Abstract
Tumor blood vessels are heterogeneous, of at least six distinct types, are induced primarily by VEGF-A, and provide a potentially useful therapeutic target. Breast cancer is characterized by changes in the microenvironment that result in altered tensional homeostasis. Also, breast cancers arise as the result of epigenetic as well as genetic changes. Tumor blood vessel pericytes result, in part, from bone marrow precursor cells, and VEGF is a negative regulator of glioblastoma tumor cell invasion.
Keywords: angiogenesis, mechano-biology, patterning
Introduction
Like normal tissues, tumors are comprised of two discrete but interactive compartments, parenchyma and stroma [1]. In tumors, the tumor cells themselves are the parenchyma, whereas the stroma is comprised of a mixture of non-malignant cells and connective tissue elements. Stromal elements include blood and lymphatic vessels, as well as fibroblasts and inflammatory cells. In addition, stroma includes a variety of extracellular macromolecules that serve to provide structural support; these include collagen, fibronectin, fibrin, various proteoglycans and hyaluronan. The quantities of these different stromal cells and extracellular deposits differ widely in different tumors. For example, in desmoplastic tumors, stroma may account for >80% of the tumor mass, whereas in other tumors (e.g., many lymphomas) stroma may account for only a tiny fraction. Tumor malignancy does not correlate closely with the amount of stroma deposited; both highly and less malignant tumors can possess abundant or limited amounts of stroma. Nonetheless, all tumors require at least some stroma to meet their needs of nutrition, waste removal, and structure. Even “liquid tumors”, i.e., leukemias, have stroma, the blood plasma in which they circulate. Though long neglected, it has become clear in recent years that stroma is essential for tumor maintenance and growth and has potential as a therapeutic target. As one example, anti-angiogenic agents have recently been found to impede tumor growth and prolong survival when used in adjuvant mode, thus proving in principle that attacking stroma can be a useful approach to therapy [2, 3]. Going forward, it is likely that other approaches will be used to target tumor stroma. Therefore, it was appropriate to begin the 3rd International Symposium on Cancer Metastasis and the Lymphovascular System with a session on the tumor microenvironment. Four speakers addressed various aspects of tumor stroma, as follows below.
Tumor blood vessels: the what, the how and the why (Harold F. Dvorak)
The tumor vasculature is an important component of the tumor microenvironment [1, 4]. The current view of tumor angiogenesis can be summarized as follows: tumors must induce the formation of new blood vessels if they are to grow beyond minimal size; they do so by secreting growth factors, particularly vascular permeability factor/vascular endothelial growth factor-A (VEGF-A); the resulting tumor blood vessels are highly abnormal; and anti-angiogenic therapy is useful as an adjuvant, though, in general, its addition to the therapeutic armament prolongs life by only a few months [2, 3]. Therefore, it is a good time to address some basic questions: What are tumor blood vessels, How do they form, and why might their diversity be important therapeutically?
What are tumor blood vessels?
While it has long been recognized that tumor blood vessels are abnormal, angiogenic blood vessels are often spoken of as being of a single type. This is certainly not the case. Studies over the last decade have established that angiogenic tumor blood vessels are highly heterogeneous and can be classified into at least four structurally and functionally distinct types (Table 1).
Table 1.
Classification of angiogenic tumor blood vessels
| Vessel type | Vessel Properties |
|---|---|
| Mother vessels | Large, thin-walled, hyperpermeable, lightly fenestrated pericyte-poor sinusoids that are engorged with red blood cells. |
| Capillaries | Resemble normal capillaries as far as is known |
| Glomeruloid Microvascular Proliferations (GMP) | Poorly organized vascular structures that macroscopically resemble renal glomeruli. They are comprised of endothelial cells and pericytes with minimal vascular lumens and reduplicated basement membranes. |
| Vascular Malformations (VM) | Mother vessels that have acquired an often asymmetric coat of smooth muscle cells and/or fibrous connective tissue. Resemble arterio-venous malformations found in other settings. |
How do angiogenic tumor blood vessels form?
