Abstract
The purpose of this phase I dose-finding randomized controlled trial was to evaluate the safe and effective dose of isoflavones to be used in future clinical trials for prostate cancer prevention. Forty-five eligible men were supplemented with 40, 60, and 80 mg of purified isoflavones or no supplement from biopsy to prostatectomy. Compliance with the study agent, toxicity, and changes in plasma isoflavones, serum steroid hormones, prostate-specific antigen (PSA), and tissue Ki-67 were analyzed from baseline to completion of the study. Forty-four subjects completed the study with a duration of intervention of 30 (± 3) days. We observed significant increases in plasma isoflavones with treatment for all doses compared with controls without producing any toxicity. Significant increases in serum total estradiol were observed in the 40 and 60 mg isoflavone-treated arms. However, a significant increase in serum free testosterone was observed in the 60 mg isoflavone-treated arm. Changes in serum sex hormone–binding globulin, PSA, and percentage of tissue Ki-67 were not statistically significant with treatment for this sample size and duration of intervention. Our results identify a safe dose of purified isoflavones for future clinical trials and establish the need for further definitive, well-powered trials to examine the role of isoflavones in prostate carcinogenesis.
Keywords: isoflavones, Ki-67, prostate cancer, prostate-specific antigen, steroid hormones
The American Cancer Society estimates that there will be about 186,320 new cases of prostate cancer (CaP) in the United States in 2008, and about 28,660 men will die of this disease.1 CaP remains the most common malignancy and the second leading cause of cancer death among men in the United States.1 The initiation and progression of CaP are a multistep process including several intermediate steps and may involve a complex series of both exogenous and endogenous factors.2–4 Although it is clear that clinical CaP incidence and mortality vary greatly between populations, the frequency of latent CaP is evenly distributed among populations, suggesting that external factors such as diet and other lifestyle factors are important in the transformation from latent into more aggressive clinical cancer.2–5 Epidemiologic and laboratory studies and a few pilot clinical trials have demonstrated that several nutrients, including isoflavones, could induce apoptosis and suppress the formation and growth of human cancers, including CaP.6–8 Epidemiologic studies have consistently reported a lower incidence of clinically evident disease in populations consuming isoflavones. An inverse relationship between dietary intake, plasma and prostatic fluid concentrations of isoflavones, and the incidence of CaP has been observed in these populations, demonstrating the potential role of isoflavones in mediating epigenetic effects.7–9 Thus, the potential preventive properties of isoflavones in CaP, as demonstrated by evidence from epidemiologic studies, although limited, appear promising.
In vitro data have consistently shown that genistein modulates cell proliferation, angiogenesis, tumor cell invasion and tumor metastasis, cell cycle regulation, antioxidants, and induction of apoptotic cell death,10–12 indicating that purified isoflavones are promising chemopreventive agents. Specifically, our previous data indicated that similar to bortezomib and velcade (PS-341), purified isoflavone, genistein, is a potent proteasome inhibitor, and the genistein-induced proteasome inhibition was accompanied by induction of apoptosis in the cell lines.13 Phase I trials have demonstrated the clinical characteristics, pharmacokinetics, and safety of whole soy and purified isoflavones with single- and multiple-dose administration in healthy, early-stage, or treated cancer patient cohorts14–17 with doses of purified soy isoflavones ranging from 1 to 16 mg/kg body weight, where some of the doses were higher than those previously administered to humans as whole soy proteins, without significant clinical toxicity. A few pilot phase I dose-finding clinical trials, including our study, have demonstrated a trend toward stabilization of or a reduction in serum prostate-specific antigen (PSA) with short-term isoflavone supplementation in CaP patient populations, without significant clinical toxicity6,14–16,18–22 with the exception of gastrointestinal symptoms.
The epidemiologic, laboratory, and early phase I and II clinical trials appear promising. Although there may be multiple pathways by which isoflavones can impact CaP progression, our central hypothesis is that the effect of supplementation with a constant dose of purified isoflavones (versus a placebo) will produce a corresponding increase in plasma levels of isoflavones, resulting in modulation in serum steroid hormone levels, indicated by an increase in serum sex hormone–binding globulin (SHBG) and estradiol and a decrease in free testosterone, thereby potentially contributing to a decrease in or stabilization of disease progression in men diagnosed with localized CaP, as indicated by a decreasing surrogate marker of proliferation, serum PSA and Ki-67% expression. The specific aim of the phase I dose-finding randomized controlled trial was to recruit and randomize men with clinically localized CaP to receive various doses (40, 60, and 80 mg/d) of purified isoflavones (Prevastein HC) versus a control arm consuming a diet without this supplement in the presurgical period prior to radical prostatectomy and to observe the effectiveness of the study agent in producing an increase in (a) plasma levels of isoflavones and corresponding (b) modulation of steroid hormones (a decrease in free testosterone, an increase in SHBG and total estradiol) and (c) reduction in or stabilization of a surrogate marker of proliferation, serum total PSA, and (d) reduction in or stabilization of tissue Ki-67, a nuclear antigen and molecular marker known to be associated with the progression of cancer. In addition, our aim was to evaluate (e) compliance with the study agent and (f) dose-related toxicity. Our overall goal was to determine the best dose of isoflavones that could be used in a well-powered, future phase I dose-finding randomized placebo-controlled clinical trial to examine the safety and effectiveness of isoflavones, in addition to exploring the potential mechanism of action of isoflavones in prostate carcinogenesis.
