Summary
The immediate and long-term outcomes, complications, recurrences and the need for retreatment were analyzed in a series of 280 consecutive patients with anterior communicating artery aneurysms treated with the endovascular technique. From October 1992 to October 2001 280 patients with 282 anterior communicating artery aneurysms were addressed to our center. For the analysis, the population was divided into two major groups: group 1, comprising 239 (85%) patients with ruptured aneurysms and group 2 comprising of 42 (15%) patients with unruptured aneurysms. In group 1, 185 (77.4%) patients had a good initial pre-treatment Hunt and Hess grade of I-III. Aneurysm size was divided into three categories according to the larger diameter: less than 4 mm, between 4 and 10 mm and larger than 10 mm. The sizes of aneurysms in groups 1 and 2 were identical but a less favorable neck to depth ratio of 0.5 was more frequent in group 2.
Endovascular treatment was finally performed in 234 patients in group 1 and 34 patients in group 2. Complete obliteration was more frequently obtained in group 2 unlike a residual neck or opacification of the sac that were more frequently seen in group 1. No peri-treatment complications were recorded in group 2. In group 1 the peri-treatment mortality and overall peri-treatment morbidity were 5.1% and 8.1% respectively. Eight patients (3.4%) in group 1 presented early post treatment rebleeding with a mortality of 88%. The mean time to follow-up was 3.09 years. In group 1, 51 (21.7%) recurrences occurred of which 14 were minor and 37 major. In group 2, eight (23.5%) recurrences occurred, five minor and three major. Two patients (0.8%) presented late rebleeding in group 1.
Twenty-seven second endovascular retreatments were performed, 24 (10.2%) in group 1 and three (8.8%) in group 2, seven third endovascular retreatments and two surgical clippings in group 1 only. There was no additional morbidity related to retreatments.
Endovascular treatment is an effective method for the treatment of anterior communicating artery aneurysms allowing late rebleeding prevention. Peri-treatment rebleeding warrants caution in anticoagulation management. This is a single center experience and the follow-up period is limited. Patients should be followed-up in the long-term as recurrences may occur and warrant additional treatment.
Keywords: brain, cerebral, anterior communicating, aneurysm
Introduction
Endovascular treatment is an increasingly used method for the treatment of ruptured and unruptured aneurysms 1, and demonstrates short-term clinical and angiographic efficacy as a therapeutic alternative to the surgical treatment of intracranial aneurysms 2-10. Anterior communicating (ACom) artery aneurysms represent the majority of aneurysms in the adult population. ACom aneurysms are diagnosed in 22% to 30% of patients with subarachnoid haemorrhage (SAH) 11,12. Our series is, to our knowledge, the largest of anterior communicating artery aneurysms treated with the endovascular technique. We analyzed the immediate and long-term outcome of the technique, associated complications, recurrences and the need for retreatment.
Patients and Techniques
Patient Population
From October 1992, we systematically included all angiographically detected aneurysms in a special registry. Information was recorded prospectively and included the patient's cardiovascular and neurological history, family history, clinical presentation, Hunt and Hess score at arrival and at the time of treatment, treatment delay, aneurysm characteristics, short, mid and long-term clinical and angiographic results. Nine hundred and forty patients had been registered with 1120 aneurysms until October 2001. Of these patients, 280 harboured 282 ACom aneurysms (25.2%). Two patients had two ACom aneurysms. The date of October 2001 was chosen to obtain at least three years of observation for each patient.
Of the patients treated, 152 were females (54.2%) and 128 males (45.7%). The age of the patients ranged from 18 years to 81 years with a mean of 51.45 years (SD+/-13.7 years).
Clinical Presentation
For the analysis, the population of 280 patients with ACom aneurysms was split into two major groups: group 1, comprising 239 (85%) patients with ruptured aneurysms and group 2, comprising 42 (15%) patients with unruptured aneurysms. The discovery of the unruptured aneurysms was fortuitous in 22 patients and secondary to subarachnoid haemorrhage from another intracranial aneurysm in 16 patients. One patient had an aneurysm on a feeding artery of an arteriovenous malformation and one patient had a previously partially clipped aneurysm.
In group 1,185 (77.4%) patients had a good initial pre-treatment Hunt and Hess grade of I-III (Table 1). The amount of blood in the subarachnoid space was quantified according to the Fisher grading scale (Table 2).
Table 1.
Hunt & Hess grade of the patient with a ruptured anterior communicating aneurysm.
| Hunt & Hess grade | n | (%) |
|---|---|---|
| I | 48 | 20,1 |
| II | 95 | 39,7 |
| III | 42 | 17,6 |
| IV | 36 | 15,1 |
| V | 18 | 7,5 |
| Total | 239 | 100 |
Table 2.
Grading of the subarachnoid hemorrhage on initial CT scans of the patients with a ruptured anterior communicating aneurysm according to the Fisher grading scale.
| Fisher Grading of SAH | n | (%) |
|---|---|---|
| 1 | 22 | 9,2 |
| 2 | 50 | 20,9 |
| 3 | 27 | 11,3 |
| 4 | 140 | 58,6 |
| Total | 239 | 100 |
Indication for Aneurysm Treatment
In general, we maintain a policy of aneurysm surgery or coiling as soon as possible (within 72h) after aneurysm rupture. Final decisions regarding the treatment are collectively taken by the members of the vascular neurosurgery and interventional neuroradiology departments. In patients presenting intracranial hypertension due to an intracranial hematoma surgical decompression is immediately performed with surgical clipping of the aneurysm. The remaining patients presenting with subarachnoid hemorrhage, and patients without subarachnoid haemorrhage, are primarily referred to the neurointerventional team to appreciate suitability for endovascular treatment. If the endovascular technique is judged unsuitable or if there is an unsuccessful attempt to treat, the patient is referred for surgical clipping.
