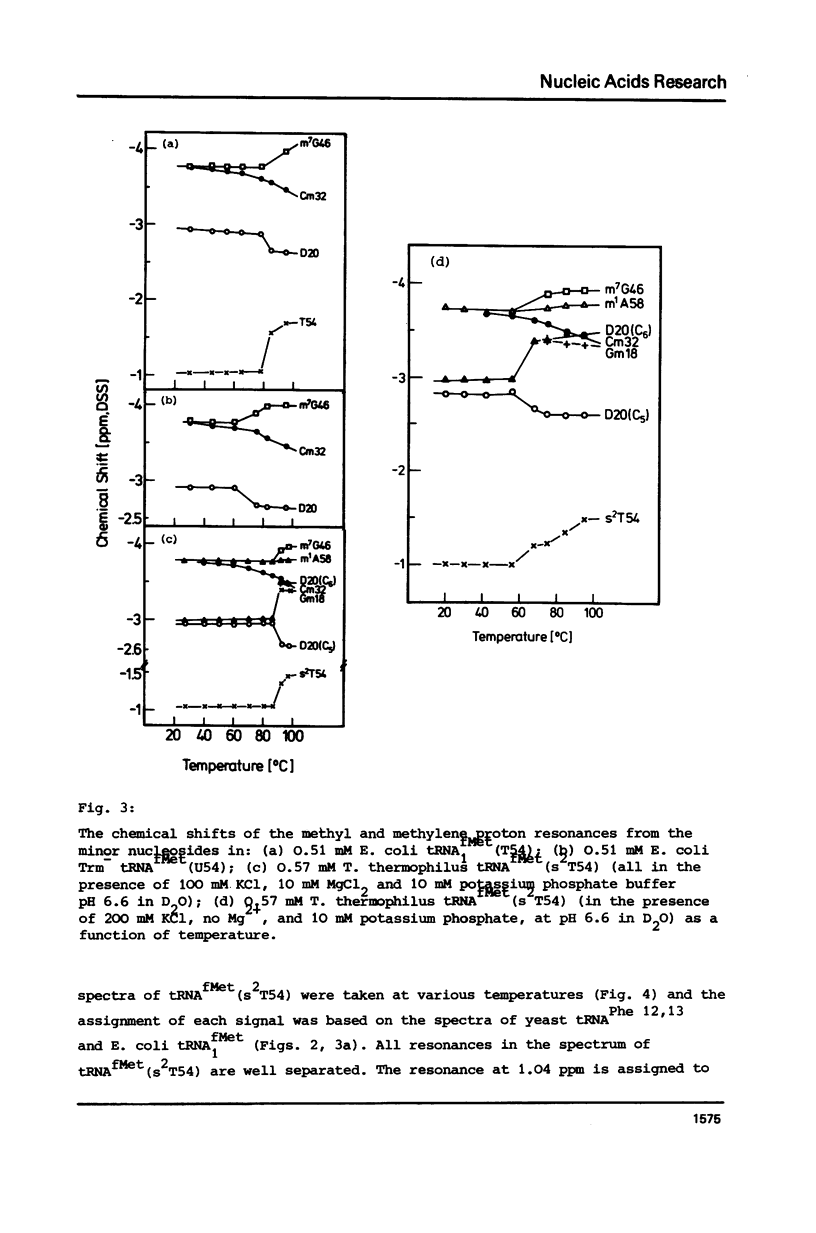
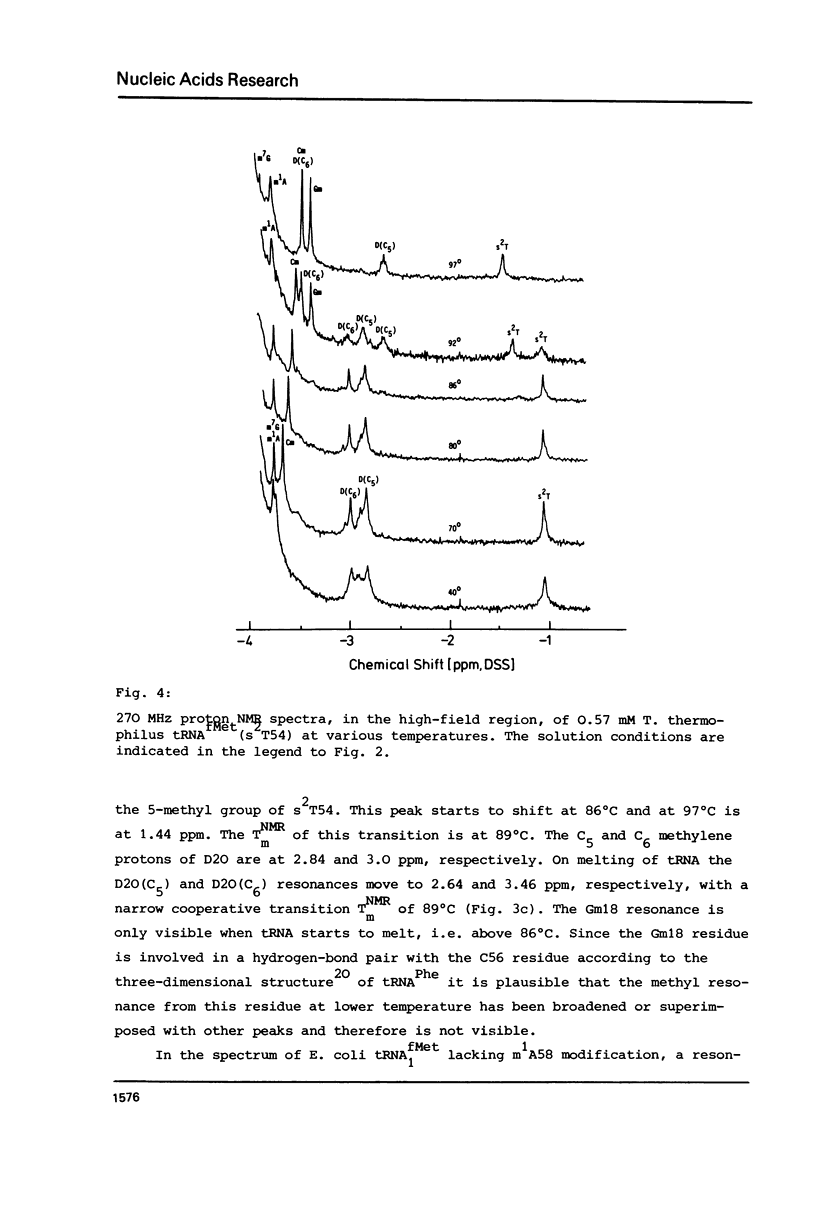
Abstract
In order to elucidate the functional role of the modified uridines at position 54 of tRNA, the 270 MHz high-field proton NMR spectra of methionine tRNAs from E. coli, from a mutant thereof, and from T. thermophilus, containing ribothymidine, uridine and 2-thioribothymidine, respectively, have been measured as a function of temperature. A comparison of the NMR melting profiles of the minor nucleosides from these tRNAs shows that the melting temperature of uridine containing tRNA is 6 degrees C lower than that of the wild type tRNA whereas that of the 2-thioribothymidine tRNA is 7 degrees C higher than that of the wild type tRNA. These results, therefore, demonstrate that these modifications serve for stabilization of the tertiary structure of tRNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):99–111. doi: 10.1128/jb.124.1.99-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers D. M., Cole P. E., Hilbers C. W., Shulman R. G. The molecular mechanism of thermal unfolding of Escherichia coli formylmethionine transfer RNA. J Mol Biol. 1974 Jul 25;87(1):63–88. doi: 10.1016/0022-2836(74)90560-9. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Ts'o P. O., Sprinzl M., vd Harr F., Cramer F. 1H nuclear magnetic resonance studies of transfer RNA: the methyl and methylene resonances of baker's yeast phenylalanine transfer RNA and its fragments. Biochemistry. 1977 Jul 12;16(14):3143–3154. doi: 10.1021/bi00633a017. [DOI] [PubMed] [Google Scholar]
- Maelicke A., von der Haar F., Sprinzl M., Cramer F. The structure of the anticodon loop of tRNAPhe from yeast as deduced from spectroscopic studies on oligonucleotides. Biopolymers. 1975 Jan;14(1):155–171. doi: 10.1002/bip.1975.360140112. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Dudock B. S. Effect of ribothymidine in specific eukaryotic tRNAs on their efficiency in in vitro protein synthesis. Nature. 1976 May 13;261(5556):159–162. doi: 10.1038/261159a0. [DOI] [PubMed] [Google Scholar]
