Abstract
The martian surface environment exhibits extremes of salinity, temperature, desiccation, and radiation that would make it difficult for terrestrial microbes to survive. Recent evidence suggests that martian soils contain high concentrations of MgSO4 minerals. Through warming of the soils, meltwater derived from subterranean ice-rich regolith may exist for an extended period of time and thus allow the propagation of terrestrial microbes and create significant bioburden at the near surface of Mars. The current report demonstrates that halotolerant bacteria from the Great Salt Plains (GSP) of Oklahoma are capable of growing at high concentrations of MgSO4 in the form of 2 M solutions of epsomite. The epsotolerance of isolates in the GSP bacterial collection was determined, with 35% growing at 2 M MgSO4. There was a complex physiological response to mixtures of MgSO4 and NaCl coupled with other environmental stressors. Growth also was measured at 1 M concentrations of other magnesium and sulfate salts. The complex responses may be partially explained by the pattern of chaotropicity observed for high-salt solutions as measured by agar gelation temperature. Select isolates could grow at the high salt concentrations and low temperatures found on Mars. Survival during repetitive freeze-thaw or drying-rewetting cycles was used as other measures of potential success on the martian surface. Our results indicate that terrestrial microbes might survive under the high-salt, low-temperature, anaerobic conditions on Mars and present significant potential for forward contamination. Stringent planetary protection requirements are needed for future life-detection missions to Mars. Key Words: Analogue—Mars—Planetary protection—Salts—Life in extreme environments. Astrobiology 12, 98–106.
1. Introduction
The martian surface is an extreme environment that challenges microbes with low temperatures, low water activities, oxidizing minerals, and high doses of radiation (Clark, 1998). To prevent forward contamination of Mars with terrestrial microbes, great efforts have been focused on understanding the diversity of microbes associated with spacecraft and their assembly facilities. Spacecraft assembly facilities are xerophilic environments maintained at low relative humidity (40%). Microbial species isolated from spacecraft closely match those from air and surfaces in the Class 100K clean rooms used for spacecraft assembly (Favero et al., 1966; Favero, 1971; Puleo et al., 1977). A broad range of microbes, including bacteria and archaea, has been detected in spacecraft assembly facilities with cultivation and molecular techniques (Foster and Winans, 1975; Puleo et al., 1977; Moissl et al., 2007, 2008; Stieglmeier et al., 2009). Many of these isolates tolerated high salinity, radiation, and peroxides (Venkateswaran et al., 2001, 2003a, 2003b; La Duc et al., 2003; Link et al., 2003; Kempf et al., 2005).
Salt tolerance is expected to be a significant requirement for survival on Mars. However, the salts found in martian soils contain a high abundance of sulfur compounds, present as sulfates of Mg, Ca, and Fe (Clark and van Hart, 1981; Clark, 1993; Wänke et al., 2001; Clark et al., 2005). Although chlorine is present as Mg, Na, or Ca chlorides or perchlorates, their concentrations (weight/weight) are significantly less than those of sulfates (S:Cl ratio of 4:1). Should water ice in permafrost regions melt (McEwen et al., 2011), these deposits could dissolve and create heavy brines dominated by MgSO4 salts, since Ca and Fe sulfates are sparingly soluble above pH 3. In fact, it has been suggested that perhaps the most extreme near-surface condition on Mars is the high concentration of MgSO4 salts (Tosca et al., 2008).
Previous characterization of halotolerant microbes from spacecraft assembly facilities and a wide range of natural hypersaline environments has focused on high concentrations of NaCl, not MgSO4. For our current investigation of life at high concentrations of MgSO4, we will use the terms epsotolerance and epsophilic to describe those microbes capable of growth at high concentrations of MgSO4 and those microbes that require high concentrations of MgSO4 for growth, respectively. Few natural environments are rich in magnesium sulfates such as Epsom salt (epsomite; MgSO4·7H2O), and none have been extensively examined microbiologically. Historical and preliminary reports provide evidence for microbial life in Hot and Basque Lakes in the Pacific Northwest, environments with saturating concentrations of epsomite (Anderson, 1958; Hyde et al., 2007; Crisler et al., 2009, 2010; Foster et al., 2010; Eberl et al., 2011; Kilmer et al., 2011). Certain stone monuments that produce epsomite efflorescences yielded microbial isolates that grew at up to 25% MgSO4 (Laiz et al., 2000; Mandrioli and Saiz-Jimenez, 2002). A few garden soil isolates have been reported to grow at 0.42 M MgSO4 (Markovitz, 1961; Markovitz and Sylvan, 1962). Finally, the greatest reported epsotolerance was for isolates from the Guerrero Negro salterns that grow at 0.9 M MgSO4 (Javor, 1984).
The current study focuses on the epsotolerance of a collection of halotolerant bacteria isolated from a natural inland thalassohaline hypersaline (NaCl) environment, the Great Salt Plains (GSP) of Oklahoma (Caton et al., 2004, 2009; Wilson et al., 2004; Litzner et al., 2006; Caton and Schneegurt, 2011). These may be the types of microorganisms of most concern for forward contamination of Mars. Previous work at this site generated a culture collection that included 46 distinct phylotypes with a wide range of environmental tolerances and physiological characteristics (Caton et al., 2004). Enrichments were performed at high salinity, at least 10% NaCl, but it is not clear how epsotolerance relates to halotolerance. In the current study, growth of GSP isolates was measured in media containing 2 M MgSO4, near saturation. A subset of isolates also was exposed to high concentrations of other magnesium and sulfate salts. Many of the halotolerant GSP isolates were epsotolerant, some growing at 2 M magnesium sulfate. Responses to combinations of salts and other stressors appear complex. It is interesting to note that many of the GSP isolates were related to halotolerant isolates from spacecraft assembly facilities (Caton et al., 2004; Caton and Schneegurt, 2011). This raises the potential that terrestrial microbial contaminants can grow in brines associated with martian soils. Preliminary accounts of portions of this work have appeared previously (Bhattarai et al., 2004; Crisler et al., 2008, 2009, 2010).
2. Methods
2.1. Bacterial isolates
Bacterial isolates were obtained as part of a previous study in which aerobic heterotrophic halotolerant microbes from the GSP were characterized (Caton et al., 2004; Wilson et al., 2004; Litzner et al., 2006; Caton et al., 2009). Briefly, nutrient-rich complex SP medium (10% salinity) was inoculated in the field with surface soil samples and maintained as shake-flasks, or SP agar plates were inoculated with soils directly. Bacterial isolates were purified by repetitive streaking on SP agar plates after serial dilution of enrichment cultures. The isolates were characterized biochemically and physiologically, and the data was summarized by Caton et al. (2004) and presented fully in an electronic supplement (online Supplement 4 of Litzner et al., 2006). A nearly full-length 16S rRNA gene sequence for each isolate appears in GenBank with accession numbers AY505499–AY505536, AY553063–AY553129, and DQ157161–DQ157162. The GSP culture collection was maintained on SP agar slants in moist chambers.