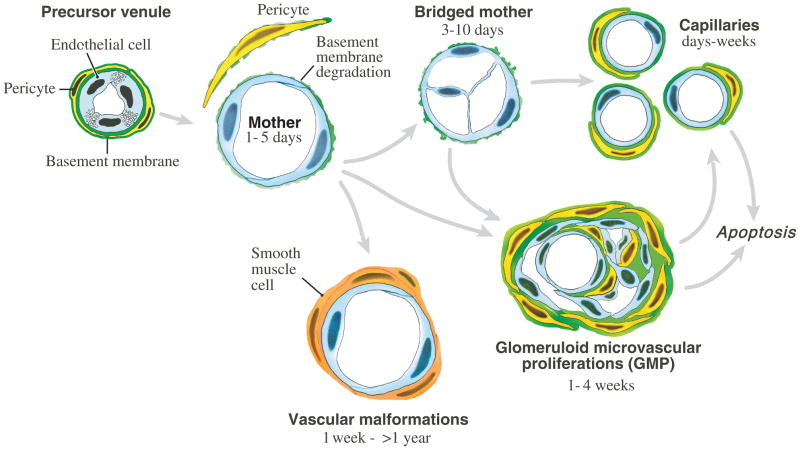
Tumor angiogenesis falls into the general category of pathological angiogenesis, i.e., the type of neovascular response induced by processes such as wound healing and chronic inflammation [5]. Further, it has been possible to induce surrogate forms of all of the types of angiogenic tumor blood vessels listed in Table 1 by expressing VEGF-A in mouse tissues with an adenoviral vector (Ad-VEGF-A164) (Fig. 1). The first new blood vessel type to develop in tumors, as well as in healing wounds and chronic inflammation, is the “mother vessel” (MV) [6, 7].
Figure 1.
Schematic diagram of tumor angiogenesis. reproduced from [32].
Mother vessels (MV) arise from preexisting venules (and to a lesser extent from capillaries) and their properties are listed in Table 1. The initial step in MV formation is degradation of venular basement membrane (VBM). VBM are rigid structures comprised of a tightly woven meshwork of type IV collagen, laminin, entactin, perlecan and heparan sulfate. VBM limit the expansion of the normal microvasculature to ~30% [8], whereas MV have lumens that are 4–5 times those of the normal vessels from which they arise. Therefore, for microvessels to enlarge to become MV, their VBM must be degraded. Chang et al recently confirmed earlier observations that Ad-VEGF-A164 and VEGF-A-expressing tumors induce VBM degradation [7], and found that they did so by upsetting the normal cathepsin-cysteine protease inhibitor (CPI) balance [9]. Cathepsins are members of the cysteine protease family and CPIs are a family of small proteins that serve as their endogenous inhibitors. Cathepsins B, S and L were selectively upregulated in the pericytes of venules and capillaries as they were developing into MV, a process that began within a day of two after Ad-VEGF-A164 or tumor cell injection into mouse tissues. Over the same time period, several CPIs were strikingly downregulated, thus freeing cathepsins from inhibition. On the other hand, the activity of matrix metalloproteases 2 and 9, proteases known to have important roles in tumor biology, actually declined during the course of MV formation. Together these data indicate that VEGF-A induces MV formation by upsetting the cathepsin-CPI balance in preexisting microvessels.
Degradation of the VBM has two important consequences. First, pericytes, having lost their VBM substrate, fall off, accounting for the dearth of pericytes associated with MV. Second, the underlying endothelium thins and expands to cover a now greatly enlarged surface area. These data indicate that the VBM and pericytes together serve as a restraint that limits the size of venules and capillaries. Further, they suggest that MV formation can result from events taking place entirely within the microvasculature itself, and that proteases, for example secreted by tumor cells, are not necessary for MV formation. Remaining to be determined is the mechanism by which VEGF-A regulates the cathepsin-CPI axis.
Very little is known about the mechanisms by which MV evolve into the other types of angiogenic blood vessels (reviewed in [1, 4, 10]).
Why is an understanding of tumor blood vessel diversity important clinically?
Anti-VEGF therapy has been found to prolong the survival of some cancer patients, but it has not been as effective as was originally hoped for, based on studies in mouse cancer models. There are a number of possible reasons for this, e.g., side effects that limit the use in cancer patients of anti-VEGF-A therapy at high doses; vascular normalization resulting from anti-angiogenic therapy [3]; neo-expression of growth factors other than VEGF-A, etc. However, another largely neglected and potentially important possibility is that the tumor vasculature is heterogeneous and that certain of the vessel subtypes listed in Table 1 have gained VEGF-A independence and no longer require VEGF-A for their maintenance.
To test this hypothesis, we have used the Ad-VEGF-A164 system to generate tumor surrogate vessels of the several types listed in Table 1 in mice. Of particular interest are vessels that arise at later times as these are most likely to have lost their VEGF-A-dependence. Our goal is to find new molecules on the surfaces of the endothelial cells that line such blood vessels and to gauge them for therapeutic potential. This approach has been promising, and we have recently identified one such potential target, the four transmembrane, tetraspanin-like protein, TM4SF1 [11]. TM4SF1 is highly expressed on the endothelial cell plasma membranes of smooth muscle coated blood vessels in several important human cancers. Knockdown of TM4SF1 inhibits division and migration of cultured endothelial cells and causes them to undergo senescence. Also, knockdown of this gene in vivo inhibited later stages of Ad-VEGF-A164–induced angiogenesis. It will be of interest to determine whether antibodies under development that target this gene will be therapeutically effective in mouse and, more importantly, in human cancers.