Embolization Technique
Endovascular treatment is always preceded by diagnostic digital angiography on a biplanar system with additional multiple views. Since 1997, 3D angiography (Butterfly System, GEMS) has been added to the protocol. Working projections are determined from the multiple views or the 3D acquisition. The final treatment result is always evaluated in the working projections with additional face and profile projections including the late venous phase to rule out thromboembolic complications.
The procedures are performed with the patients under general anaesthesia, and systemic heparinization is provided throughout. An initial dose of 2000 to 3000 U of heparin is i.v. administered, followed by continuous administration of 20 to 40 Units/kg/h with an electrical syringe throughout the treatment. At the end of the treatment the heparin is not antagonized. Postoperatively, a low molecular weight heparin is used at curative doses first and then at preventive doses for 48 hours to one week, according to the secondary thrombotic arterial risk, which is estimated as a function of the size of the neck and the possible protrusion of a coil into the parent artery. Thereafter platelet antiaggregating agents are used: acetylsalicylic acid (Aspirin, Bayer, Germany) and since 1998 Flurbiprofene (Cebutid, Shire Laboratories, France) at a daily dose of 100 mg for one month.
Besides anti-coagulants, patients receive intravenous nimodipine (Nimotop, Bayer Laboratories, Germany) throughout the procedure, at a dose of 10 mg/h, which is maintained, in the case of ruptured aneurysms, for a time period of 14 to 21 days, or interrupted at the end of the intervention in other settings. Papaverine at a dose of 300 mg diluted in 1l of physiologic saline solution is usually administered through the working catheter throughout the procedure.
In this series, when a thromboembolic complication occurred, we first used small remedies: arterial pressure augmentation, intra-arterial papaverine injection, eventually associated with pulsed injections of physiologic saline at the level of the thrombus. If this failed, we used intra-arterial administration of urokinase directly at the level of the thrombus. No patient was treated with anti-Gp IIb-IIIa.
Endovascular treatment comprised selective catheterization of the aneurysm and compaction using coils. No remodeling technique was used. The final anatomical outcome was assessed according to Raymond's classification 43: a complete obliteration was recorded if there was no filling of contrast medium in the dome, body or neck. A residual neck involved residual filling of part of the neck and a partial occlusion involved filling of contrast medium in the aneurysmal dome.
After the intervention, patients were closely monitored for vasospasm. The patients were discharged home or to their referring hospital when their condition became satisfactory.
Clinical and Angiographic Follow-up Studies
Clinical and angiographic follow-up were done six weeks, six months, one year, three years and five years post embolization and thereafter as considered suitable. Angiographic follow-up was performed with classical angiography or 3D Time of Flight (TOF) magnetic resonance angiography (MRA). All patients had at least one control with classical angiography at one year post embolization with face, profile and working projections. 3D TOF MRA was usually performed without contrast unless the imaging results were ambiguous in which case an additional 3D TOF sequence with contrast was performed.
Statistical Analysis
Statistical analysis of the results was made using the Chi-square test and the Student t test.
Results
Aneurysm Description
The number of aneurysms per patient, including other locations besides the ACom artery, ranged from one to five with a mean of 1.28 aneurysms per patient. Aneurysm size was divided into three categories according to the larger diameter: less than 4 mm, between 4 and 10 mm and larger than 10 mm. Aneurysm neck to depth ratio was also divided into three categories: less than 0.5, between 0.5 and 1, and larger than 1. As shown in Tables 3 and 4, the sizes of aneurysms in groups 1 and 2 were identical but a less favorable neck to depth ratio of 0.5 was more frequent in group 2. Size was associated with a large neck to depth ratio in group 1 (p=0.025), an association not withstanding for group 2 (p = 0.2).
Table 3.
Aneurysmal size in ruptured and unruptured anterior communicating aneurysms.
| Size | Ruptured % | Unrupture % |
|---|---|---|
| < 4 mm | 92 (38,5) | 16 (38,1) |
| 4-10 mm | 126 (52,7) | 22 (52,4) |
| > 10 mm | 21 (8,8) | 4 (9,5) |
| Total | 239 (100) | 42 (100) |
Table 4.
Aneurysmal size in ruptured and unruptured anterior communicating aneurysms.
| Neck to Depth ratio | Ruptured % | Unrupture % |
|---|---|---|
| < 0,5 | 107 (44,8) | 11 (26,2) |
| 0,5-1 | 128 (53,6) | 30 (71,4) |
| >1 | 4 (1,7) | 1 (2,4) |
| Total | 239 (100) | 42 (100) |
Immediate Anatomical Outcomes
Endovascular treatment was not performed in 12 patients, five patients in group 1 and seven patients in group 2. The reason was either a very tortuous vessel anatomy in eight cases or a very small aneurysmal size of 2 mm in four cases.
Complete obliteration was more frequently obtained in group 2 unlike a residual neck or opacification of the sac that were more frequently seen in group 1 (Table 5).
Table 5.