2.2. Growth at high salt and varied conditions
Standard SP medium was modified to reach desired salt concentrations and pHs. It was not possible to produce solid agar plates with media containing 2 M MgSO4, since gelation was disrupted. Liquid shake-tube cultures (1–2 mL in vented 13×100 mm test tubes; 150 rpm; 1 in. stroke diameter) were lightly inoculated [below 0.05 optical density (OD) units at 600 nm] for growth experiments. Turbidity of the culture was measured as A600 at the start of the incubation period and after various periods of time to follow growth at the selected temperature or pH. Positive growth was assigned to those cultures that increased in turbidity to above 0.2 OD units, although most positive cultures had much higher turbidity. Growth curves in various salts were plotted and used to report exponential growth rate (as the slope) and highest culture density (in stationary phase) as a percentage of values obtained with low-salt (0.1% NaCl) control cultures.
2.3. Desiccation and freezing tolerance
Desiccation tolerance was determined through repetitive drying and rewetting cycles. Aliquots (1 mL) of dense microbial cultures in SP medium (0.1% NaCl) supplemented with 2 M MgSO4 were desiccated in a SpeedVac (Savant) at 33.3 Pa (0.25 torr) for 15 min. The dried pellets were resuspended to their original volume in medium, and a subsample was serially diluted and spread-plated on SP medium with 10% NaCl for colony counts. Freezing tolerance was determined in a similar fashion. In this case, aliquots of dense microbial culture (1 mL) in SP medium (0.1% NaCl) supplemented with 2 M MgSO4 were frozen at −80°C with a 5100 Cryo 1°C freezing container (Nalgene) filled with ethanol, which cools at approximately 0.5°C min−1. Samples were thawed at room temperature and used for serial dilutions and standard plate counts.
2.4. Agar gelation assay
Chaotropicities of salt solutions were determined with the agar gel-point assay of Hallsworth et al. (2003). A solution of 1.5 g of agar (Bacto) in 40 mL of water was melted at 95°C and allowed to cool to 55°C before adding 60 mL of warmed chaotropic solution to bring the mixture to the desired salt concentration. A temperature probe was used to measure the temperature at which the sudden sol-gel transition was observed visually. All measurements were done in triplicate, with standard deviations being small enough to fit within the symbols on the curves presented. Chaotropic effect was calculated by using a conversion factor of 4.15 kJ g−1 °C−1 for a 1.5% agar gel (Cornillon et al., 1995).
2.5. Model of pore water salinity
By using a model of putative salts, including NaCl, MgSO4, and CaSO4, based upon previous minimum S and Cl estimates, it was determined that a salt concentration of 6.9% is expected in martian soil (Clark and van Hart, 1981; Clark, 1998). The amount of salt per cubic centimeter is calculated as the product of the weight percent salt and the density of the soil, taken as 1.1 g cm−3 (Clark et al., 1977). The amount of ice per cubic centimeter is the product of the filled pore volume and the density of ice (taken as 1.0 g cm−3). The ratio of these values determines the predicted salinity of the brines.
3. Results
3.1. Predicted salinities of martian brines
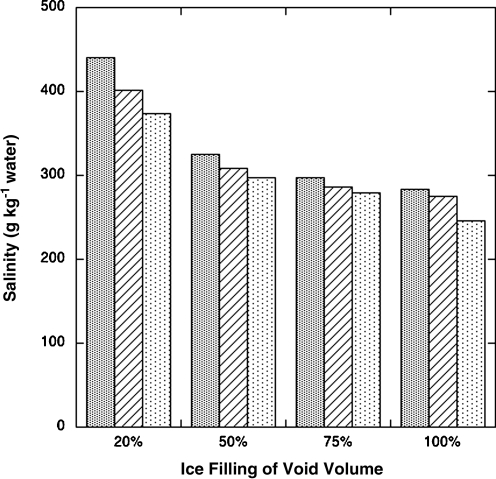
Obliquity wobbles in Mars' orbit that change the direction of solar illumination are accompanied by the redistribution of ice around the planet via the cold-trapping mechanism (Jakosky et al., 2003) and can produce conditions during which ice will temporarily exceed its melting point, creating local environments in which dormant organisms could emerge from perennating structures, proliferate, and disseminate. Warmer slopes on martian hills may harbor liquid water environments (McEwen et al., 2011). Soil salinity has been modeled for brines formed by ground ice melt in a permafrost region (Fig. 1). Within any given body of regolith, soil pore space is available to host ice (20–100% filling of void spaces spanning ice-poor to ice-rich permafrost). When pore volume is incompletely filled by water vapor, condensing via cold trapping within it, there will be less ice and hence a higher salinity, since the water/soil ratio is lower than if more ice is present. Only in the case of a pure ice lens would the initial salinity be low, but because of the intrinsic porosity of regolith, any meltwater would quickly flow and wick (by capillarity) into the surrounding dry material to equilibrize the distribution of water throughout the locally affected soil bed. The model includes compacted (40% voids) to loose (60% voids) soils. Assuming the ice melts, the resulting brine would have salinities ranging from approximately 250 to 400 g salt kg−1.
FIG. 1.
Salinity calculations are based upon a mixture of ice with typical martian soil whose minimal salt content is estimated to be 4.6% MgSO4, 1.7% CaSO4, and 0.6% NaCl (weight/weight). The pore space of the original soil bed is modeled to range from 40%, 50%, and 60% (heavy stipple, hashed, and light stipple, respectively), and this void volume is filled by various fractions of ice, resulting in the salinity indicated when the ice melts.
With its low solubility, CaSO4 contributes little to the ionic strength of the brine, except through the common-ion effect. From the standpoint of microorganisms, and except for certain areas rich in ferric sulfate (seen only in Columbia Hills, Gusev Crater), the predominant ions in solution will be Mg2+ and 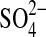 , with much lower values of Na+, Cl−, and Ca2+. In this regard, the martian environment is decidedly different from the more common brine environments on Earth, which have ion ratios similar to seawater.
, with much lower values of Na+, Cl−, and Ca2+. In this regard, the martian environment is decidedly different from the more common brine environments on Earth, which have ion ratios similar to seawater.
3.2. Epsotolerance of GSP isolates
The epsotolerance of extant GSP isolates from the group of 63 bacteria characterized by Caton et al. (2004) were measured in shake-tube cultures by using SP media (1% or 10% NaCl) supplemented with 10% (weight to volume) or 2 M (∼50% weight to volume) MgSO4 (Table 1). Most of the isolates grew in the presence of 10% MgSO4 supplemented with 1% NaCl. Those that did not grow had salinity optima above 5%, but also could grow at 0.1% salinity (Caton et al., 2004), and included six Halomonas isolates, a Staphylococcus, and a Halobacillus. It is interesting to note that comparable numbers of isolates grew at 10% MgSO4 when either 1% or 10% NaCl was added. Only GSP 9 (Bacillus) and GSP 60 (Halobacillus) did not grow in 10% MgSO4 with 10% NaCl.