Materials and Methods
This was a randomized controlled clinical trial conducted in a cohort of men recruited from member institutions of the Moffitt Community Clinical Oncology Program (CCOP) Research Base and approved by the Institutional Review Boards at these institutions. Men between the ages of 45 and 80 years with clinically localized CaP, based on pathologic assessment from biopsy specimens, with no previous or current therapy for CaP or a history of cancer except nonmelanoma skin cancer, who were scheduled for prostatectomy between 30 (± 3) days after registration were eligible. Patients who received neoadjuvant hormonal therapy, vegans, and/or nutritional supplement users, as ascertained by baseline food records, were excluded from the study. Subjects with a known history of hepatic and/or renal disease, prostatitis, and urinary tract infection within 30 days of registration or with a body mass index (BMI) > 32 kg/m2 were excluded from the study.
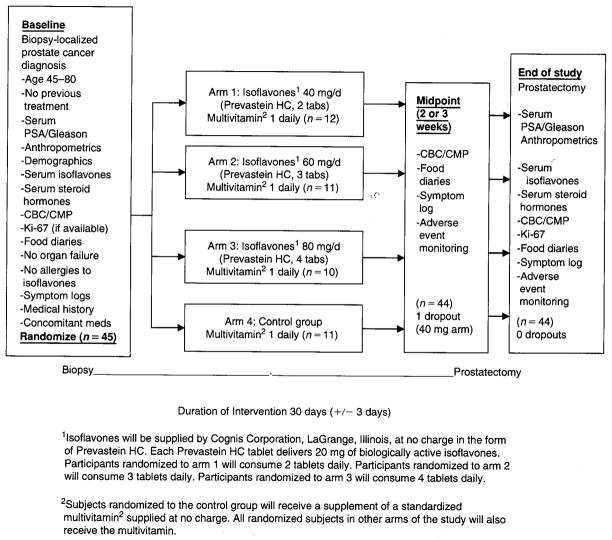
On determination of eligibility and the granting of informed consent, patients were registered using a telephone-based registration and randomization system21 determined by a preset algorithm. These assignments were stratified by Gleason score (2–4; 5–7; and 8–10). Subjects were assigned to one of four arms; the intervention arm provided supplementation with isoflavones (Prevastein HC) supplied by Cognis Corporation (LaGrange, IL). Prevastein HC is a botanical test compound containing the glycoside forms of isoflavones (genistein, daidzein, glycetin) with a ratio similar to that found in soy foods. Each tablet contains 40% soy isoflavones in the aglycone form. Participants were randomized to arms 1 to 3 and instructed to consume one (arm 1, 40 mg), two (arm 2, 60 mg), or three (arm 3, 80 mg) capsules daily. Subjects randomized to the control group received no supplementation. To avoid possible confounding owing to vitamin or mineral deficiencies and prevent the use of other nonstandardized supplements, which may contain isoflavones or other large doses of antioxidants, a standard formulation containing 100% US Recommended Dietary Allowance for vitamins (PBA Multi Vita, Pan American Lab Company, Miami, FL) was provided by the investigators to subjects in all groups, including the control group, during the entire study period. The duration of the intervention was from biopsy to prostatectomy (30 ± 3 days), depending on the duration between these procedures (Figure 1).
Figure 1.
Results of a randomized phase I dose-finding trial of several doses of isoflavones in men with localized prostate cancer: administration prior to radical prostatectomy. CBC = complete blood count; CMP = comprehensive metabolic panel; PSA = prostate-specific antigen.
During their first visit, subjects provided baseline demographic anthropometric data; medical and family histories of cancer, alcohol, and tobacco use; and a nutritional history including nutritional supplement use. Participants were instructed to complete a weekly 2-day food record of all foods, beverages, and nutritional supplements consumed, to verify compliance with the diet and the instruction to avoid isoflavone-rich foods. Diet records were analyzed using the University of Minnesota Nutrition Data System-Research version for analysis of nutrient composition at the Arizona Diet and Behavioral Assessment Center (<http://www.azdiet-behavior.azcc.arizona.edu>). Participants completed a daily study agent intake and symptom log. Weekly logs and food records were mailed to the research office. Two visits to the research office at baseline and at completion of the study on the day of prostatectomy were required. All other weekly monitoring occurred via telephone and mail. An 85% compliance rate with supplements and completion of intake and symptom logs were required. Nonfasting blood samples were drawn for analysis at the baseline visit and the end of the study visit of (a) serum PSA (baseline and end of study); (b) serum free testosterone, total estradiol, and SHBG (extraction, chromatography, radioimmunoassay) at baseline and the end of the study; (c) safety markers, including comprehensive metabolic panel (spectrophotometry, ion-selective electrode hexokinase) and a complete blood count (electronic cell sizing sorting cytometry/microscopy) at LabCorp Diagnostic Laboratories in Tampa, Florida; and (d) analysis of plasma total isoflavones using high-performance liquid chromatography, an extraction procedure developed by Craft Technologies, Inc (Wilson, NC). Immunohistochemistry (Ki-67) was performed on paraffin-embedded sections from prostate biopsies and prostatectomy tissues with prostate adenocarcinoma for evaluation of change in proliferation. Ki-67 antibody (clone MIB-1, DakoCytomation, Carpinteria, CA) was used at a dilution of 1:50. To enhance antigen retrieval, citrate buffer was used. The antigen antibody reaction was detected using DAB chromogen. The change in the percentage of Ki-67-positive tumor cells was evaluated. Although we had initially planned to evaluate other tissue biomarkers, including apoptosis, owing to competition for a representative prostate tissue sample from biopsy cores (diagnosis vs research), we used an order of biomarkers to be examined (Ki-67, apoptotic index).
