Abstract
Data was analyzed from 92 patients > 5 years after intraperitoneal (IP) radionuclide therapy (RIT) with 90Y- or 177Lu-CC49 to determine prognostic factors. Patients had CC49 antibody-reactive ovarian cancer confined to the abdominal cavity after primary debulking and chemotherapy. The first 27 patients received IP 177Lu-CC49 alone; the remainder received Interferon (IFN), to increase the expression of the tumor-associated glycoprotein-72 (TAG-72) antigen, +/− IP paclitaxel (25–100 mg/m2) 2 days before RIT. Factors assessed by univariate (and some multivariate) analysis included age, race, body size, interval between initial diagnosis and RIT, interval between 2nd look surgery and RIT, 90Y versus 177Lu, MBq dose, paclitaxel dose, grade of tumor, extent of initial surgery, size of disease deposits prior to RIT, intensity of TAG reactivity, the addition of unlabeled antibody, and the development of human anti-mouse antibody and/or serum sickness after murine antibody. A statistically significant improvement in progression-free survival (p≤0.05) was noted for less bulky disease and younger age. Administration of paclitaxel plus IFN, an immune response, and use of 90Y showed a favorable nonsignificant trend. Dose escalation of radionuclide did not change risk of progression; thus, this therapy may have therapeutic efficacy at modest dose levels.
Key words: cancer, intraperitoneal, ovarian, radioimmunotherapy
Introduction
Once patients with ovarian cancer relapse after primary cytoreductive surgery and chemotherapy they usually have progression-free survival (PFS), measured in months, to a variety of treatment options including chemotherapy, biologics, radiation, and gene therapy. In our series of phase I studies utilizing antibody-targeted radionuclide therapy (RIT), 14% of evaluable patients with small volume relapsed ovarian cancer confined to the abdominal cavity were without evidence of progression > 4 years after a single intraperitoneal (IP) infusion of 90Y- or 177Lu-CC49 antibody. To determine prognostic factors for long PFS among patients with ovarian cancer receiving IP RIT, univariate, and multivariate analysis was performed for patient/disease related and treatment factors.
Methods
Data were analyzed from 92 patients treated with 90Y- or 177Lu-CC49 IP RIT. The patients had persistent or recurrent ovarian cancer confined to the abdominal cavity after primary debulking and chemotherapy. Tumor samples from all patients reacted with CC49, an anti-TAG-72 antibody developed in the laboratory of Jeffrey Schlom at the National Cancer Institute.1 All patients had primary ovarian or extra-ovarian peritoneal origin epithelial carcinoma: no sarcomas were included. The first 27 patients received IP 177Lu-CC49 alone with dose escalation from 10 mCi/m2 to 45 mCi/m2 [370–1665 MBq/m2].2,3 The next 10 also received human recombinant Interferon (IFN) alpha 2b which was administered as four subcutaneous injections of 3×106 U on alternate days beginning 5 days before RIT. IFN was given in an effort to increase the expression of the TAG-72 antigen.4 All subsequent patients (after the first 37) also received a single IP infusion of paclitaxel at 25, 50, 75, or 100 mg/m2 2 days before IP RIT.5,6 Patients who received both IFN and paclitaxel were treated with 1184–1480 MBq/m2 177Lu-CC49 or 518–895.4 MBq/m2 90Y-CC49.7 Patients were monitored for toxicity, biokinetic parameters, and clinical plus radiographic assessment of antitumor effects.
Univariate and multivariate analysis was performed to assess the relationship of various prognostic factors to PFS. Factors evaluated included age, race, body size (m2), interval between initial diagnosis and RIT, interval between second look surgery and RIT, radionuclide MBq dose, 90Y versus 177Lu, paclitaxel dose, grade of tumor, extent of initial surgery, size of disease deposits prior to RIT, intensity of TAG reactivity, the addition of unlabeled antibody, and the development of human anti-mouse antibody (HAMA) and/or serum sickness after murine antibody. HAMA was quantitated as mg/mL in blood sera.8
The intensity of TAG reactivity was the sum of the graded intensity of +1, +2, +3, or +4 times the percent of cells at each grade. Thus the minimum score would be 5% of cells as +1, for a score of 5, and the maximum score possible would be 100% of cells at +4 intensity, for a score of 400. The actual scores varied from 10 to 300. Those patients whose intensity was not quantitated were excluded from some analyses.