Table 1.
Growth of GSP Isolates at High Salinities
| [MgSO4] | [NaCl] | Growth/Total | Growth (% of isolates) |
|---|---|---|---|
| 10% | 10% | 44/46 | 95.6 |
| 10% | 1% | 44/52 | 84.6 |
| 2 M | 10% | 5/52 | 11.5 |
| 2 M | 1% | 18/52 | 34.6 |
The number of GSP isolates exhibiting substantial growth was reduced considerably by raising the MgSO4 concentration to 2 M (Table 1). The 18 isolates that grew with 2 M MgSO4 and 1% NaCl were mainly Halomonas and Bacillus spp., and most had salinity tolerances of 15% or more and salinity optima of 5% or more. Increasing the salinity of the medium to 10% NaCl and 2 M MgSO4 reduced the number of positive isolates to six, including five Halomonas (GSP 2, 3, 4, 21, 28) and a Pseudomonas (GSP 24). It is interesting to note that two of these (GSP 24 and 28) showed no growth with 1% NaCl and 2 M MgSO4. Further, GSP 60 (Halobacillus) grew in the presence of 2 M MgSO4 and at low MgSO4 but not at 10% MgSO4. These exceptional isolates suggest that the interplay between epsotolerance and halotolerance is complex and that different mechanisms may be involved.
Growth at higher MgSO4 concentrations was tested with six select isolates from diverse taxa, namely, GSP 3 (Halomonas variabilis), GSP 10 (Bacillus megaterium), GSP 11 (Bacillus sp. str. KL-152), GSP 17 (Salibacillus marismortui), GSP 21 (Halomonas salina), and GSP 63 (Bacillus licheniformis). All these isolates were able to grow at 60% and 70% MgSO4, near saturation. Growth curves were analyzed to yield growth rates (slope in log phase) and carrying capacities (highest culture density attained), both of which are presented in Table 2 as a percentage of the low-salt (0.1% NaCl) controls for each isolate (approximate control slopes and maximum densities of 1.0 OD units d−1 and 2.8 OD units, respectively). Growth rates in the presence of 1 or 2 M MgSO4 were ≤21% of low-salt controls in all but two cases. The highest culture density observed was approximately half that of the low-salt controls.
Table 2.
Growth Rates and Carrying Capacities of GSP Isolates in High Concentrations of Magnesium and Sulfate Salts
| |
Growth of GSP isolate (% low salt controlb) |
|||||
|---|---|---|---|---|---|---|
| Mediuma | 3 | 10 | 11 | 17 | 21 | 63 |
| Growth rate | ||||||
| 1 M LiSO4 | 28 | 30 | 31 | 48 | 28 | 36 |
| 1 M MgCl2 | 36 | 34 | 43 | 31 | 44 | 54 |
| 1 M Na2SO4 | 14 | 21 | 19 | 19 | 20 | 18 |
| 1 M MgSO4 | 17 | 17 | 16 | 19 | 14 | 9 |
| 2 M MgSO4 | 17 | 19 | 34 | 21 | 32 | 11 |
| Maximum culture density | ||||||
| 1 M LiSO4 | 61 | 61 | 66 | 61 | 56 | 61 |
| 1 M MgCl2 | 57 | 58 | 59 | 56 | 63 | 69 |
| 1 M Na2SO4 | 61 | 58 | 57 | 48 | 54 | 53 |
| 1 M MgSO4 | 54 | 61 | 62 | 65 | 57 | 44 |
| 2 M MgSO4 | 42 | 41 | 38 | 60 | 38 | 36 |
SP medium with 1% NaCl supplemented with listed salts.
SP medium with 1% NaCl.
3.3. Multiple simultaneous stressors
The interesting responses to combinations of MgSO4 and NaCl led to the examination of other simultaneous stressors. Growth at various salinities was measured at pH 5 and at 7°C to further simulate potential martian surface conditions. Only five isolates (GSP 4, 5, 21, 31, 32) grew at 2 M MgSO4 and 1% NaCl at 7°C. These included three Halomonas and two Marinococcus, the latter growing poorly in this medium at room temperature. With 10% NaCl and 2 M MgSO4, five isolates (GSP 2, 21, 28, 34, 35) grew well at 7°C, including three Halomonas and two Halobacillus, the latter growing poorly in this medium at room temperature and in 10% NaCl without 2 M MgSO4. When medium containing 2 M MgSO4 and 1% NaCl was set to pH 5, six of the isolates grew, including Halomonas and Bacillus (GSP 3, 4, 5, 10, 21, 46). With 10% NaCl and 2 M MgSO4 at pH 5, seven isolates grew, including Halomonas, Halobacillus, Marinococcus, and Chromohalobacter (GSP 2, 5, 28, 31, 35, 43, 58). Several of these did not appear to grow in this medium at pH 7. Again, the response of GSP microbial isolates to multiple simultaneous stressors appears complex. The interplay of MgSO4 concentration and temperature was further examined for the group of six diverse isolates chosen for additional study above (GSP 3, 10, 11, 17, 21, 63). All these isolates demonstrated a smaller range of permissible growth temperatures in SP medium with 0.1% NaCl when it was supplemented with 2 M MgSO4. Growth was observed at 25°C and 37°C but not at 4°C, 7°C, or 45°C.
Seven GSP isolates found to be most tolerant to single environmental stressors in our earlier studies (Caton et al., 2004; Litzner et al., 2006) were tested for their ability to grow with a series of multiple simultaneous stressors (Table 3). Note that for organisms from a hypersaline alkaline environment, low-salt or low-pH conditions might be even greater stresses than high-salt or high-pH conditions. Our previous work showed that all these organisms can grow at pH 5, 7, or 11 at 25°C and 10% NaCl. These isolates also could grow at 4°C and 45°C at pH 7 and 10% NaCl, and with 0.1% or 15% NaCl at 25°C and pH 7. In the current study, all the isolates, except GSP 18, grew at 45°C with 15% salinity at both pH 7 and 11. All the isolates grew at 0.1% salinity at pH 7 and at both 4°C and 45°C, with all isolates also growing at 0.1% salinity at pH 5 and at 45°C. For five of the stressor combinations, mainly at pH 5 and 4°C, only two isolates could grow. The two Halomonas spp. isolates, GSP 21 and 28, exhibited the broadest environmental tolerances. Again, physiological responses to the interplay of multiple environmental stressors seem complex.
Table 3.