To evaluate and ensure subject safety, any change in medical condition and use of concomitant medications were monitored throughout the study period. All safety and compliance data were collected at baseline and the end of the study period. All adverse events were reported on the adverse event case report form regardless of whether they were related to the study drug. The severity of the events was determined using the National Cancer Institute Common Terminology Criteria for Adverse Events version 2.0 (<http://ctep.info.nih.gov>). All adverse events, including laboratory abnormalities, that in the opinion of the study physicians were clinically significant were followed according to good medical practices and documented. Adverse events were reported to the Moffitt CCOP Research Base Operations Center, the Food and Drug Administration, and Institutional Review Boards.
Descriptive statistics were generated for variables assessed at the baseline visit, which include race, ethnicity, age, weight, height, BMI, family history of cancer, history of smoking, history of alcohol use, and other relevant medical history. Descriptive statistics provide a detailed characterization of the study population. At the conclusion of the study, analysis of variance was used to compare the four group mean changes in plasma isoflavone levels, serum steroid hormone levels, total PSA, and tissue Ki-67 expression. Dunnett tests for comparisons of multiple treatment arms against the control were also performed, and 95% confidence intervals for the differences in these group mean changes were formed. In addition, paired t-tests were used to compare posttreatment versus pretreatment changes for each group for plasma isoflavone concentrations, serum steroid hormones, and serum total PSA levels. Furthermore, we evaluated the dose response of isoflavones using a multiple linear regression model. The serum measures at the end point were modeled as the dependent variable, including the baseline measures and dose as covariates in the model. The quadratic models were created to determine if there were any nonlinear effects. In addition, we examined the possible role of age, smoking, alcohol use, family history of cancer, presence of high-grade prostatic intraepithelial neoplasia, stage of disease, and history of benign prostatic hyperplasia in changing the plasma levels of isoflavones.
Finally, the correlation between treatment-associated changes in plasma isoflavones and changes in the surrogate marker of disease progression (serum PSA) was obtained by the Spearman nonparametric correlation method. Prior to unblinding, the incidence of toxicity was evaluated. Although compliance was monitored, we employed the intent-to-treat principle in all group comparisons. Subjects were analyzed according to the group into which they were randomized without regard to compliance or actual diet.
Tests of significance were two-tailed, and a probability (p) value < .05 was considered statistically significant. Statistical analyses were performed with SAS version 9 (SAS Institute, Cary, NC).
Results
Of men diagnosed with localized CaP, between the years 2003 and 2005, 45 men met the eligibility criteria and were consecutively admitted to the study. Forty-four men completed the study and were able to provide complete data pre- and posttreatment, including serum and plasma for analysis. One subject in the 40 mg dose group dropped out of the study, and no specific reason was provided by the subject. A 97.7% participant retention rate was achieved in the subjects recruited.
Initial comparison of baseline demographic variables such as age and race; anthropometric measurements such as height, weight, and BMI; smoking history; family history of cancer; and personal history of benign prostatic hyperplasia is displayed in Table 1. Although no significant differences were observed in the four groups on these variables, notably, 93% of the subjects had a family history of cancer, 82% had a history of benign prostatic hyperplasia, and 57% of CaP patients in both groups were former or current smokers and had a mean BMI > 26.
Table 1.
Demographic Characteristics of Subjects at Baseline
| Characteristics | Isoflavones
|
Control Group | All Subjects | ||
|---|---|---|---|---|---|
| 40 mg | 60 mg | 80 mg | |||
| n | 12 | 11 | 10 | 11 | 44 |
| Age (yr), mean (SD) | 59.90 (7.14) | 58.96 (−6.41) | 58.66 (7.14) | 60.30 (6.54) | 59.48 (6.61) |
| Weight (kg), mean (SD) | 87.93(11.85) | 85.55 (±9.82) | 91.25 (±6.4) | 82.52 (±10.03) | 86.53 (9.95) |
| Height (m), mean (SD) | 1.81 (0.06) | 1.78 (0.05) | 1.82 (0.05) | 1.77 (0.08) | 1.80 (0.06) |
| BMI (kg/m2), mean (SD) | 26.69 (3.25) | 26.79 (2.03) | 27.47(1.91) | 26.24 (1.95) | 26.78 (2.32) |
| Ethnicity, n (%) | |||||
| Hispanic | 1 (8.33) | 2(18.18) | 0 (0.00) | 1 (8.33) | 4 (9.09) |
| Non-Hispanic | 11(91.67) | 9(81.82) | 10 (100.00) | 11(91.67) | 40 (90.91) |
| Race, n (%) | |||||
| White | 11 (91.67) | 11 (100.00) | 9(90.00) | 11 (100.00) | 42 (95.45) |
| Black | 0 (0.00) | 0 (0.00) | 1 (10.00) | 0 (0.00) | 1 (2.27) |
| Other | 1 (8.33) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (2.27) |
| Former or current smoker, n (%) | |||||
| Yes | 4 (33.33) | 8(72.73) | 6(60.00) | 7 (63.64) | 25 (56.82) |
| No | 8 (66.67) | 3 (27.27) | 4 (40.00) | 4 (36.36) | 19 (43.18) |
| Former or current alcohol user, n (%) | |||||
| Yes | 8 (66.67) | 8(72.73) | 8(80.00) | 6 (54.55) | 30 (68.18) |
| No | 4 (33.33) | 3 (27.27) | 2 (20.00) | 5 (45.45) | 14 (31.82) |
| Family history of cancer, n (%) | |||||
| Yes | 12 (100.00) | 10 (90.91) | 10 (100.00) | 9(81.82) | 41 (93.18) |
| No | 0 (0.00) | 1 (9.09) | 0 (0.00) | 2(18.18) | 3 (6.82) |
| History of benign prostatic hyperplasia, n (%) | |||||
| Yes | 11(91.67) | 9 (81.82) | 7 (70.00) | 9(81.82) | 36 (81.82) |
| No | 1 (8.33) | 2(18.18) | 3 (30.00) | 2(18.18) | 8(18.18) |
Subjects in both groups reported similar average intake of macronutrients and micronutrients at baseline. Changes in nutritional intake between treatment groups and the control group did not differ significantly. No significant changes in anthropometric variables such as weight and BMI were observed during the study period. As noted in Table 2, significant increases in plasma daidzein and genistein were observed from baseline to the end of the study period in the 40 mg and 60 mg isoflavone-treated groups. However, although significant increases in plasma genistein were observed in the 80 mg isoflavone-treated arm, diadzein levels failed to increase significantly. Overall, an increase in plasma isoflavones was observed in all treated arms compared with controls, even in this short duration (30 days ± 2) of intervention.
