Abstract
Fragile X syndrome is the most frequent hereditary cause of mental retardation after Down syndrome. Expansion of CGG repeats in the 5′ UTR of the fragile X mental retardation gene 1 (FMR1) causes gene inactivation in most of the cases. The FMR1 gene is classified into normal 5–44; gray zone 45–54; premutation 55 to <200; and full mutation ≥200 repeats. Precise sizing of FMR1 alleles is important to understand their variation, predisposition, and for genetic counseling. Meta-analysis reveals prevalence of premutation carriers as 1 in 259. No such reports are available in India. About 705 women from Tamil Nadu, South India, were screened for the FMR1 allelic variation by using radioactive polymerase chain reaction–polyacrylamide gel electrophoresis (PAGE) analysis. The women who were homozygous by radioactive polymerase chain reaction (rPCR) were reanalyzed by methylation-specific polymerase chain reaction (Ms-PCR) and GeneScan analysis. The techniques were validated and compared to arrive at a correction factor. Among 122 genotypes, 35 repeat variants ranging in size from 16 to 57 were observed. The most common repeat is 30 followed by 29. One in 353 women carried the premutation. No full mutations were observed. Screening populations with low frequency of premutations may not be applicable. Ms-PCR is more suitable for routine screening and clinical testing compared with rPCR-PAGE analysis.
Introduction
Fragile X syndrome (FXS) is the second most common form of mental retardation after Down syndrome, and accounts for about 15%–20% of X-linked mental retardation cases (Turner et al., 1996). The CGG trinucleotide repeats in expanded state (≥200) at Xq27.3 or a deletion or impairment of the FMR1 gene are the most important causes of FXS. The clinical features are variable and overlap with those of autism spectrum disorders. Some premutation carriers (55 to <200 CGG repeats) were found to have fragile X-associated primary ovarian insufficiency and also fragile X-associated tremor/ataxia syndrome (FXTAS) (Hagerman and Hagerman, 2004). The number of CGG repeats at the FRAXA fragile site is highly polymorphic. The most common CGG repeats are 30 or 29, and normally range from 6 to 54 (Fu et al., 1991).
Screening of CGG repeat size in different ethnic groups helps not only in understanding the FMR1 gene variation and identification of premutation carriers who are phenotypically normal, but also in allowing the adoption of best management practices for altering the risk of having fragile X affected children. Screening a normal population with a high incidence of premutations is, therefore, a cost-effective method for managing intellectual disability in the population (Toledano-Alhadef et al., 2001).
Materials and Methods
Subjects
A total of 705 unrelated normal women (age range: 18–33 years; mean±standard deviation 21.0±2.1 years) were recruited from colleges (students) and hospitals (working staff ) located in Tamil Nadu, South India. Written informed consent was obtained from all the participants. Institutional ethical clearance was obtained for this study as per the protocol.
Genotype analysis
Genomic DNA was extracted from 5 to 10 mL of whole blood by the salting-out method (Miller et al., 1988).
Radioactive polymerase chain reaction–polyacrylamide gel electrophoresis analysis
FMR1 CGG repeats were amplified with 6 pmol of each primer c and f (Fu et al., 1991) in 15 μL reaction volume containing 1×polymerase chain reaction (PCR) buffer (including 3.5 mM MgCl2), 200 μM each of dATP, dCTP and dTTP, 50 μM dGTP, 150 μM 7-deaza-dGTP (Roche), 10% DMSO (Sigma), 0.5 U of KlenTaq (AB Peptides), 2 μCi α32PdCTP (BRIT), and 100 ng of genomic DNA. A negative control (without template) and a positive control (known premutation) were included in every PCR setup.
The reaction conditions are 95°C for 10 min, followed by 30 cycles of 95°C for 1.5 min, 65°C for 1 min, 72°C for 2 min, and a 4°C hold. 15 μL of the amplicon was denatured at 95°C for 10 min and electrophoresed at 1500 volts for 5 h in 1XTBE on 5% denaturing polyacrylamide gel. The gel was fixed, dried, and autoradiographed (Kodak).
A negative control (without template) and a positive control (known premutation) were included in every PCR setup. The FMR1 radioactive PCR products appear as a discrete series of bands on an autoradiogram (Fig. 1). Repeat lengths were established by comparison with the reference standard (29 repeats). Phenotypic classification of FMR1 alleles was based on the CGG repeat size as per the guidelines of the American College of Medical Genetics (Maddalena et al., 2001).
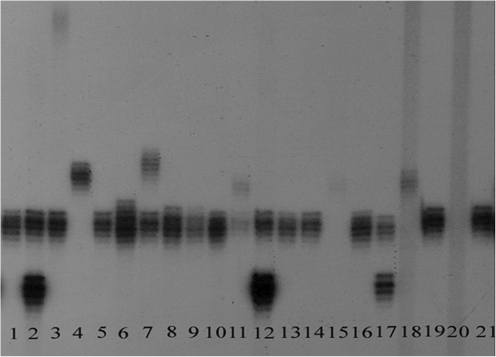
FIG. 1.
An autoradiogram of FMR1 PCR products. Lanes 1, 4, 5, 9, 10, 13, 14, 16, and 19: normal homozygous woman; lanes 2, 6, 7, 8, 11, 12, and 17: normal heterozygous woman; Lanes 15 and 18: woman with normal and full mutation allele; lane 20: full mutation male; lane 3: woman with normal and premutation FMR1 allele; Lane 21: normal homozygous woman with 29 repeats (reference standard). FMR1, fragile X mental retardation gene 1; PCR, polymerase chain reaction.
