Abstract
Background
Infliximab (IFX) therapy escalation during maintenance treatment occurs frequently in clinical practice in patients with ulcerative colitis (UC). Outcomes for these patients have not been described.
Aim
To describe the prevalence of, and outcomes after, IFX escalation during maintenance therapy in patients with moderate-severe UC.
Methods
Retrospective observational study of clinical outcomes in ambulatory patients with moderate-severe UC treated with maintenance IFX.
Results
Fifty-six ambulatory patients received IFX for moderate-severe UC; fifty (89%) responded and proceeded to maintenance therapy. Mean duration of maintenance therapy was 14 months, with mean follow-up of 38 months. Twenty-seven patients (54%) required IFX therapy escalation after a mean of 6 maintenance infusions. Clinical remission was noted in 36% of the entire cohort (18/50) at 12 months; 19% in the escalation group, and 56% in the non-escalation group. Patients who required IFX escalation were less likely to be in clinical remission at 12 months (OR 0.2, 95% CI 0.1-0.6, p=0.01) when compared to those who did not. During the follow-up period, 27% of patients required a colectomy, and the mean time to colectomy was 17 months. Patients in the escalation group required a colectomy in 33% of cases, compared with 21% of non-escalation patients.
Conclusions
A significant proportion of ambulatory patients with UC treated with maintenance IFX required therapy escalation over time. This was associated with lower remission, and higher colectomy, rates.
Background
Ulcerative colitis is a chronic inflammatory condition that affects the colon, and requires maintenance therapy to maintain remission. Although most patients are successfully managed with mesalamine formulations, about 25% of patients fail these or other therapies, and require escalation to immunomodulators, infliximab (IFX), cyclosporine or colectomy (1).
IFX (Remicade®, Janssen, Malvern, PA) was FDA-approved in the US in 2008 for the treatment of moderate-severe ulcerative colitis. The Active Ulcerative Colitis Trials (ACT), and a number of cohort studies, have reported the efficacy and safety of IFX (IFX) in induction and maintenance of remission in patients with UC in this setting in the short to medium term (2-7). In patients with Crohn’s disease (CD), the experience with maintenance IFX has been of a gradual loss of response in many patients (8,9). This has primarily been managed by escalation of IFX therapy (higher doses and / or more frequent infusions), with significant cost implications (10). Early reports in patients with UC suggest a similar need for IFX escalation in up to 60% of patients (4,11). Whether these patients have different long-term outcomes to non-escalation patients in clinical practice is unknown.
Of additional interest is the impact of azathioprine / mercaptopurine on clinical outcomes in patients with UC treated with IFX (12). The prospective SUCCESS trial reported superior steroid-free remission rates in azathioprine-naïve patients with UC treated with the combination of IFX and azathioprine, when compared to IFX or 2 azathioprine alone (13). In practice, many patients with UC who commence IFX have historically already failed azathioprine. Post-hoc analyses of randomized controlled trials, and reports from clinical practice, noted no difference in efficacy outcomes when IFX was used with azathioprine / mercaptopurine in populations enriched with azathioprine failures (5,14,15).
The goal of this study was to examine the prevalence of, and outcomes after, infliximab therapy escalation in patients with moderate-severe UC receiving maintenance treatment.
Methods
Patients
All patients who received IFX infusions for confirmed ulcerative colitis (UC) at Beth Israel Deaconess Medical Center between 2000 and 2009 were eligible for inclusion in the study. Patients were identified by a search of ICD-9 codes from infusion center attendees, and individual charts then reviewed by one of us (ER). The hospital’s electronic medical record (EMR), which includes all patients’ clinical notes and records, including infusion center notes, was accessed to retrieve data. A standardized data extraction format was used for all patients. The diagnosis of UC was confirmed by reviewing endoscopy & pathology reports. Patients who received “maintenance” IFX were initially induced at 0, 2 and 6 weeks, and then received scheduled IFX every 8 weeks thereafter. Patients continued their other maintenance therapies during the follow-up period. Prospective approval was obtained from the BIDMC IRB to review all patients’ records. All data was made anonymous after initial data extraction.