Continuous variables included factors such as age, total MBq dose, quantitative mg HAMA, time to progression and TAG-72 score, whereas dosing groups of mg/m2 or MBq/m2 were step variables. Age was also analyzed as a step variable. IFN, serum sickness, and unlabeled antibody were compared as either present or absent. The CC49 antibody was labeled at a concentration ratio of 370 MBq/mg. For the majority of patients, unlabeled antibody was added to make the total antibody dose 20 mg. For the last 42 patients, no unlabeled antibody was added. Certain factors were not available for all patients, thus the number of patients for each factor analyzed is listed in Table 1.
Table 1.
The Range, Mean, Median, and Standard Deviation for Variables is Compared
| No. | Mean | Std | Median | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Age (years) | 92 | 58 | 11 | 58 | 33 | 78 |
| MBq injected | 92 | 1998 | 639 | 1981 | 611 | 3263 |
| Mo. postsurgery | 68 | 2.5 | 2.8 | 1.8 | 0.3 | 17.4 |
| Mo. PFS | 90 | 14.1 | 27.1 | 4.3 | 1.5 | a168.0 |
| Patient size (m2) | 92 | 1.73 | 0.16 | 1.71 | 1.37 | 2.27 |
| Mo. after diagnosis | 91 | 12.2 | 8.2 | 8.7 | 5.0 | 44.1 |
| TAG score | 79 | 168 | 77 | 180 | 10 | 300 |
| HAMA (mg/mL) | 88 | 3369 | 6079 | 564 | 5 | 33692 |
| Log HAMA | 88 | 6.2 | 2.4 | 6.3 | 1.6 | 10.4 |
168 is used as maximum since that is the longest progression-free survival follow-up date.
HAMA, human anti-mouse antibody; HR, hazard ratio; PFS, progression-free survival; TAG, tumor-associated glycoprotein.
Descriptive statistics were used to summarize the data. Median survival time is provided for the whole sample and also as stratified samples (by serum sickness, use of 90Y, and bulk of disease, respectively), with survival curves obtained by using product-limit estimation. Logarithm transformation of HAMA was used in analysis because the range was wide (5–33,692). Results of log-rank test of equality over strata are presented. The Cox Proportional hazards model was used to examine the effect on time to progression of important covariates. Results from both univariate and multiple analyses are presented with hazard ratio (HR) as a measure of effect and p-values.9 Confidence intervals (CI) are provided as 95%. Available analysis of cases has been adapted in dealing with missing data.10 All analyses were performed with SAS for Windows (Version 9.2; SAS Institute, Cary, NC).
Results
Of ninety-two patients treated with IP RIT, 91 were assessable for PFS, 46 of whom had disease deposits < 2 cm. PFS survival for nonmeasureable disease was determined by physical exam and/or CT scans that were consistent with progression.
Table 1 lists descriptive statistics for continuous variables that were assessed to determine which factors affected PFS.
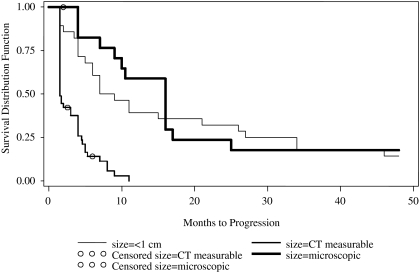
Although African-Americans had a tendency for longer PFS, they represented only 5% of the patients treated and this variable was not further analyzed. The impact of several other factors on PFS is compared in Table 2. Age and the size of tumor nodules at the time of RIT were significant prognostic factors for PFS with p<0.05. Although younger age was advantageous, the HR was more modest than the impact of the disease burden. The tendency for rapid progression with measurable disease contrasted to a fraction of patients with small volume disease who continued to be free of progression > 4 years is illustrated in Figure 1.
Table 2.