Table 2.
Plasma Isoflavone Pretreatment versus Posttreatment by Treatment Group
| Variable | Isoflavones
|
Control Group (n = 8)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 mg (n = 10)
|
60 mg (n = 10)
|
80 mg (n = 10)
|
||||||||||
| Pretreatment | Posttreatment | p* | Pretreatment | Posttreatment | p* | Pretreatment | Posttreatment | p* | Pretreatment | Posttreatment | p* | |
| Daidzein μmol/L | 0.022(0.011) | 0.100(0.063) | .014 | 0.019 (0.008) | 0.084 (0.073) | .027 | 0.025(0.020) | 0.089(0.111) | .102 | 0.031 (0.029) | 0.017 (0.006) | .196 |
| Genistein μmol/L | 0.021 (0.002) | 0.222 (0.130) | .002 | 0.018 (0.008) | 0.273 (0.229) | .007 | 0.021 (0.046) | 0.171 (0.186) | .029 | 0.025 (0.021) | 0.020 (0.014) | .492 |
Data are presented as mean (SD).
Paired t-test comparing the mean at baseline versus at the final week within each group.
The baseline and final concentrations of serum total PSA in the two groups are displayed in Table 3. Serum PSA remained stable in all isoflavone-supplemented groups, with no significant increase compared with controls. The baseline and final concentrations of serum free testosterone, SHBG, and total estradiol for the two groups are displayed in Table 3. Significant (p ≤ .05) increases in serum estradiol (27.83 pg/mL [SD 7.80] to 41.30 pg/mL [SD 12.47]) were observed in the 40 mg isoflavone-supplemented group during the study period. Serum total estradiol increased in the 60 mg isoflavone-treated arm, although no statistical significance was observed. Significant (p ≤ .005) mean increases in serum free testosterone were observed in subjects in the 60 mg isoflavone-treated group (9.88 pg/mL [SD 2.91] to 14.19 pg/mL [SD 3.37]). No significant changes in serum SHBG were observed for this duration of intervention and sample size.
Table 3.
Serum Prostate-Specific Antigen and Steroid Hormone Concentrations
| Variable | 40 mg (n = 12)
|
Isoflavones 60 mg (n = 11)
|
80 mg (n = 10)
|
Placebo Group (n = 11)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | Posttreatment | p* | Pretreatment | Posttreatment | p* | Pretreatment | Posttreatment | p* | Pretreatment | Posttreatment | p* | |
| Total PSA ng/dL | 4.88 (2.90) | 5.52 (2.92) | .173 | 6.12 (2.60) | 6.73 | .280 | 5.08 (2.58) | 5.16 (8.66) | .798 | 5.48 (3.38) | 5.12(1.86) | .734 |
| Total estradiol pmo/L | 27.83 (7.80) | 41.30 (2.47) | .023 | 28.18 (11.65) | 39.40 (18.19) | .176 | 39.50 (9.76) | 35.67 | .368 | 30.27 (12.52) | 38.40 (17.15) | .221 |
| SHBG nmol/L | 34.83 (11.95) | 35.30 (12.00) | .932 | 40.00 (9.24) | 43.10 (9.77) | .302 | 35.00 (14.31) | 37.20 (12.76) | .373 | 38.91 (16.48) | 38.09 (16.90) | .652 |
| Free testosterone (pg/mL) | 10.48 (3.00) | 11.81 (4.95) | .473 | 9.88 (2.91) | 14.19 (3.37) | .003 | 12.89 (4.40) | 13.38 (3.96) | .658 | 10.72 (2.44)) | 10.53 (3.44 | .849 |
PSA = prostate-specific antigen; SHBG = sex hormone-binding globulin.
Data are presented as mean (SD).
Paired t-test comparing the mean at baseline versus at final week within each group.
The results from the multivariate model with dose and baseline measures as the covariates are displayed in Table 4 and do not demonstrate any dose effect on any of the outcomes. Additionally, there was no nonlinear dose effect when the quadratic term was included in the model. However, the results indicate that the baseline measures were good predictors of the end point measures (p < .001), with the exception of serum total estradiol levels. The higher the levels of serum PSA, SHBG, and free testosterone at baseline, the greater were the values at the completion of the study.
Table 4.
Evaluation of Dose Response: Multiple Linear Regression Model
| Dependent Variable | Intercept
|
Baseline Measure
|
Dose
|
Dose*Dose
|
||||
|---|---|---|---|---|---|---|---|---|
| t Value | p Value | t Value | p Value | t Value | p Value | t Value | p Value | |
| PSA | −0.26 | .79 | 23.96 | < .01 | 0.47 | .64 | −0.12 | .90 |
| Total estradiol | 4.84 | < .01 | −0.16 | .87 | 0.59 | .56 | −0.69 | .50 |
| SHBG | 0.93 | .36 | 13.33 | < .01 | 0.22 | .83 | 0.17 | .87 |
| Free testosterone | 2.02 | .05 | 3.14 | < .01 | 1.50 | .14 | −1.04 | .31 |
PSA = prostate-specific antigen; SHBG = sex hormone-binding globulin.