Response of GSP Bacterial Isolates to Simultaneous Stressors
| |
|
|
GSP isolate |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Salinity (%) | pH | Temperature (°C) | 7 | 18 | 21 | 28 | 44 | 50 | 63 |
| 15 | 5 | 45 | − | − | + | + | − | − | − |
| 15 | 7 | 45 | + | − | + | + | + | + | + |
| 15 | 11 | 45 | + | − | + | + | + | + | + |
| 0.1 | 5 | 45 | + | + | + | + | + | + | + |
| 0.1 | 7 | 45 | + | + | + | + | + | + | + |
| 0.1 | 11 | 45 | + | + | + | + | − | − | + |
| 15 | 5 | 4 | − | − | − | − | − | − | − |
| 15 | 7 | 4 | − | − | + | + | − | − | − |
| 15 | 11 | 4 | − | − | + | + | − | − | − |
| 0.1 | 5 | 4 | − | − | + | + | − | − | − |
| 0.1 | 7 | 4 | + | + | + | + | + | + | + |
| 0.1 | 11 | 4 | − | − | + | + | − | − | − |
Shake-tube cultures were exposed to different combinations of growth temperature and media pH and salinity.
Positive (+) and negative (−) growth by turbidity (increases of at least 0.2 OD units) are indicated. Close phylogenetic relationships: GSP 7, Bacillus sp. AY030333; GSP 18, Bacillus marisflavi; GSP 21, Halomonas salina; GSP 28, Halomonas variabilis; GSP 44, Bacillus baekryungensis; GSP 50, Psychrobacter sp. UT85875; GSP 63, Bacillus licheniformis.
3.4. Effect of salinity on agar gelation
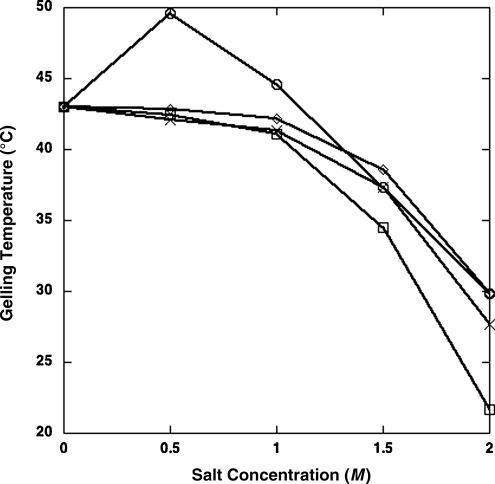
The temperature at which agar undergoes sol-gel transition is an indication of how disruptive, or chaotropic, various salt solutions are to this process (Hallsworth et al., 2003). Note that, as the salinity of a solution increases, the temperature needed for gelation is usually lower, and this can be quantified as calculated chaotropic effects. Gelation temperatures at different salinities were measured for several salts and are presented in Fig. 2. LiSO4 and Na2SO4 exhibit similar curves with increasing chaotropicity as concentrations increase (55 and 64 kJ g−1 at 2 M, respectively). MgCl2 was more disruptive than these at higher concentrations (88 kJ g−1 at 2 M). MgSO4 showed a much different pattern. Lower concentrations of MgSO4 were kosmotropic (stabilizing), raising the gelation temperature (−27 and −7 kJ g−1 at 0.5 and 1 M, respectively). At higher concentrations, the chaotropicities of MgSO4 solutions (55 kJ g−1 at 2 M) were similar to those of LiSO4 and Na2SO4. These differences may help to explain the complex physiological responses observed for GSP isolates growing at these high salinities.
FIG. 2.
The chaotropicities of different salt solutions were determined by their effects on the gelation temperature of agar. MgSO4,  ; MgCl2,
; MgCl2,  ; LiSO4,
; LiSO4, 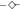 ; Na2SO4,
; Na2SO4, 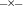 .
.
3.5. Growth in other magnesium or sulfate salts
Six isolates from diverse taxa (GSP 3, 10, 11, 17, 21, 63) were tested for the ability to grow in SP media supplemented with 1 M MgCl2, Na2SO4, or LiSO4 and compared to growth in media supplemented with 1 or 2 M MgSO4. While CaSO4 and FeSO4 are found in martian soils, these salts are sparingly soluble above pH 3 and could not be tested at 1 M. In nearly all cases, growth rates were less than 50% of low-salt (0.1% NaCl) controls (Table 2). Stationary-phase maximum densities were consistent, mainly between 50% and 70% of low-salt controls. Growth rates in 1 M MgCl2 and 1 M LiSO4 were greater than in high MgSO4, while cultures in 1 M Na2SO4 were comparable to those in high MgSO4. Note that for GSP 11 and 21, growth rates were higher in 2 M MgSO4 than in 1 M MgSO4, concurring with a trend observed in the broad survey of Table 1. It is interesting to note that a previous examination of the tolerance of the 63 GSP isolates of Caton et al. (2004) to heavy metals revealed that GSP 10 and 21 were most tolerant of 1000 ppm lead and cadmium, while GSP 26, 30, and 58 also performed well, with GSP 12 tolerant to lead but not cadmium (Bhattarai et al., 2004). Overall heavy metal tolerances in this GSP group were greater than those previously reported for halotolerant bacteria and haloarchaea (Nieto et al., 1987; Amoozegar et al., 2005).
3.6. Effect of freeze-thaw and drying-rewetting cycles on survival
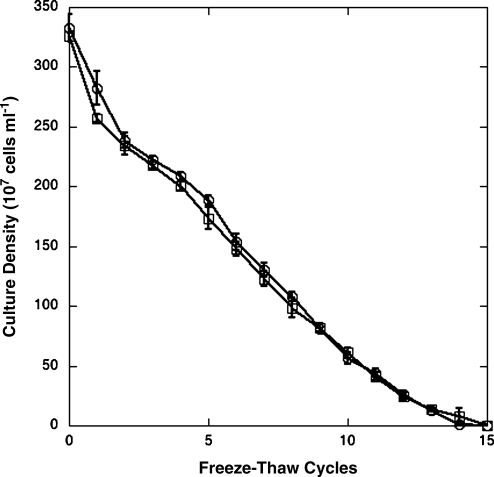
By simulating potential diurnal temperature changes at the martian surface, six isolates from diverse taxa (GSP 3, 10, 11, 17, 21, 63) were exposed to alternating freeze and thaw cycles. In an experiment that alternated between −70°C and 25°C, dilution plate counts were made after each cycle (Fig. 3). All the isolates gave approximately the same response, and only the most tolerant and least tolerant isolates are shown. The reduction in the number of viable bacteria was nearly linear with increasing cycles. After 15 cycles, no viable organisms were recovered. Note that soil quality will influence the rates of thermal change, and these may differ from those used here.
FIG. 3.