Definitions
Patients were defined as “ambulatory” if they had active UC based on clinical and / or endoscopic grounds and IFX was decided upon, and initiated as, an out-patient. Patients were categorized as having being a “responder” to IFX based on their gastroenterologists’ global assessment within 6 weeks of initiation of IFX, and their progression to maintenance therapy after induction. Clinical remission was defined as the absence of symptoms of active UC (no diarrhea, rectal bleeding or abdominal pain) as recorded by the primary gastroenterologist at 12 months from the patient’s initial IFX infusion. In addition, any patient who had undergone colectomy for UC, had discontinued infliximab due to loss of response, or required systemic steroids at the time of the 12-month visit, was considered treatment failures. “Escalation” was defined as either an increase in maintenance IFX to 10mg/kg at least every 8 weeks, or 5mg/kg every 4-6 weeks. Azathioprine or mercaptopurine use continued after the initiation of IFX was considered “concomitant”. Data on endoscopic / histologic healing after therapy was not uniformly recorded using standardized criteria, and therefore excluded as an end-point.
Outcomes
The primary outcome of interest was clinical remission at 12 months. The secondary outcome was cumulative rate of colectomy during the follow-up period. An intention-to-treat analysis was used for efficacy outcomes.
Statistical analysis
Categorical data was analyzed using the Pearson’s Chi-square test, or Fisher’s exact test if any cell number was <5, for frequencies. Continuous data was analyzed using Student’s t-test if normally distributed or Wilcoxon’s rank sum test if non-normal data. Missing data was censored for the primary analysis, but a separate analysis was performed assuming missing patients were all in remission, or all not in remission to assess if missing data biased the results to the null hypothesis. For the end-point of 5 remission at 12 months, a nominal logistic regression model was created using significantly-associated variables (p<0.1), and the odds ratio for those variables that remained significant (p<0.05) in the model determined. Predictors of colectomy from baseline demographic, disease characteristics and treatment were performed using Cox proportional hazards analysis. Time to colectomy was summarized as the cumulative incidence of colectomy through 12 months estimated by the Kaplan-Meier method; groups were compared using the log-rank test stratified by study. Failure plots were used for figures (cumulative probability of colectomy over time).
JMP software was used for statistical analysis (SAS Institute, Cary, NC). For retrospective power calculations, PS Power and Sample Size Calculations version 2.1.31, 2004 (Department of Biostatistics, Vanderbilt University) was used.
Results
Sixty-two patients who received IFX for UC, and had complete records at our institution, were identified for analysis. The outcomes of this cohort are highlighted in Figure 1. Fifty-six of these were ambulatory out-patients with moderate-severe disease, and the other 6 received IFX during a hospitalization for steroid-refractory colitis. After initial induction IFX to treat UC, 50/56 ambulatory patients (89%) responded and proceeded to maintenance therapy during the study period – this group constituted the study cohort. In contrast, only 2/6 (33%) severe, steroid-refractory patients had a clinical response noted.
Figure 1.
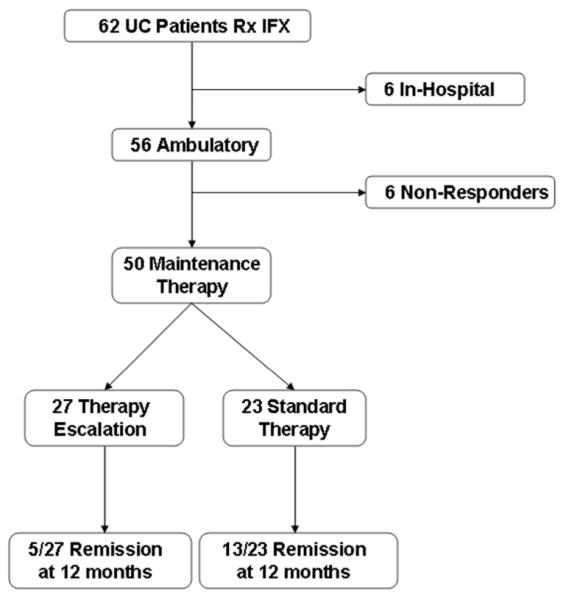
CONSORT flow chart of outcomes of patients in cohort from baseline
The characteristics of the maintenance cohort, detailed in Table 1, were similar to those in the ACT2 trial (5). They had UC for a mean of 8 years, had an equal mix of left-sided (48%) and extensive (52%) disease geography, and mean CRP and ESR were 28 and 37 respectively at the time of initiation of IFX. The majority (41/50, 80%) had exposure to azathioprine / mercaptopurine; 19/41 of these (46%) were still taking azathioprine, but 20/41 patients (49%) had discontinued due to adverse effects. Those still taking azathioprine had a mean of 12 months continuous therapy prior to initiation of IFX. Twenty-seven patients (54%) were on concomitant steroids, mostly (18/27) at oral doses of prednisone >20mg per day when IFX was initiated, and had steroid exposure for a median of 3 months. This cohort represents the typical ambulatory patient with moderate-severe UC seen in our center.