Women with two normal-size FMR1 alleles were scored as unaffected heterozygotes, whereas women with one normal-size FMR1 allele were reanalyzed using fluorescent Methylation-specific PCR (Ms-PCR) and GeneScan analysis (Zhou et al., 2006a), to find out whether they are “true homozygotes” or the other allele is expanded and beyond the amplification efficiency of rPCR, due to its high GC content.
Methylation-specific polymerase chain reaction
DNA samples were treated with sodium bisulfite as described by Zhou et al. (2006a) and purified using QIAEX II Gel Extraction Kit (Qiagen).
Three sets of primers were used to amplify the antisense strand of bisulfite modified DNA, one set targeting the nonmethylated allele–nonMet-PCR and the other two sets targeting the methylated allele–Met-Triple primed PCR (mTP-PCR) and “mTP-PCR.” Each Ms-PCR was performed in a 50 μL reaction volume containing the respective primers at concentrations described (Zhou et al., 2006a) with 0.2 mmol/L dNTPs, 2.5U HotStarTaq™ DNA polymerase (Qiagen), Q-Solution (Qiagen) at a concentration of either 0.5×(for nonMet-PCR) or 1.5×(for Met-PCR and mTP-PCR), 1×buffer (including 1.5 mM MgCl2), and 5 μL of the bisulfite-modified DNA. An enzyme activation step at 95°C for 15 min was followed by 40 cycles of 98°C for 1 min, 60°C for 1 min, 72°C for 2 min, followed by a final extension at 72°C for 10 min. Amplified Ms-PCR products were pooled, denatured, and capillary electrophoresed on an ABI Prism 3100 Genetic Analyzer, and fragment size was scored using Gene Mapper 3.7.1.
FMR1 analysis by chemiluminescence
Polymerase chain reaction was performed with 0.5 μM of each primer 1 and 3 (Brown et al., 1993) in 10 μL reaction containing 1×PCR buffer (including 0.75 mM MgCl2), 200 μM each of dNTPs, 0.25 U of KlenTaq (AB Peptides), and 100 ng of genomic DNA. Initial denaturation at 94°C for 4 min was followed by 28 cycles of 94°C for 1 min, 63°C for 1 min, 72°C for 2 min, and a 4°C hold. PCR products were denatured and electrophoresed on 6% denaturing polyacrylamide gel at 500 V for 90 min in 0.67×TBE. The gel was transferred onto a nylon membrane by electroblotting, and detection/visualization was by hybridizing on an oligonucleotide probe producing chemiluminescence (Quick Light Hybridization kit; Orchid Biosciences).
Validation
The precise sizing of FMR1 alleles is of the utmost importance in understanding FMR1 CGG repeat variation and a predisposition to FXS. The following observations in the current study prompted us to consider validation.
(a) Discordant Met and nonMet migration profiles were observed in Ms-PCR analysis with methylated DNA migrating faster than its unmethylated counterpart. This difference in migration speed could be due to the variation in the base composition of Met and nonMet alleles or to the degradative effect of sodium bisulfite on DNA. Reports on differential migration of Met and nonMet DNA on sodium bisulfite treatment are available in this context (Zhou et al., 2004; Boyd et al., 2006).
(b) In the current study, 28 (39.29%) was the most common CGG repeat observed followed by 27 (24.04%). The most common repeat in populations of Asian origin is 29 or 28, whereas in Caucasians 30 or 29 is the most common repeat. These differences in the most common repeat between populations can be attributed to ethnic specificity or may represent differences in the sensitivities and specificities of the various techniques employed in different studies.
Validation result-1
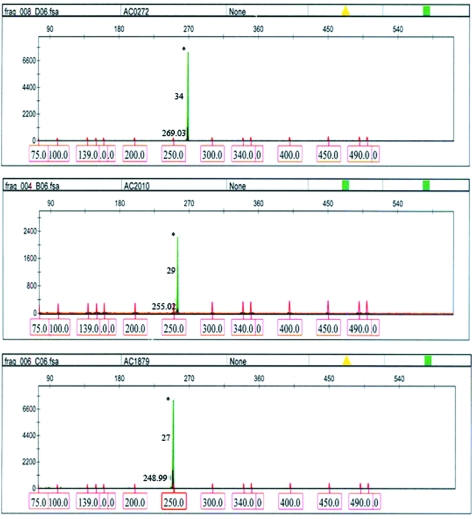
Three male samples with known CGG repeat data determined by sequencing (Zhou et al., 2006b) were validated by Ms-PCR. All the three samples showed a difference of two repeats less by Ms-PCR (Table 1 and Fig. 2).
Table 1.
Comparison of CGG Repeat Data of Methylation-Specific Polymerase Chain Reaction GeneScan Analysis and Sequencing (Validation-I)
| Sample ID | CGG repeat size by sequencing | CGG repeat size by Ms-PCR | Repeat differences observed |
|---|---|---|---|
| AC0272 | 36 | 34 | 2 |
| AC2010 | 31 | 29 | 2 |
| AC1879 | 29 | 27 | 2 |
Ms-PCR, methylation-specific polymerase chain reaction.
FIG. 2.
GeneScan profiles of three fluorescent methylation-specific (Ms)-PCR samples used in validation-I. The Met-Triplet primed, met, and nonmet-PCR products appear as black, blue (#), and green (*) peaks, respectively. FMR1 alleles are sized by using Rox-labeled internal size calibrator. The CGG repeat size calculated is indicated in the middle of the peak. The amplicon size is indicated at the bottom of the peak. Color images available online at www.liebertonline.com/gtmb
It is evident by Validation-1 that the CGG repeats are undersized in Ms-PCR. Therefore, a correction factor of +2 repeats is applied for FMR1 alleles up to 34 repeats. The “difference of two repeats” may not be common for alleles with >34 CGGs. Since the sizing error increases with the increase in repeat number, a correction factor of +2 may not be applicable for repeat size >35.