Tolerance to freeze-thaw cycles (alternating −70°C and 25°C) as measured by standard plate count. Only isolates with the greatest and least responses are shown. GSP 10,  ; GSP 17,
; GSP 17,  .
.
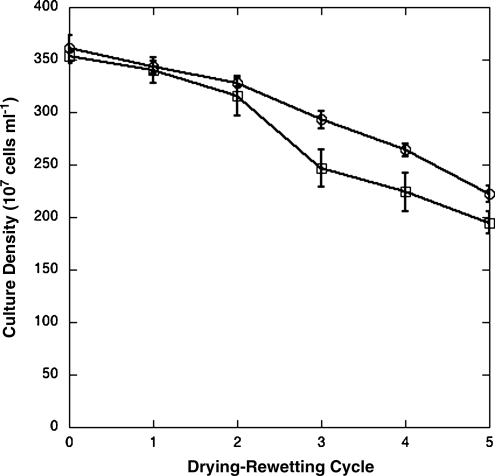
Conditions with intermittent moisture were mimicked by vacuum-drying and rewetting cell pellets (Fig. 4). All the isolates gave approximately the same response, and only the most tolerant and least tolerant isolates are shown. After five cycles, cell numbers were reduced by approximately 40%, with the reduction in cell number nearly linear with increasing cycles. It should be noted that these experiments were performed with planktonic cultures and that biofilms or organisms within soil pores may respond differently.
FIG. 4.
Tolerance to drying and rewetting cycles as measured by standard plate count. Only isolates with the greatest and least responses are shown. GSP 10,  ; GSP 17,
; GSP 17,  .
.
4. Discussion
Sulfate-dominated systems exhibit higher water activities and, hence, are expected to be more favorable to life than chloride-dominated systems at the same concentrations (Hallsworth et al., 2003; Marion et al., 2003; Grant, 2004). For instance, while Basque Lake has approximately the same salinity (25%) as the Dead Sea and Great Salt Lake, the water activity of Basque Lake (0.919) is much higher than that of the Dead Sea (0.690) or Great Salt Lake (0.776). We have demonstrated this principle, using an agar gelation assay that shows MgSO4 to be kosmotropic below 1 M and then increasingly chaotropic at higher concentrations. Note that salt concentrations which stabilized agar gel transitions did not appear to have fostered bacterial growth and, in fact, inhibited bacterial growth in the current study. Growth rates and stationary-phase maximum densities in both 1 and 2 M MgSO4 were much lower than those for low-salt control cultures. Furthermore, growth in 1 M MgCl2 and LiSO4 was better than in 1 M MgSO4, even though the former media are more chaotropic than the latter by the agar gelation assay.
Halotolerant organisms expend a great deal of energy maintaining homeostasis in high-salt media by effluxing ions and synthesizing compatible solutes (Oren, 1999). This may explain the lower stationary-phase maximum densities observed at higher salinities. Proportionally less of the available energy in the system (batch culture medium) can be apportioned for growth. Organisms that have metabolisms with lower energy yields may be particularly challenged. However, a preliminary report suggests that certain sulfate-reducing bacteria can survive or grow at 10% MgSO4, 17% FeSO4, or 48% Fe2(SO4)3 (Marnocha et al., 2011).
The interplay between combinations of stressors demonstrates the complexity of their relationships and ultimately their impacts on bacterial physiology. While fewer GSP isolates grew in media with 2 M MgSO4 supplemented with 10% NaCl than with 1% NaCl, this trend was not observed in media with 10% MgSO4. The relationships can be more difficult to predict when salinity extremes are combined with temperature and pH extremes. The data in Table 3 follow what might be predicted; a greater number of simultaneous stressors reduces growth more than fewer stressors. However, when high concentrations of MgSO4 are included in the scheme, these trends are not as clear. It is interesting to note that the growth inhibition observed cannot be simply attributed to high individual concentrations of Mg2+ or 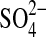 , since cultures in media supplemented with MgCl2 and LiSO4 performed better than those exposed to high MgSO4 concentrations.
, since cultures in media supplemented with MgCl2 and LiSO4 performed better than those exposed to high MgSO4 concentrations.
Substitution of other salts for NaCl has been examined previously with different findings reported for halotolerant and halophilic bacteria and archaea (Brown and Gibbons, 1955; Abram and Gibbons, 1961; Boring et al., 1963; Mullakhanbhai and Larsen, 1975). Halophilic archaea that grow above 3 M NaCl require high NaCl concentrations (at least 1.5 M), even when some of the NaCl can be replaced by other salts, such as KCl, MgCl2, and CaCl2. Substitution of NaCl by other salts for halotolerant bacteria is more common, and high NaCl concentrations are not required. The isolates described here are all halotolerant, and thus MgSO4 may be acting as a substitute for NaCl.
The mechanisms for tolerance to high concentrations of MgSO4 were not explored in the current study. Sulfate uptake systems have been studied previously in bacteria, but there has been little reason to focus on sulfate efflux systems (Kertesz, 2001; Piłsyk and Paszewski, 2009). Bacterial uptake is mainly through active ABC transporters, since sulfate anion does not readily diffuse across the cell membrane. It is not clear what the physiological consequences of high sulfates would be, as this may push the equilibrium to higher diffusion rates. Studies on microbes from the Dead Sea in high magnesium concentrations (as chlorides) were from the perspective of the requirement for high magnesium concentrations by some isolates (Oren, 1983). Magnesium uptake systems have been studied, particularly in Salmonella (Smith and Maguire, 1998; Maguire, 2006). One of the known transport complexes can act as an efflux pump, but it is not known whether these systems are operating in the GSP isolates studied here.
Environmental stresses of different types may result in similar damage to bacteria, and survival appears to involve recombination repair and oxidative-stress resistance (Mattimore and Battista, 1996; Billi et al., 2000; Battista et al., 2001; Shukla et al., 2007). In Deinococcus radiodurans, both desiccation and UV irradiation elicit the same dark DNA repair systems (Battista, 1997; Miller et al., 1999; Miller, 2000). However, salinity tolerance was not correlated to desiccation and irradiation tolerance (Shukla et al., 2007). Earlier work with GSP Halomonas spp. strains showed that desiccating high-salt conditions have uniformly selected for exceptionally high DNA-repair capacity (Wilson et al., 2004).
The current study demonstrated bacterial growth in the highest concentrations of MgSO4 yet reported. Previous work included growth studies in media containing up to approximately 1 M (25%) MgSO4 (Markovitz, 1961; Markovitz and Sylvan, 1962; Boring et al., 1963; Javor, 1984; Laiz et al., 2000; Mandrioli and Saiz-Jimenez, 2002). The current report also includes growth in the highest concentrations of magnesium ever used in laboratory media. Previous work with Dead Sea waters included up to 1.5 M MgCl2 (Volcani, 1944; Oren, 1983). Little research on growth at high lithium concentrations has been done, and the current work seems to be the first to report growth in high LiSO4. One report includes the isolation of a Micrococcus strain adapted to growth at 1.5 M LiCl, although growth was poor at 2 M LiCl, and no growth was observed at 1 M LiSO4 (Kamekura and Onishi, 1982).