Table 1.
Characteristics of patients enrolled in the study
| Characteristic | Number |
|---|---|
| Male (%) | 53 |
| Mean age in years (SEM) | 41 (2) |
| Mean disease duration in years (SEM) |
8 (1) |
| Duration of disease > 2 years at first IFX (%) |
64 |
| Current smoker (%) | 2 |
| Disease Geography | |
| Distal to splenic flexure (%) | 48 |
| Extensive colitis (%) | 52 |
| Concurrent medications | |
| Steroids (%) | 54 |
| Azathioprine / mercaptopurine (%) | 37 |
| Mean pre-Infliximab ESR (SEM) | 37 (7) |
| Mean pre-Infliximab CRP (SEM) | 28 (11) |
During maintenance therapy, this cohort received a mean of 11 scheduled infusions of IFX (SEM 1), and had a mean total follow-up of 38 months (SEM 4). Infusion history for the entire maintenance cohort is summarized in Table 2. Mean CRP was 28 (SEM 10) before starting IFX, and 11 (SEM 3) at 12 months. Five patients (9%) had a reaction to IFX, which were mostly mild (4/5) and acute (3/5). Only 2 patients had to discontinue IFX due to side-effects.
Table 2.
Infusion history of patients with UC on maintenance infliximab
| Therapy History | N=50 |
|---|---|
| Mean months on IFX (SEM) | 14 (4) |
| Mean number of infusions | 11 (1) |
| Mean months of follow-up (SEM) | 38 (4) |
| Infusion reactions | 9% |
| IFX stopped due to ADRs | 3% |
mo, months; ADRs, adverse drug reactions
IFX therapy escalation was required in 27/50 patients (54%) over time, after an average of 6 maintenance infusions. The baseline demographic and clinical characteristics of patients in both escalation and non-escalation groups at inception were similar (data not shown). In a univariate analysis, patients who required dose escalation had a longer mean duration of UC at initiation of IFX than those who did not require escalation (10.4 vs 6 years, p=0.03 by t-test). No other individual demographic or clinical factor was significantly associated with the need for dose escalation in this cohort. Patients who required dose escalation received a mean total number of 12 infusions, and had 34 months of follow-up.
Clinical remission was noted in 36% (18/50) at 12 months in the entire cohort (Table 3). Therapy-escalation patients had a remission rate of 19% at 12 months, compared with 56% in the non-escalation group. Neither concomitant therapy with azathioprine / mercaptopurine, pre-treatment CRP or ESR levels, or drop in CRP were significantly associated with the probability of clinical remission at 12 months. Patients who required IFX escalation were less likely to be in clinical remission at 12 months (OR 0.2, 95% CI 0.1-0.6, p=0.01) when compared to those who did not require escalation.
Table 3.
Clinical Outcomes of patients with moderate UC on maintenance infliximab
| Therapy Outcomes | Non-DE | DE |
|---|---|---|
| Clinical Remission at 12 months | 54% | 19% |
| Colectomy | 21% | 33% |
DE; dose-escalation
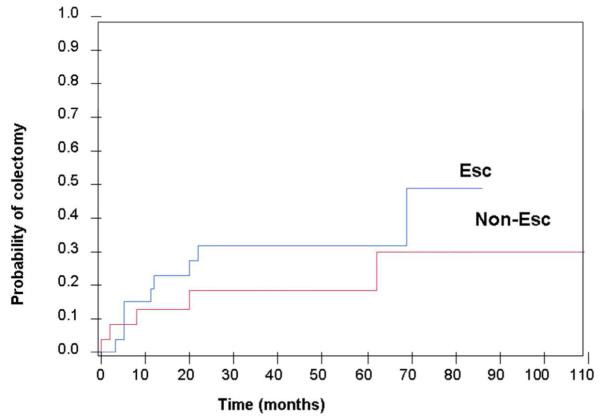
Overall, 27% of all patients with moderate UC who received maintenance IFX therapy required a colectomy during follow-up; 33% in the escalation group and 21% in the non-escalation group. The mean time to colectomy was 17 months (SEM 6) in the cohort. In a Kaplan-Meier analysis, the cumulative risk of colectomy was not significantly different between the escalation and non-escalation groups (Figure 2). In a multivariate Cox proportional hazards model, disease duration > 2 years was associated with a reduced risk of colectomy (HR 0.3, 95% CI 0.1-1.0, p=0.04).