Breast cancer as a disease of altered mechano-biology (Valerie M. Weaver)
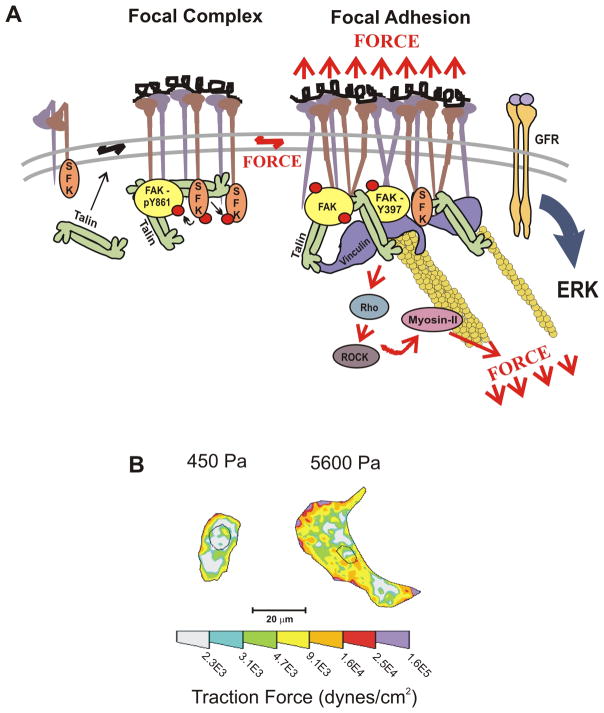
It has been known for some time that tumor and stromal cells talk to each other by secreting various cytokines (e.g., TGF–β) and other biochemical reagents. Less appreciated is the fact that tumor and stromal cells live in a microenvironment with which they interact by means of mechanical signaling. Cells in tissues are subject to a variety of forces including shear stress and compression and tension that result from their interaction with other cells or with the extracellular matrix. These mechanical forces are perceived and integrated in the cell at the molecular level through mechanically responsive sensors. Changes in these interactions may influence cell survival, motility, division, differentiation and gene expression, for example by regulating the activity of the RhoGTPases that modulate actomyosin contractility and actin dynamics. Integrins, by virtue of their extracellular interaction with the ECM and intracellular interactions with the cellular cytoskeleton are prime examples of cellular mechano-transducers, and, upon activation, may induce signaling changes that lead to the formation of focal adhesions [12, 13] (Fig. 2a). Thus, force-dependent activation of signaling cascades allows cells to respond quickly to a dynamic force environment, and the same pathways may also lead to sustained changes in cell behavior, for example, changes in gene expression, cell division, etc.
Figure 2.
A. The majority of integrins exist at the plasma membrane in a resting, inactive state in which they can be activated by inside–out or outside–in cues. With regard to outside–in activation, when cells encounter a mechanically rigid matrix or are exposed to an exogenous force, integrins become activated, which favors integrin oligomerization or clustering, talin 1 and p130Cas protein unfolding, vinculin–talin association, and Src and focal adhesion kinase (FAK) stimulation of RhoGTPase-dependent actomyosin contractility and actin remodeling. Focal adhesions mature with the recruitment of a repertoire of adhesion plaque proteins, including α-actinin to facilitate actin association, and adaptor proteins such as paxillin, which foster interactions between multiple signaling complexes to promote growth, migration and differentiation. B. Normal cells tune their contractility in response to matrix stiffness cues, but tumors exhibit altered tensional homeostasis. Cells exert actomyosin contractility and cytoskeleton-dependent force in response to matrix stiffness cues. These forces can be measured using traction force microscopy. Thus, non-malignant human mammary epithelial cells spread more and exert more force on a stiff matrix than on a soft matrix. Reproduced, with permission, from [12, 14].
Loss of homeostasis is a hallmark of disease and therefore it is not surprising that cancers should be characterized by changes in tensional homeostasis. Tumors are often detected as a palpable “stiffening” or hardening of the tissue, and approaches such as magnetic resonance imaging elastography and sono-elastography have been developed to measure this property in order to enhance cancer detection. The palpable stiffening characteristic of breast and many other cancers is attributed to desmoplasia, the synthesis of abundant dense collagenous stroma that can occupy up to 80% of the tumor mass.