Given that only postintervention tissue samples were available for staining, the differences between the treatment arms and control regarding the percentage of Ki-67 staining were estimated in these samples. Compared with the control group and other treatment arms, the 40 mg isoflavone-supplemented arm had the lowest percentage of cells expressing Ki-67 (Table 5), although this was not statistically significant for this sample size and duration of intervention.
Table 5.
Tissue Ki-67 Percentage (Postintervention) by Group
| Arm | Number of Observations | Mean Ki-67 (%) | SD |
|---|---|---|---|
| Isoflavone 40 mg | 12 | 3.20 | 2.25 |
| Isoflavone 60 mg | 11 | 4.11 | 3.52 |
| Isoflavone 80 mg | 10 | 4.63 | 2.67 |
| Control | 11 | 4.22 | 1.86 |
All anticipated and unanticipated, grades I to III, constitutional, dermatologic, gastrointestinal, metabolic, and pain symptoms of adverse events were documented on all subjects throughout the study period. Adverse events were grade I events with the exception of one grade II event reported by a subject in the 80 mg isoflavone-treated group, which was determined to be unrelated to the agent provided in the study. Grade I metabolic or laboratory changes in serum alanine transaminase (ALT), (The test is used to determine if a patient has liver damage. ALT is an enzyme involved in the metabolism of the amino acid alanine. ALT works in a number of tissues, but its highest concentration is in the liver. Injury to the liver results in release of the enzyme into the blood.) marginal elevations in lipase, amylase, phosphorus, and calcium were observed in one to two subjects in all groups and considered not related to the study agent. One subject in the 60 mg and one in the 80 mg isoflavone-supplemented arm reported grade I gastrointestinal adverse events, which were considered possibly related to the study agent.
Discussion
To our knowledge, our study was the first phase I dose-finding randomized placebo-controlled clinical trial of several doses of purified isoflavones in men with early-stage CaP to demonstrate significant increases in plasma concentrations of isoflavones, without producing clinical toxicity. In this early phase I dose-finding trial, we were able to demonstrate that in a cohort of men with localized disease, compliance with a multidose daily regimen of supplements and diet could be achieved while maintaining weekly diet and symptom records and attending follow-up visits, interviews, and blood draws to monitor compliance, safety, and toxicity, although these visits were not aligned with the normal surveillance visits. The retention rate of 97.7% indicates that this cohort was highly motivated and willing to participate in chemoprevention trials. Our study design focused on the importance of compliance with the study agent and diet regimen, a challenge inherent in all chemoprevention trials in this cohort. The following strategies were employed throughout the study period to assess and enhance compliance with and adherence to the study agent: (1) assessing adherence by pill count or returned pills at each visit, (2) completion of a daily study agent intake log to monitor and report intake of this supplement, and (3) measuring plasma levels of isoflavones at baseline and the end of the study. In addition, intake of other nutrients and compliance with instruction to avoid other supplements were monitored throughout the study using weekly two-24-hour dietary recalls and analyzing nutritional intake data using the frequently updated nutritional data system database. Subjects were provided with a comprehensive list of isoflavone-rich foods and instructed to avoid them during the entire period of this study. Compliance with this restriction was verified using data from diet recalls and interviews and reinforced at each visit. Subjects were instructed that 85% compliance with completion of the study agent and symptom log and intake of the study agent was required, although all subjects randomized were included in the final analysis. Compliance with the study agent is demonstrated by the increase in plasma daidzein and genistein, which was achieved in the isoflavone-treated groups compared with men in the placebo arm. In addition, compliance with dietary restriction of isoflavone-rich foods was demonstrated by the unchanged, low plasma levels of isoflavones observed in the control group.
Plasma levels of the isoflavones genistein and daidzein significantly increased in the 40 mg and 60 mg isoflavone-treated arms even with an average duration of intervention of 30 days. However, significant changes were observed only in plasma genistein in the 80 mg isoflavone-supplemented arm. It is interesting to note that increasing doses of isoflavones did not produce corresponding greater increases in plasma isoflavones. Given that compliance, as indicated by agent intake log and pill counts, did not demonstrate a decrease in compliance in the higher-dose groups, this observed relatively stable plasma concentration achieved even with a 40 mg dose may potentially be explained by other factors that have evolved in the recent research literature. Several epidemiologic studies have demonstrated a high correlation between increasing the dose of isoflavone intake with plasma, serum, and urinary metabolites of isoflavones.15,23,24 However, most of these studies have been limited to a single measurement of isoflavone intake and bioavailability. Variability in absorption of soy foods has been observed in human clinical trials and attributed to intake of other nutrients that alter the gut flora, seasonal changes, timing of plasma and urinary isoflavone measurements owing to the short half-life of these compounds, the composition of ethnic diets, and individual differences.6,14,15,23–27 In a more recent report to estimate the bioavailability of usual intake of isoflavones, Bakta and colleagues reported stable plasma isoflavone levels over a 1-year period in populations that consume a steady intake of food sources of isoflavones, with no significant increases over time.24 Similarly, animal models using serial measurements of plasma isoflavones have failed to observe a significant and progressive increase in plasma concentrations with a chronic dose of purified isoflavones.28 In addition, other studies have demonstrated that urinary excretion of isoflavone metabolites increases with dose, frequency, and length of time the supplement is used, demonstrating a higher elimination of metabolites of isoflavones with increasing length of use,7,27,28 indicative of a trend toward stabilization of bioavailability concentration of isoflavones with time. Our findings establish the need for further investigations into the pharmacokinetics of isoflavones in long-term clinical trials.