Immediate anatomical outcome.
| Immediate anatomical outcome |
Ruptured % | Unrupture % |
|---|---|---|
| Complete obliteration | 66 (22,8) | 13 (38,2) |
| Neck remnant | 133 (56,8) | 18 (52,9) |
| Partial obliteration | 35 (15) | 3 (8,8) |
| Total | 234 (100) | 34 (100) |
Peri-Treatment Complications
No peri-treatment complications were recorded in group 2. In group 1, however, peritreatment complications included parent vessel delamination, coil rupture or migration, aneurysm rupture and thromboembolic episodes. Peri-treatment complications occurred in 53/234 cases (22.6%).
Parent vessel delamination occurred in three cases without changing the final clinical outcome. Coil rupture occurred in two cases, where the coils were lassoed and retrieved. Coil migration into the parent artery during treatment occurred in one case. It was chosen to leave the coil in place and put the patient under anticoagulation. The patient had an excellent outcome without developing any ischemic lesion.
Aneurysmal rupture during treatment occurred in ten cases. This resulted in further worsening of the patient's clinical condition in two cases, while it was thought to have no deleterious effect in eight cases.
Thromboembolic episodes during treatment occurred in 19 patients. In thirteen cases the episode was embolic and in six it was thrombotic and occurred at the aneurysmal neck region. These episodes resulted in one death in the case of an MCA occlusion and accounted for additional neurological deficits in four patients. Two patients who survived a thromboembolic episode successfully managed with anticoagulation and fibrinolysis, presented an early rebleeding episode and died. For the remaining thrombembolic episodes, treatment (anticoagulation and/or fibrinolysis) resulted in no additional neurological deficit.
Eight patients with subarachnoid hemorrhage presented early post treatment rebleeding, defined as a rebleeding episode within 30 days after treatment (one day to 19 days, mean= 9.8 days). One patient had two rebleeding episodes: one 18 days after initial treatment, and one 90 days after retreatment. The patient was eventually operated and had a GOS of II (Table 6). Early rebleeding was associated with a subtotal occlusion in four cases and a partial occlusion in one case. In two patients with subtotal occlusion a thromboembolic event had occurred during treatment and heparin was ongoing. Another patient had a recent frontal ischemic lesion and two patients had a frontal hematoma. There was no possible explanation for the rebleeding episode in two patients who had total initial aneurysmal occlusions. In the majority of cases (87.5%) rebleeding episodes had a fatal outcome. The calculated early rebleeding rate is 3.4% (8/234).
Table 6.
Initial Hunt & Hess grade, occlusion percentage, discharge GOS (Glasgow Outcome Scale) and associated factors in patients with early rebleeding.
| Hunt & Hess Grade | Occlusion | GOS | Coexisting conditions |
|---|---|---|---|
| II | Complete | V | None |
| III | Complete | V | None |
| I* | Neck remnant | I | Rupture during treatment |
| II | Neck remnant | V | Thromboembolic episode |
| III | Neck remnant | V | Recent frontal ischemic lesion |
| IV | Neck remnant | V | Thromboembolic episode, intraparenchymal hematoma |
| II | Neck remnant | V | Intraparenchymal hematoma |
| IV | Partial opacification | V | None |
| * Patient with 2 rebleeding episodes | |||
Post-treatment thrombotic events occurred in three patients (six days to 18 days post-treatment, mean seven days). They all presented occlusion of the A2 segment of the anterior cerebral artery. This occurred in patients with an already critical clinical condition (Hunt & Hess Grade of IV) resulting in the death of one patient, while the other two patients remained in poor clinical condition.
Symptomatic vasospasm occurred in nine (3.9%) of the 234 patients with SAH: three patients presented CT lesions at the time of treatment, three developed new symptoms while hospitalized and three developed new CT lesions while sedated in the intensive care unit after the endovascular treatment. Angioplasty was performed in five of these patients. Selective intra-arterial papaverine infusion was performed in one patient. The decision not to treat was taken for three patients for whom it was estimated that the vasospasm-induced ischemic lesions where already constituted based on clinical and imaging data.
Of the five angioplasties performed, two angiplasties where done at the time of treatment and three six to eight days after treatment. Indication to treat was the occurrence of new neurologic symptoms in two patients and the appearance of new CT lesions in three patients. Angioplasty was performed on the A1 segment in one patient, on M1 and C1 segment on three patients, and on the M1, M2 segments in one patient. Restitution of the caliber of the angioplastied vessel was obtained in all cases. No angioplasty-related complications occurred. Stabilization of lesions as demonstrated on CT or clinically, was observed in four cases with one case showing complete clinical resolution. Selective intra-arterial papaverine was administered in one patient but could not prevent the development of large ischemic lesions in both parietal regions.
Morbidity and Mortality
The peri-treatment mortality and overall peri-treatment morbidity were five, 1% (12/234) and 8.1% (19/234) respectively.
Immediate Clinical Outcome
All patients were clinically evaluated before hospital discharge. The clinical status of patients in group 2 was conditioned by their initial clinical state. In group 1 73.2% of the patients with a good pre-treatment Hunt & Hess Grade (I-III) had a good clinical outcome of Glasgow Outcome Scale (GOS) of 1, while of the patients with a poor pre-treatment Hunt & Hess Grade (IV or V) only 17.8% had a good clinical outcome (GOS1) (Table 7).
Table 7.
