Summary
Malignant brainstem gliomas (BSG) are rare tumors in adults, associated with a grim prognosis and limited treatment options. Currently, radiotherapy represents the mainstay of treatment, although new studies suggest an increased role for certain chemotherapeutic agents. Intravenous (IV) administration of bevacizumab (Avastin, Genentech Pharmaceuticals) has been shown to be active in the treatment of some enhancing malignant brainstem gliomas. The IV route of administration, however, carries a risk of systemic side effects such as bowel perforation, wound disrepair and pulmonary embolism. In addition, the percentage of IV drug that reaches the tumor site is restricted by the blood brain barrier (BBB).
This technical report describes our protocol in performing superselective intra-arterial cerebral infusion (SIACI) of bevacizumab using endovascular balloon-assistance in the top of the basilar artery in a patient with a recurrent malignant brainstem glioma. It represents the first time such a technique has been performed for this disease.
This method of drug delivery may have important implications in the treatment of both adult and pediatric brainstem gliomas.
Key words: Bevacizumab, brainstem glioma, intra-arterial
Introduction
Brainstem gliomas (BSG) are rare tumors in the adult population, accounting for 1.5-2.5% of all intracranial tumors in adults1,2. Malignant BSG account for approximately 31-39% of the cases of adults with BSG3,4. Although not routinely used to make the diagnosis, histologic examination reveals a World Heath Organization (WHO) grade III or IV glioma in most cases. Radiographically, these lesions exhibit contrast enhancement, often in a ring-like appearance 3. The median survival of patients with maligant BSG tumors has been documented to be 44-74 weeks, with fractionated radiotherapy representing the only effective treatment5.
Studies incorporating bevacizumab in the treatment of recurrent supratentorial malignant tumors have demonstrated up to 50% six month progression-free survival (PSF)6. Although the role for this agent up front or at relapse in the treatment of BSG has not been fully established, a recent case report utilizing bevacizumab and irinotecan in an adult with progressive brainstem glioma demonstrated encouraging results, with clinical and radiographic results similar to those of supratentorial disease.
Recently, we published the technical feasibility of SIACI of mannitol followed by bevacizumab for the treatment of recurrent malignant supratentorial disease7. The protocol for selective infusion includes focal disruption of the BBB and high-dose first-pass infusion of the chemotherapy directly to the target. Given the preliminary data using IV bevacizumab, we wanted to test the hypothesis that SIACI of bevacizumab after BBB disruption would be effective in the treatment of malignant brainstem tumors. This report details the protocol of using endovascular balloon-assistance at the top of the basilar artery to increase brainstem penetration of the chemotherapy bevacizumab. If proven both safe and effective in larger trials, this novel delivery method may offer a better way to treat BSGs both in adults and children.
Materials and Methods
Case History
A 43-year-old male with a diagnosis of malignant BSG was treated at an outside institution with several cycles of temodar and fractionated radiotherapy. Additionally, he received 12 doses of IV bevacizumab with good tolerability and side-effects. Tissue biopsy was never performed, and diagnosis was established by radiographic and clinical criteria. The patient presented to the Weill Cornell Brain Tumor Center in December, 2009 approximately nine months after the original diagnosis with progressive complaints of diplopia and left-sided hemiparesis resulting in wheelchair dependence.
On physical examination, the patient was noted to have right VI and lower motor neuron VII nerve palsies. All other cranial nerves were intact. He was mentating well with no complaints or evidence of cognitive decline or memory impairment. He displayed a dense left-sided spastic hemiparesis, worse in the lower extremities.
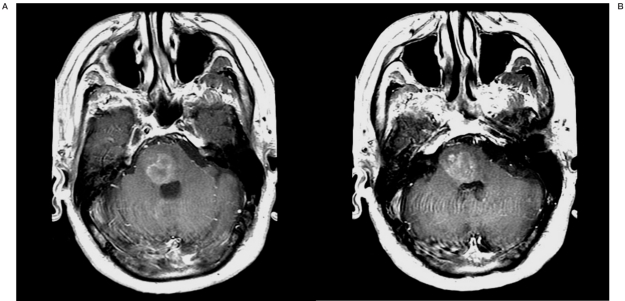
Radiographic studies including gadolinium-weighted magnetic resonance imaging (MRI) demonstrated an expansile and heterogeneously T2 hyperintense mass centered within the inferior pons which extended down to the upper medulla. In addition, a 7 mm area of focal enhancement was present on the ventral lateral aspect of the pons (Figure 1). The patient underwent balloon-assisted SIACI of mannitol and bevacizumab. The protocol was approved by the Institutional Review Board (IRB) at Weill Cornell Medical College.