Although the mechanism of action of isoflavones is not clear, it has been observed that steroid hormones play an important role in increasing or decreasing the risk of CaP. Patients with CaP have been observed to have higher free testosterone (unbound) levels and lower levels of SHBG, estrone,7 and estradiol.29 Androgens are essential for the function and growth of the prostate and are known to stimulate the proliferation of human prostatic cells.30–32 Administration of hormonal therapies has been shown to produce CaP in rodents, whereas castration, antiandrogens, and luteinizing hormone-releasing hormone agonist therapy can reduce CaP progression.29–32 It is clear from recent studies that testosterone and estradiol are important contributors of androgenic and estrogenic activity.30–32 Furthermore, SHBG, because it binds to and sequesters testosterone and estradiol, controls the bioavailability of these steroid hormones to target cells as well as their mutual balance. This effect on the active estrogen/testosterone balance may be another potential autoregulatory mechanism for the protective effect of SHBG in CaP.30 Recent evidence suggests that SHBG can function as a hormone with a direct interaction with prostate cells.31 The role of estrogens in the treatment of androgen-independent CaP was recently examined in phase II clinical trials, demonstrating that high-dose Premarin (conjugated estrogen) resulted in PSA decreases in patients with androgen-independent CaP.33,34 The only dose group in which a significant increase in serum total estradiol was observed was the 40 mg isoflavone-treated arm (p = .02). Although serum estradiol increased in the 60 mg isoflovone-treated arm, the posttreatment levels of serum estradiol in this group were similar to those observed in the placebo group and within the range of values found across the groups and time points in this study. In addition, a significant increase in serum free testosterone (p = .002) was observed in the 60 mg dose group. However, the changes in all serum steroid hormones in the 80 mg isoflavone-treated groups were not statistically significant, similar to our previous study in early CaP patients.16 No significant changes in serum SHBG were observed for this duration of intervention and sample size. Although some of the significant observations of an increase in serum steroid hormone levels may appear spurious, especially the increase in serum testosterone levels, our observation of an increase in serum estradiol levels in the 40 mg dose group may be important and similar to previous observations of mild estrogenic effects of isoflavones at steady, relatively low dose levels14,23,27,28 and thus cannot be ignored. Our observation also establishes the need for isoflavones to be evaluated in future well-powered clinical trials.
With the recent understanding of the natural history of the progression of disease2–4 in patients diagnosed with clinically localized CaP and in spite of the number of biomarkers that have been examined in predicting treatment outcomes, to date, no single marker can individually predict treatment outcome, specifically in men with localized disease. PSA, or KLK3, is a member of the human kallikrein family of serine proteases secreted by the prostatic epithelium and the epithelial lining of the periurethral glands. It is clear from a recent report of the Prostate Cancer Prevention Trial that PSA levels should be thought of as a continuous variable rather than as a dichotomous variable, such that the risk of CaP increases as the PSA level increases.35 Traditionally, clinicians have relied on PSA as a prognostic indicator in addition to tumor stage, grade, and volume. Because the serum level of PSA is proportional to the volume of tumor present, PSA has become an integral part of disease management in this population with early-stage CaP. Freedland and colleagues reported that with each 2-point increase in PSA, the risk of biochemical progression increased approximately twofold, even in men with PSA < 10 ng/mL.18 Studies have demonstrated that a greater PSA level was associated with significantly more adverse pathologic features and biochemical progression.3,18,36 However, with the current duration of intervention and sample size, we failed to observe any statistically significant changes in serum PSA with isoflavone supplementation.
Additionally, we examined tissue changes in Ki-67 expression, which provides an accurate estimate of growth fraction and in many studies has been found to be a predictor of outcome for patients treated with radical prostatectomy. Ki-67 had significantly different levels of expression in normal prostate tissue, high-grade prostatic intraepithelial neoplasia, and prostate adenocarcinoma and could potentially be applied as an intermediate end point biomarker of chemoprevention efficacy, although this has not been previously monitored serially in chemoprevention trials.37 The highest proliferation was observed in the untreated control group compared with the 80 mg isoflavone-treated arm. Although the sample size was small and adequate pre- and posttreatment tissue was unavailable in our study, when analyzed with the safety and other variables evaluated in this study, a trend was observed demonstrating that a greater number of patients showed the lowest percentage of cells expressing Ki-67 in the 40 mg isoflavone-treated arm compared with the control and other higher-dose treatment arms.
As with all endocrine therapies, previous studies using Premarin demonstrated side effects such as thromboembolism, hot flashes, reduction in bone mineral density, increase in body weight, erectile dysfunction, and gynecomastia,38 requiring intermittent use of these therapies and interruption in treatment and limiting therapies to short-term use. However, with isoflavone supplementation, all adverse events (gastrointestinal, constitutional, and metabolic) reported were grades I to II, demonstrating no clinical toxicity and thus not requiring early stopping of the study agent, similar to other phase I clinical trials using purified isoflavone preparations.14,15 Previous clinical trials using whole soy proteins or food sources of isoflavone have reported constipation and gastrointestinal symptoms such as bloating, discomfort, diarrhea, and pain in addition to lowered libido.6,39–41 Studies have also illustrated large variability in isoflavone databases and food labeling,42 thus reinforcing the need to use purified isoflavones in intervention trials. Our choice of a standardized purified isoflavone product without the soy protein was to ensure delivery of a standard dose, improve compliance, and reduce gastrointestinal and other toxicities observed in other studies.