Initial Hunt and Hess grade and final Glasgow Outcome Scale (GOS) in the group of patients with ruptured anterior communicating artery aneurysms.
| GOS | ||||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Hunt and Hess Grade |
I | 48 | 2 | 0 | 0 | 1 |
| II | 68 | 12 | 2 | 3 | 5 | |
| III | 18 | 11 | 6 | 0 | 7 | |
| IV | 6 | 5 | 12 | 1 | 9 | |
| V | 3 | 0 | 3 | 1 | 11 | |
Clinical Follow-up
The mean time of follow-up was 3.09 years (min 0, max 11 years). The patients' follow-up at each time period is shown in Table 8. Overall, the clinical evolution (GOS grade) of our patients was stable. Only two patients presented late rebleeding (0.8%). One was the patient who had two rebleeding episodes, one episode 18 days after initial treatment, and one episode 90 days after retreatment. The patient was eventually operated and had a GOS of II. The second patient presented SAH ten years after the initial SAH treatment because of a new Acom artery aneurysm that developed de novo near the initially treated aneurysm.
Table 8.
Percentage of satisfactory aneurysm occlusions in ruptured and unruptured aneurysms (complete obliteration or neck remnant) proportional to the patients followed.
| Ruptured | Unruptured | |
|---|---|---|
| Initial | (85%) 199/234 | (91,2%) 31/34 |
| 6 months | (78,6%) 132/168 | (93,3%) 28/30 |
| 1 year | (73,4%) 113/154 | (92,6%) 26/28 |
| 3 years | (74,8%) 104/139 | (92,3%) 24/26 |
| 5 years | (79%) 68/86 | (88,2%) 15/17 |
Anatomical Follow-up
The rate of satisfactory occlusions, that is complete obliterations or neck remnants, remained relatively stable throughout the follow-up period (Table 8).
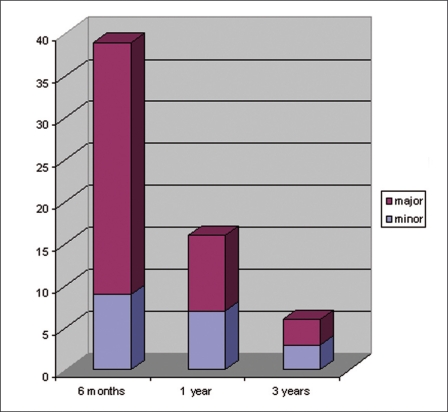
An aneurysm with complete obliteration or a neck remnant that developed an opacification during follow-up inside the sac was considered a major recurrence while a complete obliteration that developed a neck remnant was considered a minor recurrence. In group 1,51 (21.7%) recurrences occurred of which 14 were minor and 37 major. In group 2, eight (23, 5%) recurrences occurred, five minor and three major. The mean time of recurrence was 0.9 years with a minimum of six months and a maximum of three years (Figure 1). A recurrence in general was most likely to occur in previously ruptured anterior communicating aneurysms (P<0,001) and in an aneurysm with a size less than 4 mm (p=0.018). However there was no association with the neck to depth ratio (p=0.22), sex (p=0.5) or age (p=0.9). An initially complete obliteration had no effect on the probability of a major recurrence either in group 1 (p=0.25) or group 2 (p=0.678). Most recurrences (66%) were observed at the six months follow-up.
Figure 1.
Time of occurrence of major and minor recurrences after the initial treatment.
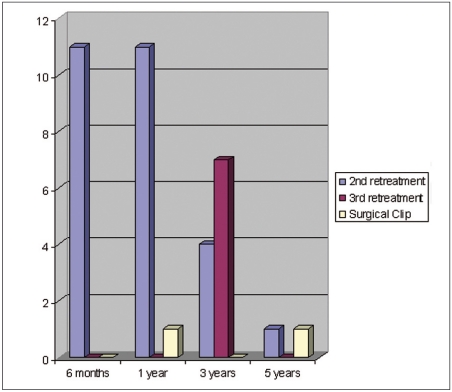
Twenty-seven second endovascular retreatments were performed, 24 (10.2%) in group 1 and three (8.8%) in group 2, seven third endovascular retreatments and two surgical clipping, in group 1 only. Most retreatments were performed during the first year after the initial treatment (Figure 2). There was no additional morbidity related to retreatments.
Figure 2.
Time and type of retreatments.
Discussion
As an alternative to the surgical treatment of ruptured and unruptured aneurysms, endovascular coiling of aneurysms has proved to be effective, with good outcomes achieved in early clinical and angiographic settings 3-10. At our institution, very early endovascular treatment has been proposed as the therapeutic intervention of first choice whatever the localization of the aneurysm.
Aneurysms located along the anterior communicating artery are the most frequently treated ones at the circle of Willis 13,14 In our series they represent 25.2% of aneurysms proposed for endovascular treatment. In the ISAT study 15, where both surgical and endovascular treatment options were available, this localization represented 45.4%. In the work of Henkes 16 which comprised 1811 aneurysms treated with the endovascular technique, they represented 9.8% of ruptured and 26.5 of unruptured aneurysms.
The surgical approach to ACom artery aneurysms in the interhemispheric fissure may be difficult because of arterial relationships, posterior fundus projection, and barriers presented by the diencephalons. Satisfactory clip occlusion of ACom artery aneurysms, while attempting to preserve the perforating arteries and maintain the patency of the ACom artery and pericallosal arteries, can be problematic. The frequent association of this aneurysm with complex ACom artery anomalies, which are encountered in 35% of cases 5,17, often complicates the perioperative analysis.