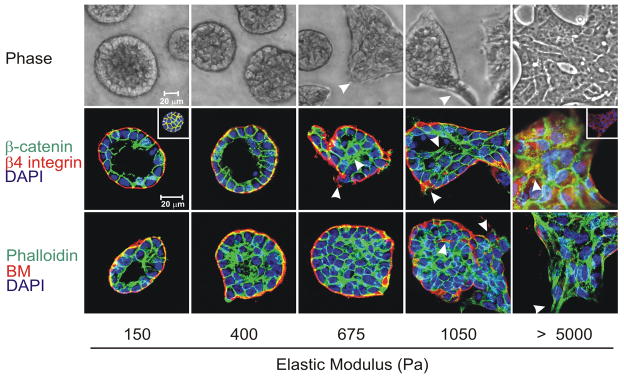
The crucial role of matrix compliance can be illustrated by studies in which mammary epithelium is grown in culture in matrices with different viscoelastic properties (Fig. 3) [14]. When grown in matrices comparable to those found in normal breast, epithelial cells proliferate until they form polarized mammary acinus-like structures with a central lumen and an external basement membrane. However, when the matrix is progressively stiffened, epithelial cell growth is enhanced, the cells lose their polarity, lose cell-cell junction proteins and exhibit irregular cell shapes with detectable actin stress fibers. Thus, focal adhesion is increased along with increased adhesion signaling. Together, the stiffened matrix promotes epithelial cell transformation toward cancer.
Figure 3.
Phase contrast microscopy and confocal immunofluorescence images of non-malignant immortalized human mammary epithelial cell (HMEC; MCF10A) colonies interacting with a three-dimensional reconstituted basement membrane (BM)-laminated polyacrylamide gel of increasing stiffness (150–5,000 Pa) showing colony morphogenesis after 20 days of culture. On compliant gels with materials properties similar to that measured in the normal murine mammary gland (150 Pa) non-malignant MECs proliferate for 6–12 days to eventually form growth-arrested, polarized acini analogous to the terminal ductal lobular units observed at the end buds of the differentiated breast. These structures have intact adherens junctions and insoluble cell–cell localized β-catenin before (main images) and after (inset a) Triton extraction, and polarity, as shown by the basal localization of (α6) β4 integrin, the apical–lateral localization of cortical actin (Phalloidin), and the assembly of an endogenous laminin 5 basement membrane. Incremental stiffening of the basement membrane gel progressively compromises tissue morphogenesis and alters EGF-dependent growth of these cells. Thus, colony size progressively increases with matrix stiffening, lumen formation is compromised, cell–cell junctions are disrupted, as revealed by loss of cell–cell-associated β-catenin (inset b), and tissue polarity is inhibited, as indicated by disorganized (α6) β4 integrin localization and loss of the endogenous laminin 5 basement membrane. Interestingly, actin stress fibers were not observed in the structures until the stiffness of the matrix reached 5,000 Pa, as has been observed in murine breast tumors in vivo. The arrows indicate loss of the endogenous basement membrane and disruption of basal polarity. Reproduced from [12, 14].
In breast cancer, tensional homeostasis is greatly altered. Breast cancers are characterized by increased tumor cell-generated force, increased compression force due to the solid state pressure exerted by the expanding tumor mass, matrix stiffening due to the desmoplastic response, and increased interstitial pressure due to a leaky vasculature and poor lymphatic drainage. Transformed cells exhibit a vastly different intermediate filament profile. They also show compromised mechano-reciprocity such that they often exert abnormally high force in response to a compliant matrix; as a result, cell-cell junctions are altered, tissue polarity is compromised, and anchorage-independent survival and invasion are promoted. The increased cell-generated forces exhibited by tumor cells enhance their growth, survival and invasion by promoting focal adhesion maturation and signaling through actomyosin contractility (Fig. 2b, c). The increased contractility of tumor cells and their associated stromal fibroblasts also induce tension-dependent matrix remodeling to promote the linear reorientation of collagen fibers surrounding the invasive front of the tumor.
The expanding tumor mass exerts compressive stress on the surrounding tissue extracellular matrix, vasculature, lymphatics and interstitial space. Tumor-associated compression stress can, in turn, induce tumor angiogenesis by directly increasing expression of VEGF-A or by indirectly blocking the existing vasculature surrounding the tumor mass to induce hypoxia and thus VEGF-A expression. Compression also increases the interstitial pressure which may exceed ten times that of normal tissue; this increased pressure induces the accumulation of fluid from leaky blood vessels and impedes lymphatic clearance. Compression force can also shrink the interstitial space surrounding the ductal structures, thereby increasing the local concentration of growth factors and cytokines that stimulate tumor cell growth. These changes in interstitial pressure can also impede the entry into tumors of chemotherapeutic drugs. In sum, tumor cells are exposed to a myriad of altered mechanical forces that dramatically modify their behavior and these findings directly implicate matrix changes in tumor evolution.