Selected Results from the Cox Model Analysis
| |
Univariate analysis |
Multiple analysis |
||||
|---|---|---|---|---|---|---|
| Covariate | No. | HR (95% CI) | p-value | No. | HR (95% CI) | p-value |
| Age group | ||||||
| < 60 years | 48 | 45 | ||||
| ≥60 years | 43 | 1.9 (1.26, 3.09) | 0.0031 | 42 | 2.89 (1.74, 4.81) | <0.0001 |
| Paclitaxel | ||||||
| ≤50 mg/m2 | 47 | 45 | ||||
| ≥75 mg/m2 | 44 | 0.86 (0.56, 1.31) | 0.4740 | 42 | 0.93 (0.27, 3.17) | 0.9073 |
| 90Y used | ||||||
| No | 71 | 69 | ||||
| Yes | 20 | 0.97 (0.58, 1.62) | 0.9024 | 18 | 0.77 (0.34, 1.75) | 0.5379 |
| MBq injected | 91 | 1.00 (0.99, 1.02) | 0.7035 | 86 | 1.01 (1.00, 1.03) | 0.1398 |
| Grade | ||||||
| 3 | 45 | 43 | ||||
| 1, 2, 4a | 46 | 0.87 (0.57, 1.33) | 0.5253 | 44 | 0.96 (0.59, 1.56) | 0.8752 |
| Disease size | ||||||
| Microscopic | 18 | 17 | ||||
| ≤ 1 cm nodules | 28 | 1.46 (0.77, 2.76) | 0.2478 | 27 | 1.67 (0.83, 3.34) | 0.1504 |
| 2 cm nodules | 45 | 6.35 (3.16, 12.74) | <0.0001 | 43 | 9.51 (4.29, 21.06) | <0.0001 |
| mg unlabeled Antibody | ||||||
| 0 | 41 | 39 | ||||
| 20 | 50 | 1.14 (0.74, 1.74) | 0.5543 | 48 | 0.87 (0.25, 2.98) | 0.8225 |
| Log HAMA | 87 | 0.96 (0.88, 1.05) | 0.3982 | 87 | 0.96 (0.86, 1.06) | 0.4004 |
Grade 4 designation for analysis is used for those that had unknown grade.
FIG. 1.
Progression-free survival is compared with size of disease deposits before treatment with intraperitoneal radionuclide therapy.
The time between second look surgery and RIT, time from diagnosis to RIT, the total MBq dose or the dose group MBq/m2 did not appear to have substantial impact on PFS with HRs (1.002–1.051). Thus, although most patients were treated with RIT within 9 weeks after second look surgery, a longer interval did not appear detrimental.
Those who had manifestation of an immune response as the development of HAMA and/or serum sickness showed a nonsignificant trend for a positive impact. Other factors showing a trend on univariate analysis that did not reach statistical significance include adjuvant administration of IFN+paclitaxel at > 75 mg/m2, optimal debulking at primary surgery versus suboptimal or unknown, and low grade tumor.
Results from multivariate analysis for selected factors are also compared in Table 2. Multivariate analysis confirmed the statistically significant effect on PFS for bulk of disease just prior to RIT and age, as was noted on univariate analysis. Dose escalation was not beneficial as noted by HR of 1.00 on univariate analysis and 1.01 on multivariate analyses; with much more narrow 95% CI than other variables. Use of 90Y resulted in a nonsignificant favorable trend on PFS over 177Lu.
Discussion
Prior studies have shown that the extent of residual disease after initial surgery affects outcome. The longest survival among ovarian cancer patients is associated with no or minimal residual tumor at initial cytoreductive surgery.11–13 Despite some difficulty in comparing studies due to the definition of optimal debulking ranging from no macroscopic to residual masses up to 3 cm., meta analysis using a continuous model for nearly 7000 Stage 3–4 ovarian cancer patients showed a 5.5% increase in median survival with every 10% increase in cytoreduction.14 Our current study indicates that bulky disease at the time of salvage therapy is similarly a poor prognostic feature. While all with measurable disease progressed in < 1 year, 14% with metastases too small to be measured by CT had PFS>4 years. Our clinical results are consistent with animal model and other clinical trial reports using several radioimmunoconjugates that found IP RIT to be most efficacious for small volume disease.15,16
In addition to the impact of tumor bulk after original surgery and at salvage, other factors of tumor grade and time since diagnosis, suggest a negative prognostic trend of more aggressive tumors but in this analysis their impact did not reach statistical significance.