Figure 2.
Kaplan Meier failure plot of cumulative colectomy rate grouped according to escalation (blue line) / non-escalation (red line)
Discussion
Infliximab has joined azathioprine / mercaptopurine as an appropriate therapeutic choice for induction and maintenance of remission in patients with moderate-severe UC (16). Much of the literature on outcomes with this agent in clinical practice has been reported in hospitalized patients with severe UC, or a mix of patients with moderate and severe disease (2,4,17). As the experience with infliximab has grown in ambulatory patients, a number of studies have reported high rates of therapy escalation during maintenance treatment (4,11,17).
In our cohort, at least half the patients required escalation of their infliximab to shorter infusion cycles, or higher doses, due to relapse of symptoms. Our results are similar to a Canadian cohort of patients with moderate-severe UC which included both hospitalized and ambulatory patients; 58% required dose interval shortening, and 21% required dose increases in this cohort over a median of 13 months (11). Infliximab escalation was also required in 16/117 patients (14%) in the open-label extension of the ACT studies, and 36/80 patients (45%) in a large French cohort (4,18). Data from US community practices suggests that up to 40% of patients with UC require shortening of their infusion cycles, which is more common than occurs in patients with Crohn’s 6 disease (19). A number of explanations for secondary loss of response to infliximab have been proposed, including antibodies to infliximab (ATIs), differences in clearance, and innate anti-TNF antibodies (8). This increase in use of infliximab has significant implications in terms of costs and cumulative exposure to infliximab.
Long-term remission and colectomy rates in patients with UC who require infliximab escalation, and how they compare to non-escalation patients, have not previously been described. Our data suggests that patients who require IFX escalation ultimately have numerically lower remission rates and higher colectomy rates over time. The colectomy rate in our therapy-escalation patients (33%) is higher than that reported in cohorts of patients who could be maintained on stable doses of infliximab (17-18%) (4,6). However, it is similar to the colectomy rates (30-40%) reported in cohorts that included hospitalized patients with more severe disease who received infliximab (11,20,21). The experience in patients with Crohn’s disease is that IFX therapy escalation regains response in most patients in the short term (22,23). However, longer term data suggests that a further proportion of these will lose response even to high-dose IFX (9,24). A cost-utility analysis concluded that IFX escalation was less cost-7 effective than other strategies in managing refractory UC (25).
We also identified disease duration as a risk factor for colectomy in this cohort. Patient with disease duration > 2 years were less likely to require a colectomy during follow-up. This is consistent with other natural history studies that the risk of colectomy is highest in the first year after diagnosis (26). It is likely that patients with rapidly progressive disease tend to proceed to colectomy at earlier times than those with more stable, chronic disease. This has implications for selection of therapy in patients with more severe disease. Surprisingly, concomitant azathioprine / mercaptopurine use did not influence colectomy or 12-month remission rates in this cohort. Although prospective studies in IBD have demonstrated superior efficacy of the IFX / azathioprine combination therapy in azathioprine-naïve patients, outcomes in cohorts with many azathioprine-experienced patients were more equivocal (5,13-15,27). The clinical benefits of continuing azathioprine when a patient with UC has failed it, and required the addition of IFX, may be smaller than those seen with the introduction of combination therapy in an azathioprine-naïve patient. This needs to be weighed against the risks of infection and lymphoma for each patient (28).
There are a number of limitations to this study. Firstly, the numbers in each group for analysis of proportions was small, and may have been under-powered to detect differences that may have been present in a larger cohort (type II error). Secondly, since data capture was retrospective, we were unable to provide disease activity scores for each patient to define remission or response as in clinical trials. Despite this, the comprehensive nature of our EHR allowed for review of all relevant clinical, infusion and surgical visits and consults for each patient, thus minimizing the possibility of missing clinically-relevant outcomes.
In conclusion, we have identified a high requirement for IFX maintenance therapy escalation in a well-characterized cohort of ambulatory patients with moderate-severe UC. The implications of the frequent need for escalation in this group warrants further study.
Acknowledgements
We would like to acknowledge the assistance of the pheresis nurses and OMR support staff in collecting patient data.
Footnotes
Authors’ declaration of personal interests
ACM has received research funding from Salix and Shire, and has served as an advisory board member for UCB and Abbott.
ASC has served as an advisory board member for UCB and Abbott, and speaker for Centocor.