A topic of great recent interest, and one dealt with in greater detail in Dr. Tlsty’s presentation, is that of the role of breast density and cancer incidence. Patients with increased breast density, as determined by mammography, have a 4–6-fold higher incidence of breast cancer. Dense breasts are characterized by increased collagen and other extracellular matrix components. Paradoxically, however, cancer incidence increases with age whereas breast density typically declines. However, although the post-menopausal breast has reduced collagen, that which remains may have abnormal properties that promote carcinogenesis.
In sum, force is a critically important determinant of tissue development and its study has been much neglected. The ability of cells to sense, respond and adapt appropriately to force contributes to disease, and particularly to cancer. Pathological changes in cells and in the architecture, topology and material properties of the matrix microenvironment constitute a positive feedback loop that propels carcinogenesis. However, many questions still need to be resolved. Such questions include how the unique material properties of specific differentiated tissues are established and maintained, how cells coordinate their function and adaptation to external cues in the microenvironment, and how physical signals might interface with and modulate the activity of biochemical signaling pathways. And, with specific regard to cancer: Does LOX-dependent collagen crosslinking stiffen the tissue and thus drive tumor progression? Would inhibiting LOX-dependent collagen crosslinking temper tissue desmoplasia, reduce mechano-transduction in mammary epithelium and thereby reduce tumor incidence and delay tumor progression?
Epigenetic and Genetic Events in Tumor Progression (Thea D. Tlsty)
It is well known that oncogenes and repressor genes play an important role in carcinogenesis and tumor progression. Less attention has been given to epigenetic events that affect these processes, i.e., heritable changes in gene expression that occur without a change in DNA sequence. Epigenetic changes can result from methylation of DNA or of histones and from the action of small RNAs (e.g., microRNAs, PIWI RNAs, etc.). The active acquisition of epigenetic changes is a poorly understood but important process in development, differentiation, and also in cancer.
The mechanisms by which epigenetic changes occur are poorly understood. However, in recent years it has become clear that tumor cells live in a stromal microenvironment that is not passive, but rather one that can actively shape and modify tumor cell behavior. In particular, it is well known that tumor progression can be markedly affected by the cells and non-cellular elements of the stromal microenvironment in which they are embedded. Signals sent to tumor cells by the microenvironment can result in cell proliferation and in DNA methylation, centrosome abnormalities, telomeric dysfunction and altered gene expression [15].
Recent work has dealt with the p16/pRB signaling pathway, a pathway that regulates the cell cycle and that is inactivated in many tumor cells [16]. When this pathway is suppressed, non-cancerous human epithelial cells in culture undergo dynamic epigenetic remodeling that results in the targeted methylation of a selected group of CpG islands. Repression of the p16/pRB pathway in primary human mammary epithelial cells activated an E2F-mediated increase in proteins that remodel chromatin and cause targeted de novo DNA methylation at a non-random collection of loci. Thus, repressing the p16/pRB pathway renders cells epigenetically plastic and results in DNA methylation in a deterministic (predictable) rather than a stochastic (random) pattern. Furthermore, the coordinated set of de novo DNA methylation events are preceded by, and dependent upon, the repression of gene expression. Thus, one can imagine that during cancer progression, tumor cells acquire epigenetic plasticity through repression of the p16/pRB pathway via mutations, deletions or methylation, which then provide the potential for programming epigenetic events. These data show that p16, a commonly inactivated tumor suppressor gene, regulates DNA methylation and that epigenetic changes occur in preneoplastic cells. In sum, DNA methylation is an active and dynamic process, and chromatin remodeling and repression precedes and is necessary for subsequent DNA hypermethylation. Finally, loss of p16 confers epigenetic plasticity, a silencing of genes important in differentiation.
To determine whether these findings were relevant to human cancer, the authors looked for p16-suppressed cells in human breast tissue. Using immunohistochemistry, her group found that normal breast tissue from cancer-free women contained foci of p16-suppressed cells, suggesting the presence of a premalignant program in these otherwise healthy women. This exciting finding directly demonstrates the relevance of p16 suppression to human cancer and offers a new marker of premalignancy [17, 18].