Endovascular treatment for ACom artery aneurysms also has some disadvantages. ACom aneurysms are technically difficult to treat when their size is 3 mm or less, in case of complex aneurysms with incorporation of adjacent ACom arteries, when the fundus of the aneurysm has a posterior projection, or when acute vessel angles have to be traversed by the microcatheter to access the aneurysm 5,17. Other causes of failure to embolize ACom artery aneurysms are the difficulty in distinguishing the aneurysmal neck 5 or the existence of vascular loops in the cervical and intracranial internal carotid artery in spite of using the cervical approach. In our series twelve aneurysms were not treated by the endovascular technique because of an unfavourable depth to neck ratio in eight patients or a very small aneurysmal size of 2 mm in four cases. Five aneurysms were judged to be unsuitable for endovascular treatment at the time of diagnostic angiography while in the other seven endovascular treatment was attempted without success. It must be noted, however, that this series encompasses all our cases from the beginning of our experience and that the remodelling techniques proposed by Moret 18 were not used. The endovascular technique allowed treatment of 108 aneurysms (40.2%) smaller than 4 mm. Of these, 11 aneurysms were smaller than 3 mm and the smallest aneurysm treated had a diameter of 2 mm. Understanding of the aneurysm angioarchitecture was substantially improved by the early introduction of 3D angiography at our institution. This improved our evaluation of the treatment risk in difficult cases, while it also resulted in a broadening of endovascular indications. Continuous improvement of interventional devices in recent years, in conjunction with the increased expertise of our interventional neuroradiologists allow us to access and treat with safety aneurysms with difficult vascular configurations 19.
Complete occlusion of an intracranial aneurysm is a necessary goal of both surgical and endovascular treatment methods. Regrowth and/or rebleeding of aneurysms incompletely treated by surgery are well documented in the literature 20-28. In our series, the percentage of complete occlusion achieved after initial treatment was 22.8% for ruptured and 38.2% for unruptured aneurysms. A partial obliteration was more common in the ruptured group (15%) than in the unruptured group (8%). In the literature the quoted rates of complete occlusion range from 35.9% to 82.5% for anterior communicating artery aneurysms and aneurysms in general 5,29-33. The respective rates for partial occlusion are 2.5% to 27.5% 5,30,31,33. The differences in ruptured and unruptured aneurysms may be explained by our different treatment strategy in each clinical setting. During the acute phase of a subarachnoid haemorrhage, we first aim to secure the aneurysm from an eventual rebleeding. Attempts for a perfect treatment are made only if this does not involve excessive additional risk. This is even more crucial in the anterior communicating artery area were the involved arteries have a very small width. This attitude is further supported by the relative ease with which a residual aneurysm may be controlled by follow-up MRA. MRA allows the early detection of re-growth and may indicate the need for re-treatment. On the other hand, in unruptured aneurysms there is a higher expectancy for a perfect anatomical result, while the operator usually feels microcatheter manoeuvres to be safer.
Immediate clinical results are in the range of those observed for the endovascular treatment of aneurysms in other localizations. For ruptured aneurysms the outcome is conditioned by the initial clinical status: in the subgroup of patients with a good pre-treatment Hunt & Hess Grade (I-III), 73.2% had a good clinical outcome (GOS 1). In the subgroup of patients with a poor pre-treatment Hunt & Hess Grade (IV or V) only 17.8% had a good clinical outcome (GOS 1). This is a low rate, which corresponds to other reports on poor pre-treatment grade patients 34,35. The surgical literature published after the introduction of CT scanning 36-44 reports good results (GOS of 1 and 2) in 74% to 87.5% of patients and excellent results (GOS 1) in up to 56.4% of patients with Acom artery aneurysms. This numbers reflect a variety of preoperative status and inclusion criteria. In our series, the percentage of good and excellent results was 77.4% and 65.2% respectively. Moreover, in our series the percentage of patients having a poor clinical grade (Hunt & Hess IV or V) before treatment was 17.6%, which is a higher rate than the 4.8% to 5.6% usually reported in the surgical literature. The peri-treatment complication rate of the ruptured aneurysms group was 22.6%, resulting in mortality of 5.1% and a permanent morbidity of 8.1%. Previous reports have documented complication rates ranging from 8% to 17%, with morbidity rates of 3% to 6.5% and mortality rates of 0% to 6.5% 6,45-48. Our mortality rate for non ruptured aneurysms was null as it was for the series of Murayama et Al 6.
The majority of peri-treatment complications were thromboembolic with a rate of 8.1%, a morbidity of 2.1% and a mortality of 0.4%. Additionally, three thrombotic episodes occurred in the post-treatment period. Thromboembolic complications occurred exclusively in patients who had initially ruptured aneurysms. In their series, Pelz et Al 47 noted thromboembolic events in 28% of patients with a permanent morbidity of 5% and no mortality. The increased incidence of thromboembolic complications during the endovascular treatment of ruptured, compared to the treatment of non ruptured aneurysms has also been noted by other authors 47,49. The occurrence of peri-treatment and post-treatment thromboembolic complications (up to 18 days post treatment in our series) prompted us to use a prevention protocol that combines the administration of heparin peri- and post-treatment and antiaggregating agents (acetylsalicylic acid and later flurbiprofene) for a month after the intervention.
The second most common peri-treatment complication was aneurysmal rupture during treatment that occurred in ten cases (4.3%), exclusively in previously ruptured aneurysms. Although it is an alarming complication, it was thought to have no deleterious effect on the patient's neurological status in 80% of cases. This good outcome has also been observed for other aneurysm localizations 50.