Validation result-2
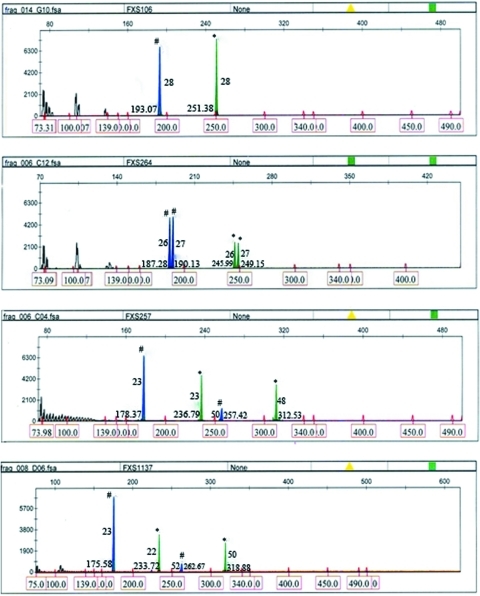
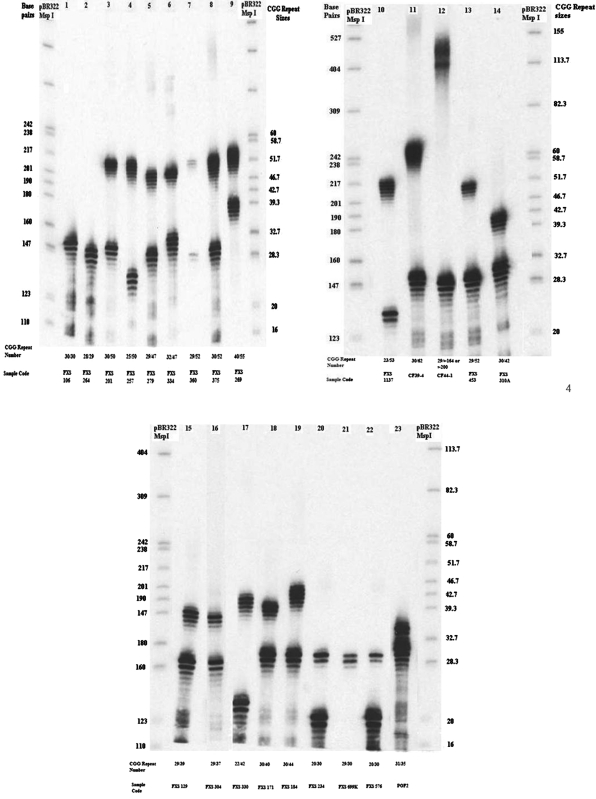
Twenty-three female samples with discordant Met and nonMet Ms-PCR profiles (Fig. 3) and known CGG repeat data by rPCR were sized by chemiluminescence (Fig. 4) at New York State Institute for Basic Research in Developmental Disabilities (Nolin et al., 2003).
FIG. 3.
Representative GeneScan profiles of four fluorescent Ms-PCR samples used in validation-II. The mTP, met, and nonmet-PCR products appear as black, blue (#), and green (*) peaks, respectively. FMR1 alleles are sized by using Rox-labeled internal size calibrator. The CGG repeat size calculated is indicated in the middle of the peak. The amplicon size is indicated at the bottom of the peak. Color images available online at www.liebertonline.com/gtmb
FIG. 4.
Chemiluminescence analysis of 23 samples used in validation-II. Lane 1: normal homozygous woman; lanes 2, 3, 4, 5, 6, 7, 8, 10, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, and 23: normal heterozygous woman; lanes 9 and 11: heterozygous woman with normal and premutation; lane 12: heterozygous woman with normal and full mutation FMR1 allele. Two unmarked lanes are pBR322 digested with MspI, a size marker.
Comparative results of the rPCR, Ms-PCR, and chemiluminescence analyses are presented in Table 2. The inferences made, based on the comparative analysis of rPCR, Ms-PCR, and chemiluminescence results, are as follows:
Table 2.