- Mazumdar S. K., Saenger W. Molecular structure of poly-2-thiouridylic acid, a double helix with non-equivalent polynucleotide chains. J Mol Biol. 1974 May 15;85(2):213–219. doi: 10.1016/0022-2836(74)90361-1. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Bierbaum J. Protein synthetic ability of Escherichia coli valine transfer RNA with pseudouridine, ribothymidine, and other uridine-derived residues replaced by 5-fluorouridine. J Mol Biol. 1974 Sep 15;88(2):313–325. doi: 10.1016/0022-2836(74)90484-7. [DOI] [PubMed] [Google Scholar]
- Oshima T., Sakaki Y., Wakayama N., Watanabe K., Ohashi Z. Biochemical studies on an extreme thermophile Thermus thermophilus: thermal stabilities of cell constituents and a bacteriophage. Experientia Suppl. 1976;26:317–331. doi: 10.1007/978-3-0348-7675-9_26. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Tarr C. E., Vosman F., Reid B. R. A nuclear magnetic resonance study of secondary and tertiary structure in yeast tRNAPhe. Biochemistry. 1977 Nov 29;16(24):5261–5273. doi: 10.1021/bi00643a016. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Tsen H. Y. Role of ribothymidine in mammalian tRNAPhe. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3696–3700. doi: 10.1073/pnas.74.9.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B., Marcu K., Dudock B. The isolation and sequence analysis of transfer RNA: the use of plaskon chromatography (RPC-5). Biochim Biophys Acta. 1973 Aug 10;319(1):25–36. doi: 10.1016/0005-2787(73)90037-3. [DOI] [PubMed] [Google Scholar]