The most important post-treatment complication was early rebleeding which occurred in eight patients (3.4%). All patients had an initially ruptured aneurysm. Early post-treatment rebleeding had a fatal outcome in 88% of cases. In five patients, rebleeding could be associated with coexisting aggravating conditions: aneurysmal rupture during treatment, peri-treatment thromboembolic episode necessitating anticoagulation, pre-existing intraparenchymal frontal hematoma or an aneurysm treated with a partial occlusion. There was no explanation for the rebleeding in two patients who had aneurysms totally occluded. Early rebleeding has also been described for other aneurysm locations at a similar rate 19,51. It is often lethal, and may occur despite a completely occluded aneurysm 19,51 as was the case in two patients of our series. The remainder of our patients with early rebleeding had an initial incomplete aneurysm occlusion and most were in a poor initial clinical condition. We believe that this potential complication warrants additional caution regarding anticoagulation management especially when thromboembolic events, intraparenchymal hematomas, or partial occlusions of ruptured aneurysms occur.
Recurrence rates are the same as for other aneurysm locations 19,31. During a mean follow-up of three years there were 40 major (14.9%) and 19 minor (7%) recurrences with however 40% of patients lost at the three-year follow-up. Raymond et Al report a recurrence rate of 33.6% with 20.7% major recurrences in a series of 501 aneurysms with a mean follow-up time of 31.3 months 31. In our series the mean time of recurrence was 0.9 years. Most recurrences occurred during the first year post-treatment, but some occurred even up to three years post treatment. Thus, extended follow-up seems to be mandatory 31. The risk of recurrence was more important for patients with an initially ruptured aneurysm (p<0.001) and an aneurysm size larger than 4 mm (p=0.018), factors that are commonly recognized as predictors for recurrences for all aneurysm locations in general 31,52,53. We failed to find any association with the depth to neck ratio or the degree of initial obliteration in both ruptured and unruptured groups of patients. While Raymond et Al reported a relationship of the degree of initial occlusion with the development of a subsequent recurrence on the short-term follow-up, this relationship no longer existed in their longest follow-up period 31. Only two patients presented late rebleeding (0.8%), one 90 days after the initial treatment and one ten years later due to the de novo formation of an aneurysm on the anterior communicating artery. In their series of 501 aneurysms on multiple localizations, Raymond et Al.31 reported a late rebleeding rate of 0.8% during a mean clinical follow-up period of 31.3 months. A satisfactory occlusion effectively protected against the risk of rebleeding. Thus, contemporary technical refinements, such as 3D coils and the balloon-assisted technique, by improving our ability to obtain complete neck occlusions should reduce the incidence of late rebleeding episodes. However, even with complete occlusions, the late rebleeding risk persists 31.
Twenty-seven (10%) second endovascular retreatments were performed, 24 concerned previously ruptured and three previously unruptured aneurysms. Seven third endovascular retreatments and two surgical clippings were recorded only in the group of ruptured aneurysms. Most retreatments were performed during the first year after the initial treatment. There was no additional morbidity or mortality related to retreatments. In a series of 2759 aneurysms on different locations, Henkes et Al.54 performed second retreatments in 350 (12.3%) aneurysms and three or more retreatments in 94 (3.4%) aneurysms with a low associated morbidity-mortality rate of 2.2%. However, as the authors point out, the risk of retreatment has to be carefully weighted against the low risk of bleeding of partially filled aneurysms. In our series the occlusion rate after a second treatment was stable in 74% (20/27). However this subgroup included a very small number of patients.
Our series included consecutive patients whose assessment was performed during a nine year period. The main limitations of our study were its retrospective nature and the lack of clinical and angiographic follow-up data for a considerable percentage of patients. Thus, we cannot exclude the possibility that some late rebleeding episodes occurred without our knowledge. These cautions notwithstanding, we believe that this study represents an accurate review of immediate and long-term outcomes as well as procedure-related complications after endovascular anterior communicating artery aneurysm treatment.
Conclusions
Our experience, with a mean follow-up of 3.09 years, shows that endovascular treatment is an effective method for the treatment of anterior communicating artery aneurysms allowing late rebleeding prevention. Peri-treatment rebleeding warrants caution in anticoagulation management. This is a single centre experience and the follow-up period is limited. Patients should be followed-up in the long-term as recurrences may occur and warrant additional treatment.