Comparison of CGG Repeat Data of rPCR, Methylation–Specific Polymerase Chain Reaction, and Chemiluminescence (Validation-II)
| |
CGG repeat data by rPCR |
CGG repeat data by Ms-PCR |
CGG repeat data by Chemiluminescence |
Repeat differences observed |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Allele 1 | Allele 2 | Allele 1 | Allele 2 | Allele 1 | Allele 2 | Normal | Gray Zone | Premutation |
| FXS 106 | 29 | ? | 28 | 28 | 30 | 30 | 1–2 | — | — |
| FXS 264 | 27 | ? | 26 | 27 | 28 | 29 | 1–2 | — | — |
| FXS 201 | 28 | ? | 27–28 | 48–49 | 30 | 50 | 2–3 | 1–3 | — |
| FXS 257 | 28a | ? | 23 | 48–50 | 25 | 50 | 2 | 0–2 | — |
| FXS 279 | 29 | ? | 27–28 | 45–47 | 29 | 47 | 1–2 | 0–2 | — |
| FXS 334 | 30 | ? | 30–31 | 45–47 | 32 | 47 | 1–2 | 0–2 | — |
| FXS 360 | 27 | PM | 27 | 49–51 | 29 | 52 | 1–2 | 0–2 | — |
| FXS 375 | 28 | ? | 28–29 | 49–51 | 30 | 52 | 1–2 | 1–3 | — |
| FXS 269 | 38 | ? | 37–39 | 52–54 | 40 | 55 | 1–3 | 1–3 | — |
| FXS 1137 | 22 | PM | 22–23 | 50–52 | 23 | 53 | 0–1 | 1–3 | — |
| CF 39–4 | NS | NS | 28 | 61–62 | 30 | 62 | 2 | 0–1 | — |
| CF 44–1 | NS | NS | 27 | PFM | 29 | ? (164 or >200) | 2 | — | Presence of either large PM or an FM |
| FXS 453 | 28 | ? | 27 | 48–50 | 29 | 52 | 1–2 | 2–4 | — |
| FXS 310A | 28 | ? | 28 | 38–40 | 30 | 42 | 2–4 | — | — |
| FXS 129 | 29 | ? | 27 | 36–38 | 29 | 39 | 0–3 | — | — |
| FXS 304 | 28 | ? | 27 | 35–37 | 29 | 37 | 0–2 | — | — |
| FXS 330 | 21 | ? | 20 | 39–41 | 22 | 42 | 1–3 | — | — |
| FXS 171 | 29 | ? | 28 | 37–39 | 30 | 40 | 1–3 | — | — |
| FXS 184 | 31 | ? | 28 | 41–43 | 30 | 44 | 1–3 | — | — |
| FXS 234 | 29 | ? | 18–19 | 28 | 20 | 30 | 1–2 | — | — |
| FXS 699K | 28 | ? | 28a | 35–36a | 29 | 30 | 1–6 | — | — |
| FXS 576 | 18 | ? | 18 | 29 | 20 | 30 | 1–2 | — | — |
| POF 2 | NS | NS | 28 | 33 | 31 | 35 | 2–3 | — | — |
PCR CGG repeat data appears as outliers.
D, difference; NL, normal; GZ, gray zone; PM, premutation; FM, full mutation; rpt, repeats; ?, repeat data not known; rPCR, radioactive polymerase chain reaction; CF, clinical family; NS, repeats not sized by radioaceive polymerase chain reaction; PFM, pre/full mutation; FXS, fragile X syndrome.
-
(a) In Ms-PCR, the nonMet-PCR repeat sizes appear smaller than the corresponding Met PCR generated sizes by 0–1 repeats for normal range up to ∼35 repeats, 1–2 repeats for alleles with repeat size from 35 up to the gray zone and small premutation alleles.
Sodium bisulfite treatment accelerates the migration of a modified nonMet allele compared with a modified Met allele of identical size (Zhou et al., 2004). In addition, the larger Ms-PCR amplicon size of the modified nonMet allele may increase secondary structure formation and further accelerate its electrophoretic migration compared with its modified Met allele counterpart.
(b) In the case of the normal alleles, repeat sizing from the Met PCR appears closer to the rPCR and chemiluminescence (validation-2) results, probably because fewer cytosines are converted by the bisulfite compared with its nonMet counterpart, thereby resulting in fewer sequence differences than in DNA not bisulfite treated. The Met PCR fragment size is also small and, thus, better represents the true repeat size during capillary electorphoresis.
(c) The repeat number difference between the three methods does not appear to follow a strict size relationship. This is probably because actual base pair sizings are not exact, for example, GeneScan base pairs come with decimal points and are not in exact multiples of 3.
(d) There appears to be a considerable level of allele dropout using the rPCR method, that is, the preferential amplification of only one allele (normal), dropping out the larger/expanded allele from amplification.
-
(e) As Boyd et al. (2006) pointed; it would not be easy to apply a correction factor, because larger amplicons deviate from the correction factor predictions for smaller fragments, possibly due to secondary structure formation. However, if necessary, a correction factor of +2 for alleles with Ms-PCR nonMet sizing of up to 35 repeats, and +3 for Ms-PCR nonMet sizes of 36–54 repeats could be considered.
Of the 705 women studied, 63.26% (446 women) were homozygous by rPCR. Reanalysis of the homozygotes by Ms-PCR revealed that 56.31% (794×chromosomes) had concordant repeat sizes by Met and nonMet-PCR, whereas 6.95% (98×chromosomes) showed discordant sizing by Met and nonMet-PCR, for example, 23 versus 22 repeats or 52 versus 50 repeats. In such cases, the upper limit of the repeat range was considered for repeat determination (Fig. 3, FXS 1137). The upper limit of the repeat range is shown to be most often generated by Met PCR and is closer to the chemiluminescence results.
Comparison of methodologies
Using rPCR-PAGE analysis, 36.73% of women (518×chromosomes) were heterozygous at the FRAXA locus. The observed heterozygosity (OH) by radioactive polymerase chain reaction (rPCR) is, thus, very low for this polymorphic gene. This could be due to the technical difficulty (stutter bands) in distinguishing the homozygous women from the heterozygous women with a single trinucleotide repeat difference. Therefore, the 63.26% of women who were homozygous by rPCR were reanalyzed by Ms-PCR to find out whether they are “homozygotes” or have an expanded allele that is beyond the amplification efficiency of the rPCR, due to its high GC content.
Ms-PCR discriminates not only normal, premutation-, and full mutation-affected men and women but also differentiates two normal alleles differing by a single trinucleotide repeat. Reanalysis by Ms-PCR of the 63.26% of women who were classified as homozygous by rPCR (892×chromosomes) revealed that 25.25% (356×chromosomes) were true homozygotes, whereas 38.01% (536×chromosomes) were heterozygotes. Thus, the observed heterozygosity (74.8%) is close to the expected heterozygosity (77.6%). The interallelic differences of the 268 rPCR-undetected but Ms-PCR detected heterozygotes and their frequencies are presented in Table 3.