Figure 1.
Contrast-enhanced Tl-weighted MRI of the brain before superselective intra-arterial cerebral infusion of bevacizumab demonstrating brainstem glioma with nodular enhancement at the ventral lateral aspect of the pons.
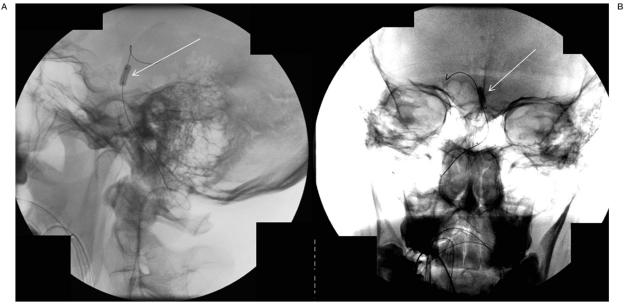
Figure 2.
Fluoroscopy in lateral (A) and AP (B) projections demonstrating balloon (arrow) inflated in the distal basilar artery during selective infusion to prevent flow of chemotherapy to non-targeted areas in the distal posterior circulation.
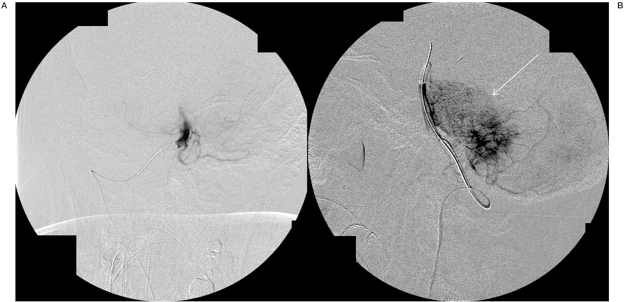
Figure 3.
Selective angiogram through the microcatheter in AP (A) and lateral (B) projections demonstrating increased vascular hyperemia (arrow) in the region of the brainstem glioma s/p infusion of mannitol, suggesting targeted blood-brain-barrier disruption.
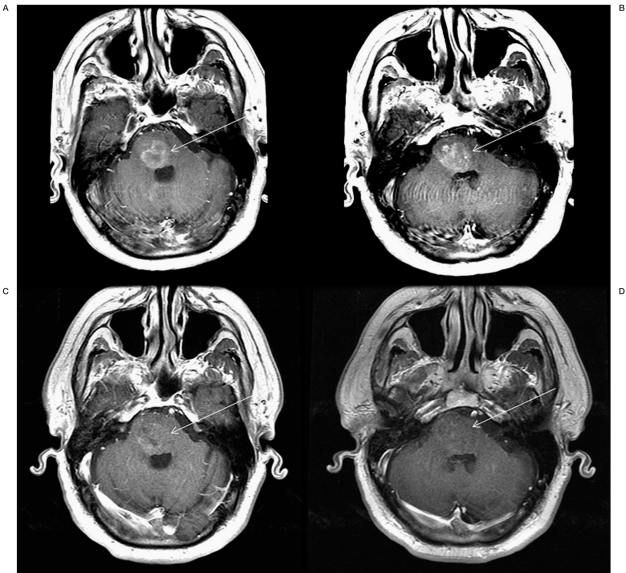
Figure 4.
Contrast-enhanced T1-weighted MRI of the brain before (A,B) and 24 hours after (C,D) superselective intra-arterial cerebral infusion of bevacizumab demonstrating a significant decrease in enhancement within the targeted region of treatment.
Follow-up MRI performed 24 hours after balloon-assisted SIACI of mannitol and bevacizumab demonstrated a marked improvement in the appearance of gadolinium enhancement in the pontine region where the chemotherapy had been targeted compared to pre-infusion sequences (Figure 4). There were no adverse events associated with the delivery of chemotherapy. The patient continues to remain clinically stable without any systemic or local sideeffects of the treatment protocol.