References
- 1.Murayama Y, Nien YL, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez N, Murayama Y, et al. Treatment of unruptured aneurysms with GDCs: clinical experience with 247 aneurysms. Am J Neuroradiol. 2004;25:577–583. [PMC free article] [PubMed] [Google Scholar]
- 3.Graves VB, Strother CM, et al. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery. 1995;37:640–647. doi: 10.1227/00006123-199510000-00006. discussion 647-648. [DOI] [PubMed] [Google Scholar]
- 4.Guglielmi G, Viñuela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 5.Moret J, Pierot L, et al. Endovascular treatment of anterior communicating artery aneurysms using Guglielmi detachable coils. Neuroradiology. 1996;38:800–805. doi: 10.1007/s002340050352. [DOI] [PubMed] [Google Scholar]
- 6.Murayama Y, Viñuela F, et al. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg. 1999;90:207–214. doi: 10.3171/jns.1999.90.2.0207. [DOI] [PubMed] [Google Scholar]
- 7.Nichols DA, Brown RD, Jr, et al. Endovascular treatment of ruptured posterior circulation aneurysms using electrolytically detachable coils. J Neurosurg. 1997;87:374–380. doi: 10.3171/jns.1997.87.3.0374. [DOI] [PubMed] [Google Scholar]
- 8.Raymond J, Roy D, et al. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg. 1997;86:211–219. doi: 10.3171/jns.1997.86.2.0211. [DOI] [PubMed] [Google Scholar]
- 9.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 10.Yundt KD, Dacey RG, Jr., Diringer MN. Hospital resource utilization in the treatment of cerebral aneurysms. J Neurosurg. 1996;85:403–409. doi: 10.3171/jns.1996.85.3.0403. [DOI] [PubMed] [Google Scholar]
- 11.Ogungbo B, Gregson BA, et al. Trends over time in the management of subarachnoid haemorrhage in Newcastle: review of 1609 patients. Br J Neurosurg. 2001;15:388–395. doi: 10.1080/02688690120082387. [DOI] [PubMed] [Google Scholar]
- 12.Sim JH. Intracranial aneurysms in Korea. Neurol Med Chir (Tokyo) 1998;38(Sup):118–121. doi: 10.2176/nmc.38.suppl_118. [DOI] [PubMed] [Google Scholar]
- 13.Kassell NF, Torner JC, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18–36. doi: 10.3171/jns.1990.73.1.0018. [DOI] [PubMed] [Google Scholar]
- 14.Le Roux PD, Elliott JP, et al. Risks and benefits of diagnostic angiography after aneurysm surgery: a retrospective analysis of 597 studies. Neurosurgery. 1998;42:1248–1254. doi: 10.1097/00006123-199806000-00026. discussion 1254-1245. [DOI] [PubMed] [Google Scholar]
- 15.Molyneux A, Kerr R, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 16.Henkes H, Fischer S, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004;54:268–280. doi: 10.1227/01.neu.0000103221.16671.f0. discussion 280-265. [DOI] [PubMed] [Google Scholar]
- 17.Proust F, Debono B, et al. Treatment of anterior communicating artery aneurysms: complementary aspects of microsurgical and endovascular procedures. J Neurosurg. 2003;99:3–14. doi: 10.3171/jns.2003.99.1.0003. [DOI] [PubMed] [Google Scholar]
- 18.Moret J, Cognard C, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol. 1997;24:30–44. [PubMed] [Google Scholar]
- 19.Willinsky RA, Peltz J, et al. Clinical and Angiographic Follow-up of Ruptured Intracranial Aneurysms Treated with Endovascular Embolization. Am J Neuroradiol. 2009;30:1035–1040. doi: 10.3174/ajnr.A1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drake CG, Peerless SJ, Hernesniemi JA. Complications of surgery for vertebrobasilar artery aneurysms and final comments. In: Drake CG, editor. Surgery of Vertebrobasilar Aneurysms: London, Ontario, Experience on 1,767 Patients. New York, NY: Springer-Verlage; 1996. pp. 300–311. [Google Scholar]
- 21.Drake CG, Friedman AH, Peerless SJ. Failed aneurysm surgery. Reoperation in 115 cases. J Neurosurg. 1984;61:848–856. doi: 10.3171/jns.1984.61.5.0848. [DOI] [PubMed] [Google Scholar]
- 22.Feuerberg I, Lindquist C, et al. Natural history of postoperative aneurysm rests. J Neurosurg. 1987;66:30–34. doi: 10.3171/jns.1987.66.1.0030. [DOI] [PubMed] [Google Scholar]
- 23.Fox AJ, Drake CG. Aneurysm neck remnant following balloon embolization. J Neurosurg. 1987;67:321–323. doi: 10.3171/jns.1987.67.2.0321. [DOI] [PubMed] [Google Scholar]
- 24.Giannotta SL, Litofsky NS. Reoperative management of intracranial aneurysms. J Neurosurg. 1995;83:387–393. doi: 10.3171/jns.1995.83.3.0387. [DOI] [PubMed] [Google Scholar]
- 25.Heilman CB, Kwan ES, Wu JK. Aneurysm recurrence following endovascular balloon occlusion. J Neurosurg. 1992;77:260–264. doi: 10.3171/jns.1992.77.2.0260. [DOI] [PubMed] [Google Scholar]
- 26.Hodes JE, Fox AJ, et al. Rupture of aneurysms following balloon embolization. J Neurosurg. 1990;72:567–571. doi: 10.3171/jns.1990.72.4.0567. [DOI] [PubMed] [Google Scholar]
- 27.Koivisto T, Vanninen R, et al. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke. 2000;31:2369–2377. doi: 10.1161/01.str.31.10.2369. [DOI] [PubMed] [Google Scholar]
- 28.Lin T, Fox AJ, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurg. 1989;70:556–560. doi: 10.3171/jns.1989.70.4.0556. [DOI] [PubMed] [Google Scholar]
- 29.Elias T, Ogungbo B, et al. Endovascular treatment of anterior communicating artery aneurysms: results of clinical and radiological outcome in Newcastle. Br J Neurosurg. 2003;17:278–286. doi: 10.1080/0268869031000153251. [DOI] [PubMed] [Google Scholar]