Epithelial to mesenchymal transition or EMT is a characteristic feature of epithelial cell tumor progression. EMT has been implicated in tumor recurrence, and is often associated with a poor prognosis in women with breast cancer. There is now evidence demonstrating a link between EMT, basal-like breast cancers, the stem-cell phenotype, and the acquisition of tumorigenic and metastatic potential. EMT is characterized by several molecular changes that include the loss of epithelial markers such as E-cadherin and ZO-1, and the induction of mesenchymal markers such as N-cadherin, fibronectin, vimentin and Snail. Though alterations in E-cadherin expression can occur through multiple mechanisms, including loss of heterozygosity and mutational inactivation, E-cadherin is frequently silenced through aberrant DNA hypermethylation of its promoter. Interestingly, when E-cadherin is silenced through promoter DNA hypermethylation, mammary cell lines often exhibit a mesenchymal morphology through the coordinated induction of a set of genes involved in EMT. In contrast, when E-cadherin is inactivated by mutation, the cells continue to exhibit an epithelial morphology, and these genes are not induced [19]. This suggests that a program of molecular alterations leading to EMT, invasion, and metastasis can be modulated epigenetically. EMT has been shown to be induced in murine cells by oncogenic ras in cooperation with factors in serum. There is also evidence that exposing cells to serum induces a gene expression pattern that resembles that of a wounding response. This wound-response signature is strongly predictive of future invasive and metastatic behavior, both of which require EMT.
The next step was to determine whether immortalized human mammary epithelial cells with repressed p16INK4A and expressing oncogenic ras (vHMEC-ras) cells could be programmed by the microenvironment to acquire epigenetic changes associated with tumorigenic phenotypes such as EMT. vHMEC-ras cells were exposed to high (10%) and low (0.5%) concentrations of serum. When cultured in serum-rich media, thought to be typical of the tumor microenvironment, human vHMEC-ras cells, like murine cells, underwent phenotypic changes indicative of EMT [20]. This morphological transition was accompanied by increased motility, increased expression of fibronectin and N-cadherin, and reduced expression of E-cadherin. E-cadherin was silenced via de novo promoter DNA methylation. As described for the previous system, transcriptional repression was found to precede DNA hypermethylation. Also, additional genes underwent de novo promoter DNA hypermethylation, including estrogen receptor alpha, Twist and CST6. Strikingly, these same genes are hypermethylated in human basal-like breast cancers that exhibit mesenchymal phenotypes and are associated with a poor prognosis but not in human luminal A, luminal B, etc. cancers. In sum, these data indicate that cultured human cells can be programmed by their microenvironment to undergo phenotypic and gene expression changes associated with epigenetic alterations important in human tumor progression. They further indicate that cultured cells can provide valuable tools to elucidate malignant cell properties that are applicable to human cancer patients. A goal for the future is to use this information to create tools that address clinical questions, such as the development of prognostic biomarkers and therapeutic targets.
Novel functions of VEGF and PDGF signaling circuits in tumor angiogenesis and invasion (Gabriele Bergers)
Dr. Bergers presented work on two topics. The first dealt with the recruitment of pericyte progenitor cells from bone marrow and their differentiation into more mature pericytes in tumor blood vessels. By way of background, the normal microvasculature is comprised of two types of cells, endothelial cells (the cells lining the blood vasculature), and perictyes, cells that surround and tightly envelop endothelial cells. Pericytes are thought to be necessary for the health of endothelial cells and so of the blood microvasculature [21, 22]. Tumors, however, include many blood vessels that lack, or have a reduced coating of pericytes, e.g. mother vessels (see Dvorak presentation and Table 1). Further, treating mice bearing the Rip1Tag2 tumor at later stages of growth with imatinib, a tyrosine kinase inhibitor that binds to the PDGF receptor (PDGFR) succeeded in regressing tumor vessels by causing pericytes to detach. These data emphasize the importance of pericytes in maintaining the tumor vasculature. However, the nature and source of tumor vessel pericytes has been little investigated. To address this question, the Bergers lab observed that, as in the developing vasculature, PDGFRβ+cells enveloped the blood vessels of Rip1Tag2 tumors and that the PDGF ligands B and D for PDGFRβ were expressed by tumor endothelial cells [23]. This suggested that a paracrine communication pathway between pericytes and endothelial cells might be operative in tumors, as in normal vascular development. Mature pericytes are known to express additional markers, namely, NG2, αSMA, and desmin. However, in tumors, not all tumor PDGFRβ vessel enveloping cells bore these mature pericyte markers. Flow cytometry and immunohistochemical studies revealed three distinct types of such cells: PDGFRβ+ NG2− cells, PDGFRβ+ NG2+ cells, and PDGFRβ−+ NG2+ cells. Thus, in Rip1Tag2 tumors, only a subset of PDGFRβ+ pericytes expressed mature pericyte markers and a subset of mature pericytes did not express detectable levels of PDGFRβ likely reflecting distinct differentiation stages. Indeed, further work demonstrated that PDGFRβ NG2− cells represent a population of progenitor pericytes, some of which are recruited from the bone marrow and bear markers (e.g., Sca1, CD45) characteristic of hematopoietic stem cells [23]. When PDGFRβ cells were mixed with endothelial cells in three-dimensional cultures, endothelial cells formed tubes with pericytes attaching, particularly at branch points. In addition, these pericytes differentiated, acquiring markers of mature pericytes (NG2, αSMA and desmin). These studies demonstrated that tumor blood vessel pericytes derive, at least in part from hematopoietic bone marrow progenitors and that progenitor pericytes undergo maturation when apposed to endothelial cells. Part of the maturation effect, but only that leading to αSMA expression, could be replicated by exposing percyte progenitors to TGF-β.