Conclusion
Evaluation of the effectiveness of intervention in this phase I dose-finding trial was based on the magnitude of change in plasma levels of isoflavones in the isoflavone-supplemented group compared with the placebo group and a corresponding modulation of steroid hormones producing stabilization or reduction in a surrogate marker of proliferation (total PSA) that could be achieved. Based on this criterion, we observed that although plasma isoflavones increased in all supplemented arms, the only arm in which we observed a modulation of serum estradiol without a significant increase in serum free testosterone or serum PSA was the 40 mg isoflavone-supplemented arm. This was also the group demonstrating the least mean percentage of cells in proliferation at the end of study with no clinical toxicity. Given that these findings of significant changes in a small sample and the short duration of intervention may have been due to chance, based on these preliminary observations, it may be important that we examine further a standardized dose of 40 mg of purified isoflavone formulation in a definitive clinical trial, powered to examine the safety and effectiveness of isoflavone in inhibiting the progression of CaP in men at high risk, with early-stage CaP (watchful waiting), or with biochemical disease progression. In addition, although the antiproliferative properties affected via the steroid hormone pathway of isoflavones have been hypothesized to be primarily responsible for isoflavone’s beneficial effects, evidence is accumulating to suggest that other mechanisms may also be involved. Future studies must use a combination of validated prognostic biochemical, morphologic, and molecular intermediate end point biomarkers to evaluate chemoprevention efficacy. Until the results from larger well-conducted phase II clinical trials have demonstrated efficacy and safety, it is critical for practitioners to exercise caution prior to recommending supplemental doses to their patients for cancer prevention or treatment.
Acknowledgments
Financial disclosure of authors: This research study was funded by the National Institutes of Health-National Cancer Institute (U10 CA81920).
Footnotes
Financial disclosure of reviewers: None reported.
Contributor Information
Nagi B. Kumar, Department of Interdisciplinary Oncology, H. Lee Moffitt Cancer Center & Research Institute, University of South Florida College of Medicine, Tampa, FL.
Loveleen Kang, Department of Pathology, University of South Florida College of Medicine, Tampa, FL.
Julio Pow-Sang, Department of Surgery, University of South Florida College of Medicine, Tampa, FL.
Ping Xu, Division of Biostatistics and Informatics, University of South Florida College of Medicine, Tampa, FL.
Kathy Allen, Department of Interdisciplinary Oncology, H. Lee Moffitt Cancer Center & Research Institute, University of South Florida College of Medicine, Tampa, FL.
Diane Riccardi, Department of Interdisciplinary Oncology, H. Lee Moffitt Cancer Center & Research Institute, University of South Florida College of Medicine, Tampa, FL.
Karen Besterman-Dahan, Department of Interdisciplinary Oncology, H. Lee Moffitt Cancer Center & Research Institute, University of South Florida College of Medicine, Tampa, FL.
Jeffrey P. Krischer, Division of Biostatistics and Informatics, University of South Florida College of Medicine, Tampa, FL.
References
- 1.American Cancer Society. Cancer facts and figures. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 2.Holmberg L, Bill-Axelson A, Garmo H, et al. SPCG-4 Study Group. Prognostic markers under watchful waiting and radical prostatectomy. Hematol Oncol Clin North Am. 2006;20:845–55. doi: 10.1016/j.hoc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Johansson JE, Andren O, Andersson SO, et al. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–19. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 4.Kelloff GJ, Leiberman R, Steele VE, et al. Chemoprevention of prostate cancer. Eur Urol. 1999;35:342–50. doi: 10.1159/000019906. [DOI] [PubMed] [Google Scholar]
- 5.Klotz L. Active surveillance versus radical treatment for favorable-risk localized prostate cancer. Curr Treat Options Oncol Rev. 2006;7:355–62. doi: 10.1007/s11864-006-0003-z. [DOI] [PubMed] [Google Scholar]
- 6.Kumar NB, Cantor A, Allen K, et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59:141–7. doi: 10.1002/pros.10362. [DOI] [PubMed] [Google Scholar]
- 7.Adlercreutz H, Honjo H, Higashi A, et al. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr. 1991;54:1093–100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu H, Ross RK, Bernstein L, et al. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–6. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morton MS, Chan PS, Cheng C, et al. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate. 1997;32:122–8. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Ouchi H, Ishiguro H, Ikeda N, et al. Genistein induces cell growth inhibition in prostate cancer through the suppression of telomerase activity. Int J Urol. 2005;12:73–80. doi: 10.1111/j.1442-2042.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 11.Adlercreutz H, Mousavi Y, Clark J, et al. Dietary phytoestrogens and cancer: in vitro and in vivo studies. J Steroid Biochem Mol Biol. 1992;41:331–7. doi: 10.1016/0960-0760(92)90359-q. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Chen S, Xu L, et al. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005;65:3470–8. doi: 10.1158/0008-5472.CAN-04-2807. [DOI] [PubMed] [Google Scholar]
- 13.Kazi A, Daniel KG, Smith DM, et al. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem Pharmacol. 2003;66:965–76. doi: 10.1016/s0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 14.Fischer L, Mahoney C, Jeffcoat AR, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer. 2004;48:160–70. doi: 10.1207/s15327914nc4802_5. [DOI] [PubMed] [Google Scholar]