Technical aspects of the balloon-assisted superselective intra-arterial procedure
The procedure was performed with the patient intubated under general anesthesia. The common femoral artery was accessed with a 19-gauge needle which was exchanged for a 6 French Sheath. A 6 French guide catheter was then advanced into the mid-cervical segment of the right vertebral artery. Biplane angiography over the cranium from a right vertebral artery injection demonstrated the basilar artery and its branches in the region of the pontine glioma. After administering intravenous weightbased heparin, a Hyperglide 7x7 balloon (ev3 Neurovascular, Irvine, CA, USA) was advanced over a microwire into the basilar artery distal to the site of planned intra-arterial infusion of mannitol/bevacizumab. Next, a microcatheter was advanced over a microwire through the guide catheter into the lower aspect of the basilar artery at the right vertebrobasilar junction. The catheter was positioned to give maximal infusion along that segment of the basilar artery where the perforators supplying the pons arise. Next, 10 cc of 25% mannitol was infused through the microcatheter over two minutes with the balloon inflated in the distal basilar artery to occlude flow of the medication to non-targeted areas in the distal posterior circulation (Figure 2). Post-infusion angiography demonstrated increased hyperemia in the vasculature supplying the tumor-infiltrated pons as compared to pre-infusion sequences, suggesting that the BBB had been disrupted (Figure 3). The hyperemia following disruption of the blood brain barrier has been observed in the other participants of the trial. Next, 8 mg/kg of bevacizumab in 36 cc was selectively infused through the microcatheter at 1 cc/min with the balloon inflated in the distal basilar artery to occlude flow of chemotherapy to non-targeted areas in the distal posterior circulation. The bevacizumab was infused over the course of three minutes with the balloon inflated, the balloon was then deflated for approximately two minutes to allow blood flow to the posterior circulation, and reinfusion with the balloon inflated was resumed. This was repeated for 36 minutes until all 36 cc of solution had been selectively infused. At the end of the procedure, all catheters were removed. There was no evidence of vascular injury.
Discussion
Bevacizumab is a monoclonal antibody that binds to vascular endothelial growth factor (VEGF) and blocks the formation of new blood vessels, but also affects existing vasculature. Anti-angiogenic therapy may normalize the structure and function of blood vessels and decrease tumor-related edema. Recent studies demonstrating the effectiveness of bevacizumab has established it as a standard, both alone or in combination with other agents, in the treatment of recurrent malignant supratentorial tumors. Despite the encouraging results from phase II trials utilizing IV bevacizumab for recurrent disease 6,8, systemic toxicity has been noted with its use, including infections, venous thromboembolic events, and wound-healing complications occurring in 9.5%, 2.4%, and 3.6%, respectively9-13. In addition, a 46.4% rate of Grade 3 or higher toxicity was seen in patients who received bevacizumab as monotherapy 14. Although the FDA has approved its systemic use in recurrent malignant disease, we have used SIACI delivery of the drug both to improve clinical efficacy rates and decrease systemic side-effect profiles.
The use of intra-arterial delivery of chemotherapy has been shown to be advantageous to IV dosing in cerebral disease in both animal models of glioma and human studies 15. In addition, techniques for BBB disruption with osmotic agents have been developed and safely utilized with chemotherapy infusion for over 20 years 16-19. Hall et Al. described eight patients who received monthly intra-arterial infusion of mannitol followed by carboplatin or methotrexate in addition to IV antineoplastic treatment protocols and radiation therapy. Despite their small sample size, the results were encouraging, with median PFS times of 15 months and median survival times of 27 months20. Their study, however, did not utilize microcatheters for selective delivery of the osmotic agent and antineoplastic drug to the pontine region or distal balloon occlusion to prevent washout to the posterior circulation.
This is the first case report describing balloon-assisted SIACI of an osmotic agent and bevacizumab for malignant BSG. The efficacy of the balloon was demonstrated by no distal contrast flow on the angiogram during inflation, and the efficacy of BBB disruption represented by increased vascular hyperemia in the region of interest. This brief hyperemia allows for increased exposure of the monoclonal antibody bevacizumab to tumor vascular endothelial growth factor targets. The immediate decrease in tumor enhancement following this treatment protocol confirms accurate delivery to the area of interest, and argues for follow-up studies utilizing larger cohorts of patients to define therapeutic efficacy. In addition, future trials with the application of radiolabeled tracers such as 64Cu-DOTA-bevacizumab will afford more precise localization of CNS distribution of chemotherapy after SIACI.