A second series of studies demonstrated that VEGF is a negative regulator of cell invasion in the case of glioblastoma multiforme (GBM), rapidly growing and highly aggressive grade IV astrocytomas (brain tumors) [24]. GBM are characterized by zones of necrosis and hypoxia and, as a result, typically express high levels of HIF-1 and downstream angiogenic factors such as VEGF, angiopoietin-2 and SDF-1α [25–27]. They disrupt the blood-brain barrier and are characterized by extensive edema and an influx of inflammatory cells. GBM spread with several distinct dispersion patterns: subpial spread, white matter infiltration, perivascular spread, and ventricular spread.
To investigate the functional significance of hypoxia and angiogenesis in astrocytoma progression, initial studies were performed with transformed astrocytes genetically engineered from murine primary culture astrocytes in which the hypoxia-responsive transcription factor HIF-1α or its target gene, the angiogenic factor VEGF, was deleted [28]. Genetic deletion of VEGF blocked tumor angiogenesis and increased vascular cell apoptosis, but, paradoxically, increased tumor invasion. When HIF-1α was knocked out in GBM cells, the new vessels that formed remained slim and regularly shaped, more closely resembling those of the normal brain vasculature. However, HIF-1α k/o GBM adapted to their inability to grow new blood vessels by co-opting and moving along preexisting blood vessels, a phenomenon described as perivascular spread, and were actually more invasive than wild type GBM cells [28].
Matrix metalloproteases (MMP), a large family of zinc-dependent endopeptidases, have been implicated in many aspects of tumor growth and progression. Of these, MMP-2 has been particularly implicated in tumor angiogenesis. To investigate the role of MMP-2 in GBM tumor cell survival and invasion, genetically engineered MMP-2 knockout GBM cells were prepared and their growth properties studied in MMP-2 knockout (k/o) mice [29]. Wild type GBM cells grew as invasive, highly angiogenic tumors with a leaky, tortuous vasculature and with hypoxic centers. In contrast, GBM-MMP-2k/o cells developed a markedly increased vasculature. Paradoxically, however, the tumor cells grew slower and were more prone to apoptosis and the mice exhibited a longer mean survival time. Apparently the dense and highly branched network of tumor blood vessels induced by these cells were not able to support tumor growth. In support of this hypothesis, tumor vessels exhibited substantially less VEGFR-2, pericytes were greatly reduced, and vessels were poorly perfused. Also, the pattern of tumor cell invasion was different in the absence of MMP-2. MMP-2 k/o GBM grew more diffusely by migrating along preexisting blood vessels into the brain parenchyma, a pattern also observed when both HIF-1 and VEGF deficient GBM were implanted in brain (such tumors did not induce proper neovascularization). Apparently, MMP-2 acts as a negative regulator of vascular patterning and angiogenesis in GBM. Investigating the mechanisms for these findings, HIF-1α expressed by GBM cells was found to induce SDF1α, and, in this way recruit bone marrow-derived CD45+ myeloid cells containing Tie2+, VEGFR1+, CD11b+, and F4/80+ subpopulations, as well as endothelial and pericyte progenitor cells to promote neovascularization [29]. Further, MMP-9 activity of bone marrow derived CD45+ cells was found to be sufficient and essential to initiate angiogenesis by increasing VEGF bioavailability (releasing it from its bound state to matrix or cells). Conversely, in the absence of HIF-1α, SDF1α levels decreased and fewer bone-marrow derived cells were recruited to tumors, thus decreasing MMP-9 and mobilization of VEGF.
Finally, it was found that VEGF is a direct and negative regulator of tumor cell invasion [29]. VEGF reduced the ability of VEGFR-expressing GBM cells to migrate and invade in vitro and in vivo. When VEGF activity was impaired, tumor cells invaded deep into the brain in the perivascular compartment. Further support for this finding has come from studies in which tumors were subjected to anti-angiogenesis therapies aimed at targeting the VEGF pathway. GBM and Rip1Tag2 tumors treated with tyrosine kinase inhibitors targeting VEGFR-2 showed initial vascular dropout and tumor stasis, only to be followed by a tumor adaptive-evasive response, mediated by other growth factors such as fibroblast growth factor and leading to augmented invasion and, in some cases, dissemination and distant metastasis [30, 31]. Obviously these findings in animal models have implications for treating cancer patients with anti-angiogenic drugs, and particularly those targeting the VEGF pathway.