Initially unlabeled antibody was added to improve targeting; it also provided more foreign antigen to which the immune system could react. This analysis showed that the addition of unlabeled antibody was favorable on multivariate, but not univariate analysis, and evidence of an immune response as evidenced by the development of HAMA and serum sickness had a favorable trend. This result supports the prior suggestion that development of serum sickness in response to antibody administration has potential for a positive therapeutic effect.17
The addition of IFN +/− paclitaxel to RIT also showed a trend toward improved PFS that did not reach statistical significance. Paclitaxel is a chemotherapeutic with proven activity against ovarian cancer and is known to have radiosensitizing effects. Because patient numbers were small for each dose level, it is possible that the low dose group did not have adequate paclitaxel to provide substantial therapeutic benefit; even the highest level may have been suboptimal as it is still less than the standard IV treatment level.
The rationale for the study of 90Y after success with 177Lu-CC49 was the longer effective penetration of the beta radiation (effective range 1–2 mm vs. 0.2–0.3 mm), shorter half life and lack of a gamma component (that adds mainly to toxicity but not efficacy). Also of concern in this change was that the more penetrating radiation from 90Y could result in higher risk of bowel complications. The 90Y group had the potential advantage of the higher paclitaxel dose. With 90Y-CC49 bowel wall toxicity was not apparent and there was a favorable trend on PFS that did not reach statistical significance.
CA 125 was not officially used as a measure of disease response or progression. Because more than one-third of the patients studied were treated after a second look surgery found disease despite normal CA 125 levels, the marker levels were not felt to be a good indicator of disease status. It is noted, however, that some patients had an increase in CA 125 several weeks before detection of recurrence by clinical or radiographic criteria. One patient with nonmeasurable disease had a complete response by Rustin criteria for normalization of CA 125.18 The search for predictive markers continues in ovarian cancer as means of detection and disease status monitoring. Other predictive markers have been reported that were not tested in this cohort such as BRCA1.19
This study represents additional analysis that leads to changes in some conclusions about the influential factors since the prior abstract report.20
Summary and Conclusions
Targeted RIT for recurrent ovarian cancer confined to the abdominal cavity is a promising therapy for selected patients. These results, consistent with those of others, suggest that phase II or III studies would best be conducted among patients with minimal residual disease because patients with CT-measureable disease tend to have short intervals to progression. Use of adjuvant agents for radiosensitization may be helpful and 90Y is a good candidate radionuclide for use in those patients. This therapy may have therapeutic efficacy at modest dose levels that do not result in severe toxicity.
Acknowledgments
Supported by NCI CM87215, NIH RO1 CA.OD67828-01, and M01 RR-00032. Comprehensive Cancer Center resources were used, including radiolabeling and data management service. We appreciate contributions of Shoulan Ding, Tracey Cotton-Young, Gayle Elliot Hines, Alma Del Grosso, Cherita Fagan, Pamela Dixon, and Jennifer Thornton and Gynecologic Oncology group, including Warner Huh, J. Max Austin, Larry Kilgore, and Mack Barnes.
Disclosure Statement
All authors involved in the article have been informed of their obligations under federal regulations governing disclosure of significant financial interests and have no conflicts of interest or potential conflicts of interest that have not been disclosed.