We have demonstrated that halotolerant bacteria from the GSP can grow at the high concentrations of MgSO4 expected in martian soils. Brines generated by condensation of water vapor from permafrost or melting of permafrost would be nearly saturated with MgSO4, as has been suggested by recent remote sensing (McEwen et al., 2011). It should be noted that the suspected oceans under the ice crust of Europa also might be rich in MgSO4 salts (Kargel et al., 2000; Marion et al., 2003). The demonstration that GSP isolates can grow at 2 M MgSO4, acidic pH, and low temperature suggests that terrestrial microbes may be capable of living in near-surface martian regolith. Additional work needs to be done to isolate and examine epsotolerant anaerobic bacteria under simulated martian surface conditions, but there is no reason to believe that anaerobic isolates will be less epsotolerant than the aerobes examined here. Tolerance to cyclic drying and rewetting or freezing and thawing seems to be a limiting factor for the growth of these terrestrial organisms on Mars.
Initial work on a related project has characterized epsotolerant isolates from Hot and Basques Lakes, environments essentially saturated with respect to MgSO4 (Crisler et al., 2008, 2009, 2010; Eberl et al., 2011; Kilmer et al., 2011). Psychrotolerant and anaerobic microbes have been detected at these sites and grow in the laboratory at 2 M MgSO4. Preliminary work also has detected epsotolerant microbes that are present in oligosaline turf soils (Schneegurt, unpublished observation). It appears that terrestrial microbes on spacecraft might be able to survive and grow on Mars. Further investigation is needed to evaluate the epsotolerance of the microbial community in spacecraft assembly facilities.
Acknowledgments
The authors appreciate the contributions of Leela Bhattarai, Todd Caton, Amy Gray, Roger Kern, Evan Moody, Hieu Nguyen, Ashley Peppers, and Noah Schneegurt. Awards from Kansas NASA EPSCoR (KNEP), NASA ROSES, and NIH NCRR KINBRE supported this work.
Abbreviations
GSP, Great Salt Plains; OD, optical density.
References
- Abram D. Gibbons N.E. The effect of chlorides of monovalent cations, urea, detergents, and heat on morphology and the turbidity of suspensions of red halophilic bacteria. Can J Microbiol. 1961;7:741–750. doi: 10.1139/m61-088. [DOI] [PubMed] [Google Scholar]
- Amoozegar M.A. Hamedi J. Dadashipour M. Shariatpanahi S. Effect of salinity on the tolerance to toxic metals and oxyanions in native moderately halophilic spore-forming bacilli. World J Microbiol Biotechnol. 2005;21:1237–1243. [Google Scholar]
- Anderson G.C. Some limnological features of a shallow saline meromictic lake. Limnol Oceanogr. 1958;3:259–270. [Google Scholar]
- Battista J.R. Against all odds: the survival strategies of Deinococcus radiodurans. Annu Rev Microbiol. 1997;51:203–224. doi: 10.1146/annurev.micro.51.1.203. [DOI] [PubMed] [Google Scholar]
- Battista J.R. Park M.J. McLemore A.E. Inactivation of two homologues of proteins presumed to be involved in the desiccation tolerance of plants sensitizes Deinococcus radiodurans R1 to desiccation. Cryobiology. 2001;43:133–139. doi: 10.1006/cryo.2001.2357. [DOI] [PubMed] [Google Scholar]
- Bhattarai L. Caton T.M. Wilson C. Miller R.V. Schneegurt M.A. Environmental tolerance of bacterial isolates from the Salt Plains Microbial Observatory [abstract N-269]. 104th General Meeting of the American Society for Microbiology Abstracts, American Society for Microbiology; Washington, DC. 2004. [Google Scholar]
- Billi D. Wright D.J. Helm R.F. Prickett T. Potts M. Crowe J.H. Engineering desiccation tolerance in Escherichia coli. Appl Environ Microbiol. 2000;66:1680–1684. doi: 10.1128/aem.66.4.1680-1684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring J. Kushner D.J. Gibbons N.E. Specificity of the salt requirement of Halobacterium cutirubrum. Can J Microbiol. 1963;2:143–154. [Google Scholar]
- Brown H.J. Gibbons N.E. The effect of magnesium, potassium, and iron on the growth and morphology of red halophilic bacteria. Can J Microbiol. 1955;1:486–494. doi: 10.1139/m55-062. [DOI] [PubMed] [Google Scholar]
- Caton I.R. Schneegurt M.A. Culture-independent analysis of the soil bacterial assemblage at the Great Salt Plains of Oklahoma. J Basic Microbiol. 2011;51:1–11. doi: 10.1002/jobm.201100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton T.M. Witte L.R. Ngyuen H.D. Buchheim J.A. Buchheim M.A. Schneegurt M.A. Halotolerant aerobic heterotrophic bacteria from the Great Salt Plains of Oklahoma. Microb Ecol. 2004;48:449–462. doi: 10.1007/s00248-004-0211-7. [DOI] [PubMed] [Google Scholar]
- Caton T.M. Caton I.R. Witte L.R. Schneegurt M.A. Archaeal diversity at the Great Salt Plains of Oklahoma described by cultivation and molecular analyses. Microb Ecol. 2009;58:519–528. doi: 10.1007/s00248-009-9507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B.C. Geochemical components in martian soil. Geochim Cosmochim Acta. 1993;57:4575–4581. [Google Scholar]
- Clark B.C. Surviving the limits to life at the surface of Mars. J Geophys Res. 1998;103:28545–28556. [Google Scholar]
- Clark B.C. van Hart D. The salts of Mars. Icarus. 1981;45:370–378. [Google Scholar]
- Clark B.C., III Baird A.K. Rose H.J., Jr. Toulmin P., III Christian R.P. Kelliher W.C. Castro A.J. Rowe C.D. Keil K. Huss G.R. The Viking X-ray fluorescence experiment: analytical methods and early results. J Geophys Res. 1977;82:4577–4594. [Google Scholar]
- Clark B.C. Morris R.V. McLennan S.M. Gellert R. Jolliff B. Knoll A.H. Squyres S.W. Lowenstein T.K. Ming D.W. Tosca N.J. Yen A. Christensen P.R. Gorevan S. Brückner J. Calvin W. Dreibus G. Farrand W. Klingelhoefer G. Waenke H. Zipfel J. Bell J.F., III Grotzinger J. McSween H.Y. Rieder R. Chemistry and mineralogy of outcrops at Meridiani Planum. Earth Planet Sci Lett. 2005;240:73–94. [Google Scholar]