Table 3.
Interallelic Difference of Methylation-Specific Polymerase Chain Reaction Heterozygotes and Their Frequencies
| Interallelic difference | No. of individuals | % |
|---|---|---|
| 1 | 176 | 65.67 |
| 2 | 25 | 9.33 |
| 3 | 11 | 4.1 |
| 4 | 3 | 1.12 |
| 5 | 5 | 1.87 |
| 6 | 7 | 2.61 |
| 7 | 1 | 0.37 |
| 8 | 6 | 2.24 |
| 9 | 6 | 2.24 |
| 10 | 6 | 2.24 |
| 11 | 4 | 1.49 |
| 12 | 2 | 0.75 |
| 13 | 1 | 0.37 |
| 14 | 2 | 0.75 |
| 15 | 4 | 1.49 |
| 16 | 2 | 0.75 |
| 18 | 1 | 0.37 |
| 19 | 1 | 0.37 |
| 21 | 2 | 0.75 |
| 22 | 1 | 0.37 |
| 23 | 1 | 0.37 |
| 27 | 1 | 0.37 |
| Total | 268 | 100 |
An interallelic difference of one repeat is observed in a greater percentage of heterozygotes (65.67%). Heterozygotes with an interallelic difference of 1–5 repeats (82.09%) were unrecognizable by rPCR due to the “stutter/shadow band artifacts.” These shadow bands are commonly seen in PCR amplification of microsatellites probably due to replication slippage (Hauge and Litt, 1993). They cause difficulty in differentiating single or double bands, thus leading to inaccuracy in CGG repeat estimation and heterozygosity determination in women. A wide interallelic difference of 6–27 CGG repeats in 17.91% of the Ms-PCR heterozygotes indicates “allelic dropout” by rPCR.
Comparative analysis of FMR1 heterozygotes repeat sizing by rPCR and Ms-PCR Met profiles generated among 50 women showed that 31% of the alleles are consistent in repeat sizing by both the methodologies.
About 33% of the alleles showed one extra repeat by rPCR, 9% of the alleles showed two extra repeats by rPCR, and 1% of the alleles showed three extra repeats by rPCR, compared with Ms-PCR. The over/excess sizing (43% of alleles) of repeat size in rPCR is observed among the lower end alleles within the heterozygotes or in the heterozygotes with an interallelic difference of 1–10.
About 18%, 6%, 1%, and 1% of the heterozygote alleles show one, two, three, and four repeats less by rPCR, respectively. This undersizing (26% of alleles) of repeat data by rPCR compared with Ms-PCR is observed among heterozygotes with an interallelic difference of >10 repeats. In addition to stutter bands, inaccuracy in the sizing of heterozygotes by rPCR could be due to the faster electrophoretic migration of lower end alleles and heteroduplexing.
Thus, rPCR-generated repeat data are under- or overestimated in heterozygotes compared with the Ms-PCR data. A common correction factor is not applicable as per the comparative analysis of the FMR1 heterozygote repeat sizing by rPCR and Ms-PCR profiles (Table 4). However, to reduce false negativity, we propose a correction factor to rPCR heterozygotes as per validation results. Thus, Ms-PCR and GS analysis of FXS is a suitable alternative technique to radioactive PCR-PAGE analysis.
Table 4.
Percentage Distribution of the Comparative Analysis of Radioactive Polymerase Chain Reaction and Methylation-Specific Polymerase Chain Reaction CGG Repeat Data of 50 Heterozygotes
| rPCR versus Ms-PCR repeat data | Allele 1 | Allele 2 | Total no. of alleles | % | Observations–allele 1 | Observations–allele 2 |
|---|---|---|---|---|---|---|
| Consistent repeat data by both Ms-PCR and rPCR | 18 | 13 | 31 | 31 | Repeat range 19–30; normal | Repeat range 28–35 |
| rPCR data with one repeat extra than Ms-PCR data | 19 | 14 | 33 | 33 | Repeat range 19–30; normal | Repeat range 19–30; normal |
| rPCR data with two repeats extra than Ms-PCR data | 6 | 3 | 9 | 9 | Repeat range 17–30; normal | Repeat range 29–36; normal but >35 repeats |
| rPCR data with three repeats extra than Ms-PCR data | 0 | 1 | 1 | 1 | Not applicable | Repeat number 31; normal |
| rPCR data with one repeat less than Ms-PCR data | 6 | 12 | 18 | 18 | Repeat range 17–32; normal | Repeat range 22–32; normal |
| rPCR data with two repeats less than Ms-PCR data | 1 | 5 | 6 | 6 | Repeat number 19; normal | Repeat range 22–37; normal but >35 repeats |
| rPCR data with three repeats less than Ms-PCR data | 0 | 1 | 1 | 1 | Not applicable | Repeat number 37; normal but >35 repeats |
| rPCR data with four repeats less than Ms-PCR data | 0 | 1 | 1 | 1 | Not applicable | Repeat number 34; normal |
| Total | 50 | 50 | 100 | 100 |
Results
FMR1 CGG repeat variation
A total of 1410×chromosomes (705 women) were analyzed for CGG repeat variation. The distribution of chromosomes by the number of CGG repeats is given in Table 5. Thirty-five repeat variants ranging in size from 16 to 57 repeats were observed. The most frequently occurring repeat was 30 (in 39.08% of the chromosomes). The next most frequent repeat size was 29 (in 24.40% of the chromosomes). Of the total variants, three repeats, 30, 29, and 31, accounted for 71.49% of the chromosomes. Six of the 35 repeat sizes were observed only once (repeat size: 16, 37, 43, 52, 55, and 57) in the sample set. Five repeat sizes (repeat size 18, 46, 50, 53, and 54) were observed twice in the sample set. Two variants (repeat size 19 and 44) were observed thrice (0.21%, each). A frequency of 0.28% was observed for repeat size 42. The remaining 18 repeat sizes varied from as low as 0.71% (repeat size 28) to as high as 3.4% (repeat size 32).