- Salemink P. J., Yamane T., Hilbers C. W. Demonstration of a tertiary interaction in solution between the extra arm and the D-stem in two different transfer RNA's by NMR. Nucleic Acids Res. 1977 Nov;4(11):3727–3741. doi: 10.1093/nar/4.11.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Grüter F., Gauss D. H. Collection of published tRNA sequences. Nucleic Acids Res. 1978 May;5(5):r15–r27. [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Wagner T., Lorenz S., Erdmann V. A. Regions of tRNA important for binding to the ribosomal A and P sites. Biochemistry. 1976 Jul 13;15(14):3031–3039. doi: 10.1021/bi00659a015. [DOI] [PubMed] [Google Scholar]
- Svensson I., Isaksson L., Henningsson A. Aminoacylation and polypeptide synthesis with tRNA lacking ribothymidine. Biochim Biophys Acta. 1971 May 13;238(2):331–337. doi: 10.1016/0005-2787(71)90100-6. [DOI] [PubMed] [Google Scholar]
- Uziel M., Koh C. K., Cohn W. E. Rapid ion-exchange chromatographic microanalysis of ultraviolet-absorbing materials and its application to nucleosides. Anal Biochem. 1968 Oct 24;25(1):77–98. doi: 10.1016/0003-2697(68)90083-3. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Nishimura S. CD spectra of 5-methyl-2-thiouridine in tRNA-Met-f from an extreme thermophile. Nucleic Acids Res. 1976 Jul;3(7):1703–1713. doi: 10.1093/nar/3.7.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Shinma M., Oshima T., Nishimura S. Heat-induced stability of tRNA from an extreme thermophile, Thermus thermophilus. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1137–1144. doi: 10.1016/s0006-291x(76)80250-1. [DOI] [PubMed] [Google Scholar]
- Yang S., Reinitz E. R., Gefter M. L. Role of modifications in tyrosine transfer RNA. II. Ribothymidylate-deficient tRNA. Arch Biochem Biophys. 1973 Jul;157(1):55–62. doi: 10.1016/0003-9861(73)90389-5. [DOI] [PubMed] [Google Scholar]