- 30.Gallas S, Pasco A, et al. A multicenter study of 705 ruptured intracranial aneurysms treated with Guglielmi detachable coils. Am J Neuroradiol. 2005;26:1723–1731. [PMC free article] [PubMed] [Google Scholar]
- 31.Raymond J, Guilbert F, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 32.Turjman F, Massoud TF, et al. Predictors of aneurysmal occlusion in the period immediately after endovascular treatment with detachable coils: a multivariate analysis. Am J Neuroradiol. 1998;19:1645–1651. [PMC free article] [PubMed] [Google Scholar]
- 33.Yu SC, Chan MS, et al. Intracranial aneurysms treated with Guglielmi detachable coils: midterm clinical and radiological outcome in 97 consecutive Chinese patients in Hong Kong. Am J Neuroradiol. 2004;25:307–313. [PMC free article] [PubMed] [Google Scholar]
- 34.Bracard S, Lebedinsky A, et al. Endovascular treatment of Hunt and Hess grade IV and V aneuryms. Am J Neuroradiol. 2002;23:953–957. [PMC free article] [PubMed] [Google Scholar]
- 35.Weir RU, Marcellus ML, et al. Aneurysmal subarachnoid hemorrhage in patients with Hunt and Hess grade 4 or 5: treatment using the Guglielmi detachable coil system. Am J Neuroradiol. 2003;24:585–590. [PMC free article] [PubMed] [Google Scholar]
- 36.Diraz A, Kobayashi S, et al. Surgical approaches to the anterior communicating artery aneurysm and their results. Neurol Res. 1993;15:273–280. doi: 10.1080/01616412.1993.11740148. [DOI] [PubMed] [Google Scholar]
- 37.French LA, Zarling ME, Schultz EA. Management of aneurysms of the anterior communicating artery. J Neurosurg. 1962;19:870–876. doi: 10.3171/jns.1962.19.10.0870. [DOI] [PubMed] [Google Scholar]
- 38.Hori S, Suzuki J. Early and late results of intracranial direct surgery of anterior communicating artery aneurysms. J Neurosurg. 1979;50:433–440. doi: 10.3171/jns.1979.50.4.0433. [DOI] [PubMed] [Google Scholar]
- 39.Keogh AJ, Sharma RR, Vanner GK. The anterior interhemispheric trephine approach to anterior midline aneurysms: results of treatment in 72 consecutive patients. Br J Neurosurg. 1993;7:5–12. doi: 10.3109/02688699308995050. [DOI] [PubMed] [Google Scholar]
- 40.Nathal E, Yasui N, et al. Intraoperative anatomical studies in patients with aneurysms of the anterior communicating artery complex. J Neurosurg. 1992;76:629–634. doi: 10.3171/jns.1992.76.4.0629. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa A, Suzuki M, et al. Vascular anomalies associated with aneurysms of the anterior communicating artery: microsurgical observations. J Neurosurg. 1990;72:706–709. doi: 10.3171/jns.1990.72.5.0706. [DOI] [PubMed] [Google Scholar]
- 42.Sengupta RP. Anterior communicating aneurysm: its surgical approach and results. In: Suzuki J, editor. Advances in Surgery for Cerebral Stroke. Berlin: Springer-Verlag; 1988. pp. 269–274. [Google Scholar]
- 43.Sengupta RP, Chiu JS, Brierley H. Quality of survival following direct surgery for anterior communicating artery aneurysms. J Neurosurg. 1975;43:58–64. doi: 10.3171/jns.1975.43.1.0058. [DOI] [PubMed] [Google Scholar]
- 44.Yasargil MG. Microneurosurgery. 4 Volumes. New York: Thieme; 1984. pp. 180–185. [Google Scholar]
- 45.Gruber A, Killer M, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery. 1999;45:793–803. doi: 10.1097/00006123-199910000-00013. discussion 803-794. [DOI] [PubMed] [Google Scholar]
- 46.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single-center experience. Neurosurgery. 1998;43:1016–1025. doi: 10.1097/00006123-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Pelz DM, Lownie SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. Am J Neuroradiol. 1998;19:1541–1547. [PMC free article] [PubMed] [Google Scholar]
- 48.Solander S, Ulhoa A, et al. Endovascular treatment of multiple intracranial aneurysms by using Guglielmi detachable coils. J Neurosurg. 1999;90:857–864. doi: 10.3171/jns.1999.90.5.0857. [DOI] [PubMed] [Google Scholar]
- 49.Iijima A, Piotin M, et al. Endovascular treatment with coils of 149 middle cerebral artery berry aneurysms. Radiology. 2005;237:611–619. doi: 10.1148/radiol.2372041015. [DOI] [PubMed] [Google Scholar]
- 50.Brisman JL, Niimi Y, et al. Aneurysmal rupture during coiling: low incidence and good outcomes at a single large volume center. Neurosurgery. 2005;57:1103–1109. doi: 10.1227/01.neu.0000185631.20246.1a. discussion 1103-1109. [DOI] [PubMed] [Google Scholar]
- 51.Sluzewski M, van Rooij WJ. Early rebleeding after coiling of ruptured cerebral aneurysms: incidence, morbidity, and risk factors. Am J Neuroradiol. 2005;26:1739–1743. [PMC free article] [PubMed] [Google Scholar]
- 52.Cognard C, Weill A, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212:348–356. doi: 10.1148/radiology.212.2.r99jl47348. [DOI] [PubMed] [Google Scholar]
- 53.Thornton J, Debrun GM, et al. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002;50:239–249. doi: 10.1097/00006123-200202000-00003. discussion 249-250. [DOI] [PubMed] [Google Scholar]
- 54.Henkes H, Fischer S, et al. Repeated endovascular coil occlusion in 350 of 2759 intracranial aneurysms: safety and effectiveness aspects. Neurosurgery. 2006;58:224–232. doi: 10.1227/01.NEU.0000194831.54183.3F. discussion 224-232. [DOI] [PubMed] [Google Scholar]