Table 5.
Distribution of Chromosomes by the Number of CGG Repeats Among Normal Unrelated Women
| CGG Repeat size | No. of Chromosomes | % |
|---|---|---|
| 16 | 1 | 0.07 |
| 18 | 2 | 0.14 |
| 19 | 3 | 0.21 |
| 20 | 26 | 1.84 |
| 21 | 15 | 1.06 |
| 22 | 11 | 0.78 |
| 23 | 21 | 1.49 |
| 24 | 47 | 3.33 |
| 25 | 35 | 2.48 |
| 26 | 22 | 1.56 |
| 27 | 11 | 0.78 |
| 28 | 10 | 0.71 |
| 29 | 344 | 24.40 |
| 30 | 551 | 39.08 |
| 31 | 113 | 8.01 |
| 32 | 48 | 3.40 |
| 33 | 16 | 1.13 |
| 34 | 14 | 0.99 |
| 35 | 13 | 0.92 |
| 36 | 24 | 1.70 |
| 37 | 1 | 0.07 |
| 38 | 20 | 1.42 |
| 39 | 15 | 1.06 |
| 40 | 16 | 1.13 |
| 41 | 12 | 0.85 |
| 42 | 4 | 0.28 |
| 43 | 1 | 0.07 |
| 44 | 3 | 0.21 |
| 46 | 2 | 0.14 |
| 50 | 2 | 0.14 |
| 52 | 1 | 0.07 |
| 53 | 2 | 0.14 |
| 54 | 2 | 0.14 |
| 55 | 1 | 0.07 |
| 57 | 1 | 0.07 |
| Total | 1410 | 100 |
Genotype frequencies of CGG repeats
From the 35 “CGG repeat” alleles, a total of 122 genotypes were observed. Fifteen genotypes occurred with a frequency of 1% or more. Together, they accounted for about 72% of the women.
Genotype 29/30 accounted for 21.56% of the women and is the most common. The 30/30 genotype was the second most common (14.61%), followed by the 29/29 genotype (9.22%). Sixty-two of the 122 genotypes occurred only once. Of the 35 allele sizes, only seven occurred in the homozygous state, that is, 20, 24, 29, 30, 36, 38, and 41 repeats. Two of these homozygous genotypes, 30/30 and 29/29, were the most frequent. The observed heterozygosity of 74.8% (527/705) was close to the expected heterozygosity of 77.6%.
Frequencies of gray zone and premutation alleles
Repeat sizes ranging from 45 to 54 are considered to be gray zone alleles, and they account for 0.63% of the total chromosomes. Repeat sizes from 55 to <200 are considered to be premutations, and two chromosomes were of this phenotype (0.14%). Thus, in our study, the premutation carrier frequency was 1 in 353 women. No full mutations with repeat sizes of 200 or higher were found.
Discussion
FXS is the second most common genetic cause of mental retardation (Chiurazzi et al., 2004). The variable nature of the FMR1 CGG repeats and clinical features of the FXS and its association with autism, premature ovarian failure, and FXTAS highlight the importance of studying the CGG repeat variation in the normal population, as well as in various disease states. The current study of CGG repeat variation in 705 normal women revealed 122 genotypes from 35 different alleles ranging in size from 16 to 57 repeats. Full mutation alleles were not observed.
Gray zone allele frequency
In our sample set, gray zone repeat sizes (range: 45–54) accounted for 0.64% of the chromosomes screened. Therefore, one in 78 women carries a gray zone allele. Murray et al. (1996) reported gray zone allele frequency among women to be as high as 1 in 52. Our observed frequency (0.64%) is lower than the values reported among Chinese (0.75%), Brazilian (2.0%), and Caucasian (1.72%) populations (Brown et al., 1996; Sucharov et al., 1999; Poon et al., 2000). The frequency of gray zone alleles appears to be low in Asians compared with the Caucasian, Finnish, and Basque populations (Brown et al., 1996; Ryynanen et al., 1999; Faradz et al., 2000; Penagarikano et al., 2004).
In several reports of Asian origin, the gray zone allele is nearly absent, thus indicating a low frequency or absence of premutation alleles (range 55 to <200 repeats) that would predictably expand to pathologic mutations in the forthcoming generations (Arinami et al., 1993; Zhong et al., 1994; Chiurazzi et al., 1996; Chen et al., 1997; Tzeng et al., 1999; Poon et al., 2000; Wang et al., 2000).
Premutation carrier frequency
The premutation repeat sizes accounted for 0.14% of the chromosomes, screened. Therefore, 1 in 353 women carried a premutation repeat. The observed premutation allele frequency (0.14%) in Tamil Nadu is lower compared with Mexican (1.7%), Brazilian (1.0%), Israeli (0.67%), Basque (0.63%), Indonesian (0.38%), and American Caucasian (0.24%) populations (Brown et al., 1996; Sucharov et al., 1999; Faradz et al., 2000; Toledano-Alhadef et al., 2001; Penagarikano et al., 2004; Rosales-Reynoso et al., 2005). No premutation alleles were reported among Chinese, Africans, Eskimo, and Mixtec (Chiurazzi et al., 1996; Kunst et al., 1996; Larsen et al., 1999; Poon et al., 2000) populations.