- Cornillon P. Andrieu J. Duplan J.C. Laurent M. Use of nuclear-magnetic-resonance to model thermophysical properties of frozen and unfrozen model food gels. J Food Eng. 1995;25:1–19. [Google Scholar]
- Crisler J.D. Newville T.M. Schneegurt M.A. Bacterial growth at concentrations of magnesium sulfate found in martian soils [abstract N-143]. 108th General Meeting of the American Society for Microbiology Abstracts, American Society for Microbiology; Washington DC. 2008. [Google Scholar]
- Crisler J.D. Newville T.M. Schneegurt M.A. Bacterial growth at concentrations of magnesium sulfate found in martian soils. Trans Kans Acad Sci. 2009;112:136. doi: 10.1089/ast.2011.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisler J.D. Kilmer B.R. Cunderla B. Madu B.E. Schneegurt M.A. Isolation and characterization of microbes from Basque Lake, BC, and Hot Lake, WA, environments with high magnesium sulfate concentrations [abstract N-298]. 110th General Meeting of the American Society for Microbiology Abstracts, American Society for Microbiology; Washington DC. 2010. [Google Scholar]
- Eberl T. Rowe K. Kilmer B.R. Cunderla B. Schneegurt M.A. Isolation and characterization of epsotolerant aerobic heterotrophic microbes from the saturating magnesium sulfate environment of Hot Lake, WA. 111th General Meeting of the American Society for Microbiology Abstracts, American Society for Microbiology; Washington DC. 2011. [Google Scholar]
- Favero M.S. Microbiologic assay of space hardware. Environ Biol Med. 1971;1:27–36. [PubMed] [Google Scholar]
- Favero M.S. Puleo J.R. Marshall J.H. Oxborrow G.S. Comparative levels and types of microbial contamination detected in industrial clean rooms. Appl Microbiol. 1966;14:539–551. doi: 10.1128/am.14.4.539-551.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster I.S. King P.L. Hyde B.C. Southam G. Characterization of halophiles in natural MgSO4 salts and laboratory enrichment samples: astrobiological implications for Mars. Planet Space Sci. 2010;58:599–615. [Google Scholar]
- Foster T.L. Winans L. Psychrophilic microorganisms from areas associated with the Viking spacecraft. Appl Microbiol. 1975;30:546–550. doi: 10.1128/am.30.4.546-550.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W.D. Life at low water activity. Philos Trans R Soc Lond B Biol Sci. 2004;359:1249–1267. doi: 10.1098/rstb.2004.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallsworth J.E. Heim S. Timmis K.N. Chaotropic solutes cause water stress in Pseudomonas putida. Environ Microbiol. 2003;5:1270–1280. doi: 10.1111/j.1462-2920.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- Hyde B.C. Foster I.S. King P.L. Southam G. Nushaj D. Limits of detection for life on Mars: an example using IR spectroscopy of sulfate salts and halophiles from lakes in British Columbia, Canada [abstract 2278]. 38th Lunar and Planetary Science Conference Abstracts, Lunar and Planetary Institute; Houston. 2007. [Google Scholar]
- Jakosky B.M. Nealson K.H. Bakermans C. Ley R.E. Mellon M.T. Subfreezing activity of microorganisms and the potential habitability of Mars' polar regions. Astrobiology. 2003;3:343–350. doi: 10.1089/153110703769016433. [DOI] [PubMed] [Google Scholar]
- Javor B.J. Growth potential of halophilic bacteria isolated from solar salt environments: carbon sources and salt requirements. Appl Environ Microbiol. 1984;48:352–360. doi: 10.1128/aem.48.2.352-360.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamekura M. Onishi H. Cell-associated cations of the moderate halophile Micrococcus variansi ssp. halophilus grown in media of high concentrations of LiCl, NaCl, KCl, RbCl, or CsCl. Can J Microbiol. 1982;28:155–161. [Google Scholar]
- Kargel J.S. Kaye J.Z. Head J.W., III Marion G.M. Sassen R. Crowley J.K. Ballesteros O.P. Grant S.A. Hogenbloom D.L. Europa's crust and ocean: origin, composition, and the prospects for life. Icarus. 2000;148:226–265. [Google Scholar]
- Kempf M.J. Chen F. Kern R. Venkateswaran K. Recurrent isolation of hydrogen peroxide-resistant spores of Bacillus pumulis from a spacecraft assembly facility. Astrobiology. 2005;5:391–405. doi: 10.1089/ast.2005.5.391. [DOI] [PubMed] [Google Scholar]
- Kertesz M.A. Bacterial transporters for sulfate and organosulfur compounds. Res Microbiol. 2001;152:279–290. doi: 10.1016/s0923-2508(01)01199-8. [DOI] [PubMed] [Google Scholar]
- Kilmer B.R. Eberl T. Rowe K. Cunderla B. Schneegurt M.A. Characterization of the microbial assemblage from the saturating magnesium sulfate environment of Hot Lake, Washington. Trans Kans Acad Sci. 2011;114:163. [Google Scholar]
- La Duc M.T. Nicholson W. Kern R. Venkateswaran K. Microbial characterization of the Mars Odyssey spacecraft and its encapsulation facility. Environ Microbiol. 2003;5:977–985. doi: 10.1046/j.1462-2920.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- Laiz L. Recio D. Hermosin B. Saiz-Jimenez C. Microbial communities in salt efflorescences. In: Ciferri O., editor; Tiano P., editor; Mastromei G., editor. Of Microbes and Art: The Role of Microbial Communities in the Degradation and Protection of Cultural Heritage. Kluwer; New York: 2000. pp. 77–88. [Google Scholar]
- Link L. Sawyer J. Venkateswaran K. Nicholson W. Extreme spore UV resistance of Bacillus pumulis isolates obtained from ultraclean spacecraft assembly facility. Microb Ecol. 2003;47:159–163. doi: 10.1007/s00248-003-1029-4. [DOI] [PubMed] [Google Scholar]
- Litzner B.R. Caton T.M. Schneegurt M.A. Carbon substrate utilization, antibiotic sensitivity, and numerical taxonomy of bacterial isolates from the Great Salt Plains of Oklahoma. Arch Microbiol. 2006;185:286–296. doi: 10.1007/s00203-006-0096-6. [DOI] [PubMed] [Google Scholar]
- Maguire M.E. Magnesium transporters: properties, regulation and structure. Front Biosci. 2006;11:3149–3163. doi: 10.2741/2039. [DOI] [PubMed] [Google Scholar]