Conclusions
In view of recent studies suggesting the improved efficacy profile of IV bevacizumab on survival in patients with recurrent malignant supratentorial glioma, we have combined this treatment protocol with endovascular techniques for accurate and selective disruption of the BBB before chemotherapy infusion. This technical report describes the method of balloon-assisted SIACI of bevacizumab after BBB disruption for malignant brainstem gliomas. If proven safe and effective in a larger cohort of patients, this paradigm may significantly alter the way chemotherapies are delivered to patients with both diffusely infiltrating low grade, and high grade malignant brainstem gliomas.
References
- 1.Packer RJ. Brain tumors in children. Arch Neurol. 1999;56(4):421–425. doi: 10.1001/archneur.56.4.421. [DOI] [PubMed] [Google Scholar]
- 2.Freemand CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40(2):265–271. doi: 10.1016/s0360-3016(97)00572-5. [DOI] [PubMed] [Google Scholar]
- 3.Guillamo JS, Monjour A, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain. 2001;124:2528–2539. doi: 10.1093/brain/124.12.2528. [DOI] [PubMed] [Google Scholar]
- 4.Salmaggi A, Fariselli L, et al. Natural history and management of brainstem gliomas in adults. A retrospective Italian study. J Neurol. 2008;255:117–177. doi: 10.1007/s00415-008-0589-0. [DOI] [PubMed] [Google Scholar]
- 5.Schild SE, Stafford SL, et al. The results of radiotherapy for brainstem tumors. J Neurooncol. 1998;40(2):171–177. doi: 10.1023/a:1006193306286. [DOI] [PubMed] [Google Scholar]
- 6.Vredenburgh JJ, Desjardins A, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 7.Riina HA, Fraser JF, et al. Superselective intraarterial cerebral infusion of bevacizumab: a revival of interventional neuro-oncology for malignant glioma. J Exp Therapeut Oncol. in press. [PubMed] [Google Scholar]
- 8.Cloughesy TF, Prados MD, et al. A phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) J Clin Oncol. 2008;26:91s. [Google Scholar]
- 9.Pope WB, Lai A, et al. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 10.Nghiemphu PL, Green RM, et al. Safety of anticoagulation use and bevacizumab in patients with glioma. Neuro Oncol. 2008;10(3):355–360. doi: 10.1215/15228517-2008-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai A, Filka E, et al. Phase II pilot study of bevacizumab in combination with temozolamide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008;71(5):1372–1380. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 12.Ananthnarayan S, Bahng, et al. Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol. 2008;88(3):339–347. doi: 10.1007/s11060-008-9573-x. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Delaloye S, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25(30):4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 14.Friedman HS, Prados MD, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 15.Gobin YP, Cloughesy TF, et al. Intraarterial chemotherapy for brain tumors by using a spatiall dose fractionation algorithm and pulsatile delivery. Radiology. 2001;218(3):724–732. doi: 10.1148/radiology.218.3.r01mr41724. [DOI] [PubMed] [Google Scholar]
- 16.Neuwelt EA, Barnett PA, et al. Osmotic blood-brain barrier modification: monoclonal antibody, albumin, and methotrexate delivery to cerebrospinal fluid and brain. Neurosurgery. 1985;17(3):419–423. doi: 10.1227/00006123-198509000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Neuwelt EA, Gilmer-Knight K, et al. Toxicity profile of delayed high dose sodium thiosulfate in children treated with carboplatin in conjunction with blood-brain-barrier disruption. Pediatr Blood Cancer. 2006;47(2):174–182. doi: 10.1002/pbc.20529. [DOI] [PubMed] [Google Scholar]
- 18.Neuwelt EA, Howieson J, et al. Therapeutic efficacy of multiagent chemotherapy with drug delivery enhancement by blood-brain barrier modification in glioblastoma. Neurosurgery. 1986;19(4):573–582. doi: 10.1227/00006123-198610000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Neuwelt EA, Dahlborg SA. Chemotherapy administered in conjunction with osmotic blood-brain barrier modification in patients with brain metastases. J Neurooncol. 1987;4(3):195–207. doi: 10.1007/BF00150611. [DOI] [PubMed] [Google Scholar]
- 20.Hall WA, Doolittle ND, et al. Osmotic blood-brain barrier disruption chemotherapy for diffuse pontine gliomas. J Neurooncol. 2006;77(3):279–284. doi: 10.1007/s11060-005-9038-4. [DOI] [PubMed] [Google Scholar]