An analysis of five published studies established a premutation allele frequency of 1 per 273. In a retrospective study, of 10,624 women, the estimated premutation carrier frequency (>54 repeats) was 1 per 259 women. Analysis of the polymorphic markers in the vicinity of the FMR1 gene showed an association of premutation with specific haplotypes. The investigators suggested that founder effect was partly responsible for the high carrier frequency observed in this population (Rousseau et al., 1995; Murray et al., 1997).
In a comprehensive study among Israeli women, the premutation carrier frequency was found to be 1:70 for alleles with ≥52 repeats and 1:152 for alleles with >54 repeats (Pesso et al., 2000). In a prospective study among 1477 Finnish women, the estimated carrier frequency was 1:246 for alleles with >60 repeats (Ryynanen et al., 1999). In yet another study, the carrier frequency was 1:113 with >54 repeats and 1:220 with >60 repeats among 14,334 Israeli women. The authors recommended screening to identify female carriers in the general population, as they found it to be more cost effective than caring for intellectually disabled patients (Toledano-Alhadef et al., 2001).
None of the studies, including our own, has reported a full mutation among normal women. However, in a prospective intervention study of the general population, one full mutation in 8426 low-risk women and three full mutations among 1033 high-risk normal women were reported (Ryynanen et al., 1999). The high-risk cases comprised all women who had a positive family history. In yet another study from Israel, three full mutations were reported among 14,334 (0.02%) women screened (Toledano-Alhadef et al., 2001). All of our other results besides those just discussed are in consonance with earlier findings.
Conclusion
In summary, fragile X CGG repeat variation among the South Indian population is reported with lower frequencies of gray zone (1 in 62) and premutation alleles (1 in 353) than reported in studies of other populations around the world. We, thus, conclude that screening all normal women for FXS carrier or full mutation status in populations with a low frequency of premutations may not be applicable. However, screening should be imperative for young women of reproductive age who belong to high-risk families to ascertain their carrier status.
Among the techniques, Ms-PCR is less time consuming and more useful in routine screening and clinical testing in both men and women than 32P-labeled radioactive PCR-PAGE analysis. Some confirmation tests and the respective correction factors, however, have to be applied for the Ms-PCR strategy.
Acknowledgments
This work was supported by UGC Research Scientist Scheme [F1-5/83(SA-II)] and a UGC Major Grant [F3-21/2002/SR(II)] to CR Srikumari Srisailapathy, National Medical Research Council of Singapore (NMRC/1079/2006) to SS Chong, Lady Tata Memorial Trust Junior Research Fellowship, and Darobji Tata Trust Travel Grant (DTT:EM:TG:M:197:1712:2005-2006) to N. Indhumathi.
The authors are grateful to Dr. Sarah L Nolin, Assistant Director, Fragile X lab, Department of Human Genetics, New York State Institute for Basic Research in Developmental Disabilities for help in validation. The authors acknowledge all the participants for their cooperation in this study.
Disclosure Statement
No competing financial interests exist.
References
- Arinami T. Asano M. Kobayashi K. Yanagi Y. Data on the CGG repeat at the fragile X site in the non-retarded Japanese population and family suggest the presence of a subgroup of normal alleles predisposing to mutate. Hum Genet. 1993;92:431–436. doi: 10.1007/BF00216445. [DOI] [PubMed] [Google Scholar]
- Boyd VL. Moody KI. Karger AE, et al. Methylation-dependent fragment separation: direct detection of DNA methylation by capillary electrophoresis of PCR products from bisulfite-converted genomic DNA. Anal Biochem. 2006;354:266–273. doi: 10.1016/j.ab.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Brown WT. Houck GE., Jr. Jeziorowska A, et al. Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA. 1993;270:1569–1575. [PubMed] [Google Scholar]
- Brown WT. Nolin S. Houck G., Jr, et al. Prenatal diagnosis and carrier screening for fragile X by PCR. Am J Med Genet. 1996;64:191–195. doi: 10.1002/(SICI)1096-8628(19960712)64:1<191::AID-AJMG34>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chen TA. Lu XF. Che PK. Ho WK. Variation of the CGG repeat in FMR-1 gene in normal and fragile X Chinese subjects. Ann Clin Biochem. 1997;34:517–520. doi: 10.1177/000456329703400504. [DOI] [PubMed] [Google Scholar]
- Chiurazzi P. Genuardi M. Kozak L, et al. Fragile X founder chromosomes in Italy; a few initial events and possible explanation for their heterogeneity. Am J Med Genet. 1996;64:209–215. doi: 10.1002/(SICI)1096-8628(19960712)64:1<209::AID-AJMG38>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Chiurazzi P. Tabolacci E. Neri G. X-Linked mental retardation (XLMR): from clinical conditions to cloned genes. Crit Rev Clin Lab Sci. 2004;41:117–158. doi: 10.1080/10408360490443013. [DOI] [PubMed] [Google Scholar]
- Faradz SM. Pattiiha MZ. Leigh DA, et al. Genetic diversity at the FMR1 locus in the Indonesian population. Ann Hum Genet. 2000;64:329–339. doi: 10.1017/S0003480000008204. [DOI] [PubMed] [Google Scholar]