Declaration of funding interests
ACM is supported by NIH grant K23DK084338
Some data presented at American College of Gastroenterology Annual Scientific Meeting, October 15-20 2010, San Antonio, Texas
References
- 1.Moss AC, Peppercorn MA. Steroid-refractory severe ulcerative colitis: what are theavailable treatment options? Drugs. 2008;68:1157–1167. doi: 10.2165/00003495-200868090-00001. [DOI] [PubMed] [Google Scholar]
- 2.Gies N, Kroeker KI, Wong K, Fedorak RN. Treatment of ulcerative colitis with adalimumabor infliximab: long-term follow-up of a single-centre cohort. Aliment.Pharmacol.Ther. 2010;32:522–528. doi: 10.1111/j.1365-2036.2010.04380.x. [DOI] [PubMed] [Google Scholar]
- 3.Gisbert JP, Gonzalez-Lama Y, Mate J. Systematic review: Infliximab therapy in ulcerativecolitis. Aliment.Pharmacol.Ther. 2007;25:19–37. doi: 10.1111/j.1365-2036.2006.03131.x. [DOI] [PubMed] [Google Scholar]
- 4.Oussalah A, Evesque L, Laharie D, Roblin X, Boschetti G, Nancey S, Filippi J, Flourie B, Hebuterne X, Bigard MA, Peyrin-Biroulet L. A multicenter experience with infliximab forulcerative colitis: outcomes and predictors of response, optimization, colectomy, andhospitalization. Am.J Gastroenterol. 2010;105:2617–2625. doi: 10.1038/ajg.2010.345. [DOI] [PubMed] [Google Scholar]
- 5.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N.Engl.J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Rutgeerts P, Feagan BG, Reinisch W, Olson A, Johanns J, Lu J, Horgan K, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Colectomy rate comparison after treatment of ulcerative colitis with placeboor infliximab. Gastroenterology. 2009;137:1250–1260. doi: 10.1053/j.gastro.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Zabana Y, Domenech E, Manosa M, Garcia-Planella E, Bernal I, Cabre E, Gassull MA. Infliximab safety profile and long-term applicability in inflammatory bowel disease: 9-yearexperience in clinical practice. Aliment.Pharmacol.Ther. 2010;31:553–560. doi: 10.1111/j.1365-2036.2009.04206.x. [DOI] [PubMed] [Google Scholar]
- 8.Danese S, Fiorino G, Reinisch W. Review article: Causative factors and the clinicalmanagement of patients with Crohn’s disease who lose response to anti-TNF-alpha therapy. Aliment.Pharmacol.Ther. 2011;34:1–10. doi: 10.1111/j.1365-2036.2011.04679.x. [DOI] [PubMed] [Google Scholar]
- 9.Chaparro M, Martinez-Montiel P, Van Domselaar M. Intensification of infliximab (IFX)therapy in Crohn’s disease: efficacy and safety. Gastroenterology. 2008;134(S1):A664. doi: 10.1016/j.crohns.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan GG, Hur C, Korzenik J, Sands BE. Infliximab dose escalation vs. initiation ofadalimumab for loss of response in Crohn’s disease: a cost-effectiveness analysis. Aliment.Pharmacol.Ther. 2007;26:1509–1520. doi: 10.1111/j.1365-2036.2007.03548.x. [DOI] [PubMed] [Google Scholar]
- 11.Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Troughserum infliximab: a predictive factor of clinical outcome for infliximab treatment in acuteulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 12.Lawlor G, Moss AC. Cause for controversy? Infliximab in the treatment of ulcerative colitis:an update. Clin.Exp.Gastroenterol. 2009;2:149–161. [PMC free article] [PubMed] [Google Scholar]
- 13.Panccione R, Ghosh S, Middleton S, Marquez J, Khalif I, Flint L, van Hoogstraten H, Zheng H, Danese S, Rutgeerts P. Infliximab, Azathioprine, or Infliximab plus Azathioprine forTreatment of Moderate to Severe Ulcerative Colitis: The UC Success Trial. Gastroenterology. 2011;140(5):S134. [Google Scholar]
- 14.Lichtenstein GR, Diamond RH, Wagner CL, Fasanmade AA, Olson AD, Marano CW, Johanns J, Lang Y, Sandborn WJ. Clinical trial: benefits and risks of immunomodulatorsand maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment.Pharmacol.Ther. 2009;30:210–226. doi: 10.1111/j.1365-2036.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- 15.Moss AC, Kim KJ, Fernandez-Becker N, Cury D, Cheifetz AS. Impact of concomitantimmunomodulator use on long-term outcomes in patients receiving scheduled maintenanceinfliximab. Dig.Dis.Sci. 2010;55:1413–1420. doi: 10.1007/s10620-009-0856-7. [DOI] [PubMed] [Google Scholar]