- 15.Busby MG, Jeffcoat AR, Bloedon LT, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002;75:126–36. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 16.Kumar NB, Krischer JP, Allen K, et al. A phase II randomized, placebo-controlled clinical trial of purified isoflavones in modulating steroid hormones in men diagnosed with prostate cancer. Int J Nutr Cancer. 2007;59:163–8. doi: 10.1080/01635580701432678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar NB, Krischer JP, Allen K, et al. Safety of purified isoflavones in men with early stage prostate cancer. Int J Nutr Cancer. 2007;59:169–75. doi: 10.1080/01635580701432660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedland SJ, Mangold LA, Walsh PC, Partin AW. The prostatic specific antigen era is alive and well: prostatic specific antigen and biochemical progression following radical prostatectomy. J Urol. 2005;174(4 Pt 1):1276–81. doi: 10.1097/01.ju.0000173907.84852.ec. discussion 1281. [DOI] [PubMed] [Google Scholar]
- 19.Dalais FS, Meliala A, Wattanapenpaiboon N, et al. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology. 2004;64:510–15. doi: 10.1016/j.urology.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 20.deVere White RW, Hackman RM, Soares SE, et al. Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer. Urology. 2004;63:259–63. doi: 10.1016/j.urology.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 21.Schroder FH, van der Cruijsen-Koeter I, de Koning HJ, et al. Prostate cancer detection at low prostate specific antigen. J Urol. 2000;163:806–12. [PubMed] [Google Scholar]
- 22.Urban D, Irwin W, Kirk M, et al. The effect of isolated soy protein on plasma biomarkers in elderly men with elevated serum prostate specific antigen. J Urol. 2001;165:294–300. doi: 10.1097/00005392-200101000-00082. [DOI] [PubMed] [Google Scholar]
- 23.Lampe JW, Gustafson DR, Hutchins AM, et al. Urinary isoflavonoid and lignan excretion on a Western diet: relation to soy, vegetable, and fruit intake. Cancer Epidemiol Biomarkers Prev. 1999;8:699–707. [PubMed] [Google Scholar]
- 24.Bhakta D, dos Santos Silva I, Higgins C, et al. A semi-quantitative food frequency questionnaire is a valid indicator of the usual intake of phytoestrogens by south Asian women in the UK relative to multiple 24-h dietary recalls and multiple plasma samples. J Nutr. 2005;135:116–23. doi: 10.1093/jn/135.1.116. [DOI] [PubMed] [Google Scholar]
- 25.Seow A, Shi CY, Chung FL, et al. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol Biomarkers Prev. 1998;7:135–10. [PubMed] [Google Scholar]
- 26.Chen Z, Zheng W, Causter LJ, et al. Usual dietary consumption of soy foods and its correlation with the excretion rate of isoflavonoids in overnight urine samples among Chinese women in Shangai. Nutr Cancer. 1999;33:82–7. doi: 10.1080/01635589909514752. [DOI] [PubMed] [Google Scholar]
- 27.Maskarinec G, Singh S, Meng L, et al. Dietary soy intake and urinary isoflavone excretion among women from a multiethnic population. Cancer Epidemiol Biomarkers Prev. 1998;7:613–19. [PubMed] [Google Scholar]
- 28.King RA, Broadbent JL, Head RJ. Absorption and excretion of the soy isoflavone genistein in rats. J Nutr. 1996;126:176–82. doi: 10.1093/jn/126.1.176. [DOI] [PubMed] [Google Scholar]
- 29.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125(3 Suppl):777S–83S. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 30.Adlercreutz H, Mazur W, Bartels P, et al. Phytoestrogens and prostate disease. J Nutr. 2000;130(3):658S–9S. doi: 10.1093/jn/130.3.658S. [DOI] [PubMed] [Google Scholar]
- 31.Parsons JK, Carter HB, Platz EA, et al. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005;9:2257–60. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 32.Carruba G. Estrogens and mechanisms of prostate cancer progression. Ann N Y Acad Sci. 2006;1089:201–17. doi: 10.1196/annals.1386.027. [DOI] [PubMed] [Google Scholar]
- 33.Pomerantz M, Manola J, Taplin ME, et al. Phase I dose-finding study of low dose and high dose conjugated estrogen for androgen independent prostate cancer. J Urol. 2007;177:2146–50. doi: 10.1016/j.juro.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 34.Daskivich TJ, Oh WK. Recent progress in hormonal therapy for advanced prostate cancer. Curr Opin Urol. 2006;3:173–8. doi: 10.1097/01.mou.0000193392.77469.e2. [DOI] [PubMed] [Google Scholar]
- 35.Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–34. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 36.Papaconstantinou C, Krischer JP. An automated patient registration and treatment randomized system. J Med Syst. 1995;19:445–56. doi: 10.1007/BF02260848. [DOI] [PubMed] [Google Scholar]
- 37.Mucci NR, Rubin MA, Strawderman MS, et al. Expression of nuclear antigen Ki-67 in prostate cancer needle biopsy and radical prostatectomy specimens. J Natl Cancer Inst. 2000;92:1941–2. doi: 10.1093/jnci/92.23.1941. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Shelley M, Harrison C, et al. Neo-adjuvant and adjuvant hormone therapy for localized and locally advanced prostate cancer. Cochrane Database Syst Rev. 2006;(4):CD006019. doi: 10.1002/14651858.CD006019.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kranse R, Dagnelie PC, van Kemenade MC, et al. Dietary intervention in prostate cancer patients: PSA response in a randomized double-blind placebo-controlled study. Int J Cancer. 2005;113:835–40. doi: 10.1002/ijc.20653. [DOI] [PubMed] [Google Scholar]
- 40.Hussain M, Banerjee M, Sarkar FH, et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111–17. doi: 10.1207/s15327914nc4702_1. [DOI] [PubMed] [Google Scholar]
- 41.Dalais FS, Melialla A, Wattanapenpaiboon N, et al. Effects of a diet rich in phytoestrogens on prostate-specific antigen and sex hormones in men diagnosed with prostate cancer. Urology. 2004;64:510–15. doi: 10.1016/j.urology.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Setchell KD, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131 (4 Suppl):1362S–75S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]