- Fu YH. Kuhl DPA. Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ. Hagerman RJ. The fragile-X premutation: a maturing perspective. Am J Hum Genet. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge XY. Litt M. A study of the origin of ‘shadow bands' seen when typing dinucleotide repeat polymorphisms by the PCR. Hum Mol Genet. 1993;2:411–415. doi: 10.1093/hmg/2.4.411. [DOI] [PubMed] [Google Scholar]
- Kunst CB. Zerylnick C. Karickhoff L, et al. FMR1 in global populations. Am J Hum Genet. 1996;58:513–522. [PMC free article] [PubMed] [Google Scholar]
- Larsen LA. Armstrong JS. Gronskov K, et al. Analysis of FMR1 (CGG)n alleles and FRAXA microsatellite haplotypes in the population of Greenland: implications for the population of the new world from Asia. Eur J Hum Genet. 1999;7:771–777. doi: 10.1038/sj.ejhg.5200374. [DOI] [PubMed] [Google Scholar]
- Maddalena A. Richards CS. McGinniss MJ, et al. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the standards and guidelines for clinical genetics laboratories of the American college of medical genetics. Quality assurance subcommittee of the laboratory practice committee. Genet Med. 2001;3:200–205. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA. Dykes DD. Polesky HF. A simple salting out procedure for extraction of DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. Youings SA. Dennis N, et al. Population screening at the FRAXA and FRAXE loci: molecular analyses of boys with learning difficulties and their mothers. Hum Mol Genet. 1996;5:727–735. doi: 10.1093/hmg/5.6.727. [DOI] [PubMed] [Google Scholar]
- Murray J. Cuckle H. Taylor G. Hewison J. Screening for fragile X syndrome: information needs for health planners. J Med Screen. 1997;4:60–94. doi: 10.1177/096914139700400204. [DOI] [PubMed] [Google Scholar]
- Nolin SL. Dobkin C. Brown WT. Unit-5 Molecular Analysis of Fragile X Syndrome. Current Protocols in Human Genetics. John Wiley & Sons Inc.; New Jersey: 2003. [DOI] [PubMed] [Google Scholar]
- Penagarikano O. Gil A. Telez M, et al. A new insight into fragile X syndrome among Basque population. Am J Med Genet A. 2004;128A:250–255. doi: 10.1002/ajmg.a.30116. [DOI] [PubMed] [Google Scholar]
- Pesso R. Berkenstadt M. Cuckle H, et al. Screening for fragile X syndrome in women of reproductive age. Prenat Diagn. 2000;20:611–614. doi: 10.1002/1097-0223(200008)20:8<611::aid-pd881>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Poon PM. Zhao Z. Wu XQ, et al. Rapid analysis of CGG repeat length in the FMR1 gene. Clin Chem Lab Med. 2000;38:935–938. doi: 10.1515/CCLM.2000.137. [DOI] [PubMed] [Google Scholar]
- Rosales-Reynoso MA. Mendoza-Carrera F. Troyo-Sanroman R, et al. Genetic diversity at the FMR1 locus in Mexican population. Arch Med Res. 2005;36:412–417. doi: 10.1016/j.arcmed.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rousseau F. Rouillard P. Morel ML, et al. Prevalence of carriers of premutation-size alleles of the FMR1 gene and implications for the population genetics of the fragile X syndrome. Am J Hum Genet. 1995;57:1006–1018. [PMC free article] [PubMed] [Google Scholar]
- Ryynanen M. Heinonen S. Makkonen M, et al. Feasibility and acceptance of screening for fragile X mutations in low-risk pregnancies. Eur J Hum Genet. 1999;7:212–216. doi: 10.1038/sj.ejhg.5200285. [DOI] [PubMed] [Google Scholar]
- Sucharov CC. Silva R. Rondinelli E. Moura-Neto S. Fragile X trinucleotide repeats from a normal population in Rio de Janeiro, Brazil. Hereditas. 1999;130:189–190. doi: 10.1111/j.1601-5223.1999.00189.x. [DOI] [PubMed] [Google Scholar]
- Toledano-Alhadef H. Basel-Vanagaite L. Magal N, et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am J Hum Genet. 2001;69:351–360. doi: 10.1086/321974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. Webb T. Wake S. Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64:196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tzeng CC. Cho WC. Kuo PL. Chen RM. Pilot fragile X screening in normal population of Taiwan. Diagn Mol Pathol. 1999;8:152–156. doi: 10.1097/00019606-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Wang YC. Li C. Lin WH. Li SY. Molecular diagnosis of fragile X syndrome and distribution of CGG repeats in the FMR-1 gene in Taiwanese. J Formos Med Assoc. 2000;99:402–407. [PubMed] [Google Scholar]
- Zhong N. Liu X. Gou S, et al. Distribution of FMR-1 and associated microsatellite alleles in a normal Chinese population. Am J Med Genet. 1994;51:417–422. doi: 10.1002/ajmg.1320510423. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Law YH. Boehm CD, et al. Robust fragile X (CGG)n genotype classification using a methylation specific triple PCR assay. J Med Genet. 2004;41:e45. doi: 10.1136/jmg.2003.012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. Lum JM. Yeo GH, et al. Simplified molecular diagnosis of fragile X syndrome by fluorescent methylation-specific PCR and GeneScan analysis. Clin Chem. 2006a;52:1492–1500. doi: 10.1373/clinchem.2006.068593. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Tang K. Law HY, et al. FMR1 CGG repeat patterns and flanking haplotypes in three Asian populations and their relationship with repeat instability. Ann Hum Genet. 2006b;70:784–796. doi: 10.1111/j.1469-1809.2006.00265.x. [DOI] [PubMed] [Google Scholar]