Acknowledgments
This work was supported by U.S. Public Health Service grants HL-64402, P01 CA92644, and by a contract from the National Foundation for Cancer Research (to hfd); by PSOC U54CA143836-01 and DOD W81XWH-05-1-0330 from the US Department of Defense (to V.M.W); and by U.S. National Cancer Institute grants R01 CA113382 and R01 CA109390 (to G.B).
References
- 1.Dvorak H. Tumor blood vessels. In: Aird W, editor. Endothelial Biomedicine. Cambridge: Cambridge University Press; 2007. pp. 1457–1478. [Google Scholar]
- 2.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 4.Nagy JA, Chang SH, Dvorak AM, Dvorak HF. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer. 2009;100:865–869. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvorak HF. Rous-Whipple Award Lecture. How tumors make bad blood vessels and stroma. Am J Pathol. 2003;162:1747–1757. doi: 10.1016/s0002-9440(10)64309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paku S, Paweletz N. First steps of tumor-related angiogenesis. Laboratory investigation; a journal of technical methods and pathology. 1991;65:334–346. [PubMed] [Google Scholar]
- 7.Pettersson A, Nagy JA, Brown LF, Sundberg C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey VS, Eckelhoefer IA, Feng D, Dvorak AM, Mulligan RC, Dvorak HF. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Laboratory investigation; a journal of technical methods and pathology. 2000;80:99–115. doi: 10.1038/labinvest.3780013. [DOI] [PubMed] [Google Scholar]
- 8.Swayne GT, Smaje LH, Bergel DH. Distensibility of single capillaries and venules in the rat and frog mesentery. Int J Microcirc Clin Exp. 1989;8:25–42. [PubMed] [Google Scholar]
- 9.Chang SH, Kanasaki K, Gocheva V, Blum G, Harper J, Moses MA, Shih SC, Nagy JA, Joyce J, Bogyo M, Kalluri R, Dvorak HF. VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res. 2009;69:4537–4544. doi: 10.1158/0008-5472.CAN-08-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]
- 11.Shih SC, Zukauskas A, Li D, Liu G, Ang LH, Nagy JA, Brown LF, Dvorak HF. The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res. 2009;69:3272–3277. doi: 10.1158/0008-5472.CAN-08-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engler AJ, Humbert PO, Wehrle-Haller B, Weaver VM. Multiscale modeling of form and function. Science. 2009;324:208–212. doi: 10.1126/science.1170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds PA, Sigaroudinia M, Zardo G, Wilson MB, Benton GM, Miller CJ, Hong C, Fridlyand J, Costello JF, Tlsty TD. Tumor suppressor p16INK4A regulates polycomb-mediated DNA hypermethylation in human mammary epithelial cells. J Biol Chem. 2006;281:24790–24802. doi: 10.1074/jbc.M604175200. [DOI] [PubMed] [Google Scholar]
- 17.Crawford YG, Gauthier ML, Joubel A, Mantei K, Kozakiewicz K, Afshari CA, Tlsty TD. Histologically normal human mammary epithelia with silenced p16(INK4a) overexpress COX-2, promoting a premalignant program. Cancer Cell. 2004;5:263–273. doi: 10.1016/s1535-6108(04)00023-6. [DOI] [PubMed] [Google Scholar]
- 18.Holst CR, Nuovo GJ, Esteller M, Chew K, Baylin SB, Herman JG, Tlsty TD. Methylation of p16(INK4a) promoters occurs in vivo in histologically normal human mammary epithelia. Cancer Res. 2003;63:1596–1601. [PubMed] [Google Scholar]
- 19.Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de Water B, Cornelisse CJ, Cleton-Jansen AM. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94:661–671. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci U S A. 2008;105:14867–14872. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 23.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger M, Wilson C. The gliomas. Philadelphia: 1999. [Google Scholar]
- 25.Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 26.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holash J, Thurston G, Rudge JS, Yancopoulos GD, Adjei AA, Bergers G, Pytowski B, Pegram M, Gordon MS. Inhibitors of growth factor receptors, signaling pathways and angiogenesis as therapeutic molecular agents. Cancer Metastasis Rev. 2006;25:243–252. doi: 10.1007/s10555-006-8504-6. [DOI] [PubMed] [Google Scholar]
- 28.Blouw B, Song H, Tihan T, Bosze J, Ferrara N, Gerber HP, Johnson RS, Bergers G. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4:133–146. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 29.Du R, Petritsch C, Lu K, Liu P, Haller A, Ganss R, Song H, Vandenberg S, Bergers G. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro Oncol. 2008;10:254–264. doi: 10.1215/15228517-2008-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol Mech Dis. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. [DOI] [PubMed] [Google Scholar]