References
- 1.Schlom J. Siler K. Milenic DE, et al. Monoclonal antibody-based therapy of a human tumor xenograft with a 177lutetium-labeled immunoconjugate. Cancer Res. 1991;51:2889. [PubMed] [Google Scholar]
- 2.Meredith RF. Partridge EE. Alvarez RD, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with lutetium-177-CC49. J Nucl Med. 1996;37:1491. [PubMed] [Google Scholar]
- 3.Alvarez RD. Partridge EE. Khazaeli MB, et al. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: A phase I/II study. Gynecol Oncol. 1997;65:94. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 4.Greiner JW. Guadagni F. Hand PH, et al. Augmentation of tumor antigen expression by recombinant human interferons: Enhanced targeting of monoclonal antibodies to carcinomas. Cancer Treat Res. 1990;51:413. doi: 10.1007/978-1-4613-1497-4_21. [DOI] [PubMed] [Google Scholar]
- 5.Meredith R. Alvarez R. Khazaeli MB, et al. Intraperitoneal radioimmunotherapy for refractory epithelial ovarian cancer with 177 Lu-CC49. Minerva Biotecnologica. 1998;10:100. [Google Scholar]
- 6.Meredith RF. Alvarez RD. Partridge EE, et al. Intraperitoneal radioimmunochemotherapy of ovarian cancer: A phase I study. Cancer Biother Radiopharm. 2001;16:305. doi: 10.1089/108497801753131381. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez RD. Huh WK. Khazaeli MB, et al. A Phase I study of combined modality (90) Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2002;8:2806. [PubMed] [Google Scholar]
- 8.LoBuglio AF. Wheeler RH. Trang J, et al. Mouse/human chimeric monoclonal antibody in man: Kinetics and immune response. Proc Natl Acad Sci U S A. 1989;86:4220. doi: 10.1073/pnas.86.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allison P. Survival Analysis Using SAS®: A Practical Guide. SAS Publishing; Cary, NC: 1995. [Google Scholar]
- 10.Allison P. Missing Data (Sage University Series on Quantitative Applications in the Social Sciences, series no. 07–136) Sage Publications; Thousand Oaks, CA: 2001. [Google Scholar]
- 11.Makar AP. Baekelandt M. Trope CG, et al. The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol. 1995;56:175. doi: 10.1006/gyno.1995.1027. [DOI] [PubMed] [Google Scholar]
- 12.Eisenkop SM. Friedman RL. Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: A prospective study. Gynecol Oncol. 1998;69:103. doi: 10.1006/gyno.1998.4955. [DOI] [PubMed] [Google Scholar]
- 13.Le T. Krepart GV. Lotocki RJ. Heywood MS. Malignant mixed mesodermal ovarian tumor treatment and prognosis: A 20-year experience. Gynecol Oncol. 1997;65:237. doi: 10.1006/gyno.1997.4625. [DOI] [PubMed] [Google Scholar]
- 14.Bristow RE. Tomacruz RS. Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J Clin Oncol. 2002;20:1248. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 15.Elgqvist J. Andersson H. Back T, et al. Alpha-radioimmunotherapy of intraperitoneally growing OVCAR-3 tumors of variable dimensions: Outcome related to measured tumor size and mean absorbed dose. J Nucl Med. 2006;47:1342. [PubMed] [Google Scholar]
- 16.Epenetos AA. Munro AJ. Stewart S, et al. Antibody-guided irradiation of advanced ovarian cancer with intraperitoneally administered radiolabeled monoclonal antibodies. J Clin Oncol. 1987;5:1890. doi: 10.1200/JCO.1987.5.12.1890. [DOI] [PubMed] [Google Scholar]
- 17.Courtenay-Luck NS. Epenetos AA. Sivolapenko GB, et al. Development of anti-idiotypic antibodies against tumour antigens and autoantigens in ovarian cancer patients treated intraperitoneally with mouse monoclonal antibodies. Lancet. 1988;2:894. doi: 10.1016/s0140-6736(88)92482-8. [DOI] [PubMed] [Google Scholar]
- 18.Rustin GJ. Use of CA-125 to assess response to new agents in ovarian cancer trials. J Clin Oncol. 2003;21(Suppl10):187s. doi: 10.1200/JCO.2003.01.223. [DOI] [PubMed] [Google Scholar]
- 19.Quinn JE. James CR. Stewart GE, et al. BRCA1 mRNA expression levels predict for overall survival in ovarian cancer after chemotherapy. Clin Cancer Res. 2007;13:7413. doi: 10.1158/1078-0432.CCR-07-1083. [DOI] [PubMed] [Google Scholar]
- 20.Meredith R. Ding S. Alvaraz R, et al. Predictors of long term outcome from intraperitoneal radioimmunotherapy for ovarian cancer. Am J Clin Oncol. 2010;32:205. doi: 10.1089/cbr.2011.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]