- Mandrioli P. Saiz-Jimenez C. EC Advanced Study Course Technical Notes. UCL Centre for Sustainable Heritage; London: 2002. Biodeterioration: macromonitoring and microeffects on cultural heritage and the potential benefits of research to society; pp. 1–5. Sessions 7–8. [Google Scholar]
- Marion G.M. Fritsen C.H. Eicken H. Payne M.C. The search for life on Europa: limiting environmental factors, potential habitats, and Earth analogues. Astrobiology. 2003;3:785–811. doi: 10.1089/153110703322736105. [DOI] [PubMed] [Google Scholar]
- Markovitz A. Method for the selection of bacteria that synthesize uronic acid-containing polysaccharides. J Bacteriol. 1961;82:436–441. doi: 10.1128/jb.82.3.436-441.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovitz A. Sylvan S. Effect of sodium sulfate and magnesium sulfate on heteropolysaccharide synthesis in Gram-negative soil bacteria. J Bacteriol. 1962;83:483–489. doi: 10.1128/jb.83.3.483-489.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnocha C.L. Chevrier V.F. Ivey D.M. Growth of sulfate-reducing bacteria in sulfate brines and the astrobiological implications for Mars [abstract 1604]. 42nd Lunar and Planetary Science Conference Abstracts, Lunar and Planetary Institute; Houston. 2011. [Google Scholar]
- Mattimore V. Battista J.R. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol. 1996;178:633–637. doi: 10.1128/jb.178.3.633-637.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen A.S. Ojha L. Dundas C.M. Mattson S.S. Byrne S. Wray J.J. Cull S.C. Murchie S.L. Thomas N. Gulick V.C. Seasonal flows on warm martian slopes. Science. 2011;333:740–743. doi: 10.1126/science.1204816. [DOI] [PubMed] [Google Scholar]
- Miller R.V. recA: The gene and its protein product. In: Luria S., editor. Encyclopedia of Microbiology. 2nd. Academic Press; San Diego: 2000. pp. 43–54. [Google Scholar]
- Miller R.V. Jeffrey W. Mitchell D. Elasri M. Bacterial response to solar ultraviolet light. ASM News. 1999;65:535–541. [Google Scholar]
- Moissl C. Osman S. La Duc M.T. Dekas A. Brodie E. DeSantis T. Venkateswaran K. Molecular bacterial community analysis of clean rooms where spacecraft are assembled. FEMS Microbiol Ecol. 2007;61:509–521. doi: 10.1111/j.1574-6941.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- Moissl C. Bruckner J.C. Venkateswaran K. Archaeal diversity analysis of spacecraft assembly clean rooms. ISME J. 2008;2:115–119. doi: 10.1038/ismej.2007.98. [DOI] [PubMed] [Google Scholar]
- Mullakhanbhai M.F. Larsen H. Halobacterium volcanii spec. nov., a Dead Sea halobacterium with a moderate salt requirement. Arch Microbiol. 1975;104:207–214. doi: 10.1007/BF00447326. [DOI] [PubMed] [Google Scholar]
- Nieto J.J. Ventosa A. Ruiz-Berraquero F. Susceptibility of halobacteria to heavy metals. Appl Environ Microbiol. 1987;53:1199–1202. doi: 10.1128/aem.53.5.1199-1202.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. Halobacterium sodomense sp. nov., a Dead Sea halobacterium with an extremely high magnesium requirement. Int J Syst Bacteriol. 1983;33:381–386. [Google Scholar]
- Oren A. Bioenergetic aspects of halophilism. Microbiol Mol Biol Rev. 1999;63:334–348. doi: 10.1128/mmbr.63.2.334-348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piłsyk S. Paszewski A. Sulfate permeases—phylogenetic diversity of sulfate transport. Acta Biochim Pol. 2009;56:375–384. [PubMed] [Google Scholar]
- Puleo J.R. Fields N.D. Bergstrom S.L. Oxborrow G.S. Stabekis P.D. Koukol R. Microbiological profiles of the Viking spacecraft. Appl Environ Microbiol. 1977;33:379–384. doi: 10.1128/aem.33.2.379-384.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla M. Chaturvedi R. Tamhane D. Vyas P. Archana G. Apte S. Bandekar J. Desai A. Multiple-stress tolerance of ionizing radiation-resistant bacterial isolates obtained from various habitats: correlation between stresses. Curr Microbiol. 2007;54:142–148. doi: 10.1007/s00284-006-0311-3. [DOI] [PubMed] [Google Scholar]
- Smith R.L. Maguire M.E. Microbial magnesium transport: unusual transporters searching for identity. Mol Microbiol. 1998;28:217–226. doi: 10.1046/j.1365-2958.1998.00810.x. [DOI] [PubMed] [Google Scholar]
- Stieglmeier E. Wirth R. Kminek G. Moissl-Eichinger C. Cultivation of anaerobic and facultatively anaerobic bacteria from spacecraft-associated clean rooms. Appl Environ Microbiol. 2009;75:3483–3491. doi: 10.1128/AEM.02565-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosca N.J. Knoll A.H. McLennan S.M. Water activity and the challenge for life on early Mars. Science. 2008;320:1204–1207. doi: 10.1126/science.1155432. [DOI] [PubMed] [Google Scholar]
- Venkateswaran K. Satomi M. Chung R. Koukol R. Basic C. White D. Molecular microbial diversity of a spacecraft assembly facility. Syst Appl Microbiol. 2001;24:311–320. doi: 10.1078/0723-2020-00018. [DOI] [PubMed] [Google Scholar]
- Venkateswaran K. Kempf M. Chen F. Satomi M. Nicholson W. Kern R. Bacillus nealsonii sp. nov., isolated from a spacecraft assembly facility, whose spores are gamma-radiation resistant. Int J Syst Evol Microbiol. 2003a;53:165–172. doi: 10.1099/ijs.0.02311-0. [DOI] [PubMed] [Google Scholar]
- Venkateswaran K. Hattori N. La Duc M.T. Kern R. ATP as a biomarker of viable microorganisms in clean-room facilities. J Microbiol Methods. 2003b;52:367–377. doi: 10.1016/s0167-7012(02)00192-6. [DOI] [PubMed] [Google Scholar]
- Volcani B.E. Papers Collected to Commemorate the 70th Anniversary of Dr. Chaim Weizmann. Daniel Sieff Research Institute; Rehovoth, Israel: 1944. The microorganisms of the Dead Sea; pp. 71–85. [Google Scholar]
- Wänke H. Brückner G. Dreibus G. Rieder R. Ryabchikov I. Chemical composition of rocks and soils at the Pathfinder site. Space Sci Rev. 2001;96:317–330. [Google Scholar]
- Wilson C. Caton T.M. Buchheim J.A. Buchheim M.A. Schneegurt M.A. Miller R.V. DNA-repair potential of Halomonas spp. from the Salt Plains Microbial Observatory of Oklahoma. Microb Ecol. 2004;48:541–549. doi: 10.1007/s00248-004-0243-z. [DOI] [PubMed] [Google Scholar]