- 16.Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S. Guidelines for the management ofinflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 17.Mortensen C, Caspersen S, Christensen NL, Svenningsen L, Thorsgaard N, Christensen LA, Bendtsen F. Treatment of acute ulcerative colitis with infliximab, a retrospective studyfrom three Danish hospitals. J Crohns.Colitis. 2011;5:28–33. doi: 10.1016/j.crohns.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Reinisch W, Sandborn WJ, Rutgeerts P, Feagan BG, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Blank M, Lang Y, Johanns J, Colombel JF, Present D, Sands BE. Long-term infliximab maintenance therapy for ulcerative colitis: The ACT-1 and extension studies. Inflamm.Bowel.Dis. 2011 doi: 10.1002/ibd.21697. [DOI] [PubMed] [Google Scholar]
- 19.Waters H, Vanderpoel J, McKenzie S, Lunacsek O, Franklin M, Lennert B, Goff J, Augustyn DH. Stability of infliximab dosing in inflammatory bowel disease: results from a multicenterUS chart review. J Med.Econ. 2011;14:397–402. doi: 10.3111/13696998.2011.583152. [DOI] [PubMed] [Google Scholar]
- 20.Jarnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, Granno C, Vilien M, Strom M, Danielsson A, Verbaan H, Hellstrom PM, Magnuson A, Curman B. Infliximab as rescuetherapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlledstudy. Gastroenterology. 2005;128:1805–1811. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Filippi J, Allen PB, Hebuterne X, Peyrin-Biroulet L. Does anti-TNF therapy reduce therequirement for surgery in ulcerative colitis? A systematic review. Curr.Drug Targets. 2011;12:1440–1447. doi: 10.2174/138945011796818153. [DOI] [PubMed] [Google Scholar]
- 22.Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification inCrohn’s disease: a review. Am.J Gastroenterol. 2009;104:760–767. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 23.Kopylov U, Mantzaris GJ, Katsanos KH, Reenaers C, Ellul P, Rahier JF, Israeli E, Lakatos PL, Fiorino G, Cesarini M, Tsianos EV, Louis E, Ben Horin S. The efficacy of shortening thedosing interval to once every six weeks in Crohn’s patients losing response to maintenancedose of infliximab. Aliment.Pharmacol.Ther. 2011;33:349–357. doi: 10.1111/j.1365-2036.2010.04523.x. [DOI] [PubMed] [Google Scholar]
- 24.Regueiro M, Siemanowski B, Kip KE, Plevy S. Infliximab dose intensification in Crohn’sdisease. Inflamm.Bowel.Dis. 2007;13:1093–1099. doi: 10.1002/ibd.20177. [DOI] [PubMed] [Google Scholar]
- 25.Xie F, Blackhouse G, Assasi N, Gaebel K, Robertson D, Goeree R. Cost-utility analysis ofinfliximab and adalimumab for refractory ulcerative colitis. Cost.Eff.Resour.Alloc. 2009;7:20. doi: 10.1186/1478-7547-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression ofulcerative colitis. A long-term follow-up of 1116 patients. Dig.Dis.Sci. 1993;38:1137–1146. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 27.Sokol H, Seksik P, Carrat F, Nion-Larmurier I, Vienne A, Beaugerie L, Cosnes J. Usefulness of co-treatment with immunomodulators in patients with inflammatory boweldisease treated with scheduled infliximab maintenance therapy. Gut. 2010;59:1363–1368. doi: 10.1136/gut.2010.212712. [DOI] [PubMed] [Google Scholar]
- 28.Melmed GY, Spiegel BM, Bressler B, Cheifetz AS, Devlin SM, Harrell LE, Irving PM, Jones J, Kaplan GG, Kozuch PL, Velayos FS, Baidoo L, Sparrow MP, Siegel CA. Theappropriateness of concomitant immunomodulators with anti-tumor necrosis factor agentsfor Crohn’s disease: one size does not fit all. Clin.Gastroenterol.Hepatol. 2010;8:655–659. doi: 10.1016/j.cgh.2010.04.023. [DOI] [PubMed] [Google Scholar]