Summary
We describe a case of a ruptured basilar bifurcation aneurysm that thrombosed during preparation for endovascular therapy as a complication of diagnostic angiogaphy, and showed a favorable evolution during long-term follow-up.
Endogenous thrombosis of ruptured, non giant aneurysms is uncommon. The persistence of occlusion over time in such cases is not well established.
Two weeks after rupture, a 6 x 8 mm basilar bifurcation aneurysm was referred for endovascular treatment. During preparation for endovascular coil occlusion, without having any endovascular material at the level of the basilar artery, a complete thrombotic occlusion of the basilar bifurcation and aneurysm was observed. Given the good collateral circulation for both posterior cerebral arteries no thrombolysis was undertaken. The early follow-up of seven days, three and six months showed a complete recanalization of the basilar artery and remodeling of the basilar bifurcation. The 20 months imaging follow-up demonstrated a small aneurysm regrowth at the prevoius location that remained stable during the follow-up of seven years. Unchanged biological and hemodynamic characteristics. however, may pose an elevated risk of a new aneurysm formation over time, making long-term imaging follow-up, and in case of progression, aneurysm occlusion necessary for the patient.
Key words: cerebral aneurysm, subarachnoid hemorrhage, spontaneous thrombosis, wessel wall remodeling, aneurysm regrowth
Introduction
Spontaneous, endogenous thrombosis of intracranial aneurysms rarely happens. It may occur after subarchnoid hemorrhage (SAH) and is usually presumed to be related to vasospasm or antifibrinolytic treatment. Fusiform or giant unruptured aneurysms have also a higher tendency for spontaneous thrombosis, which, in the majority of cases, remains partial. However, the persistence of occlusion over time is not well documented, and it might be influenced by the location of the aneurysm, the morphology and by hemodynamics. We present a case of a ruptured basilar tip aneurysm that thrombosed during preparation for endovascular treatment. This resulted in long-lasting occlusion of the aneurysm that showed a remodeling phenomenon in the early phase, and a small regrowth over seven years of follow-up.
Case Report
A 52-year-old woman was admitted with sudden onset of headache, vomiting and nuchal rigidity. The initial brain CT scan revealed severe SAH of Fisher grade III (Figure 1A). From her medical history severe obesity, unstable chronic hypertension, type 2 diabetes treated with insulin and smoking were relevant. The patient was transferred to our institution 13 days following symptom onset. At the time of admission she was alert and oriented showing no focal neurological symptoms. Digital subtraction angiography (DSA) revealed an aneurysm of the basilar bifurcation of 6 x 8 mm in size, without any evidence of vasospasm (Figure 1B). Coil embolization of the aneurysm was decided and general anesthesia was induced. Anticoagulation was provided by continuous heparinized saline flush lines connected to the introducer sheath and the guiding catheter, and yet no bolus dose of heparin was given.
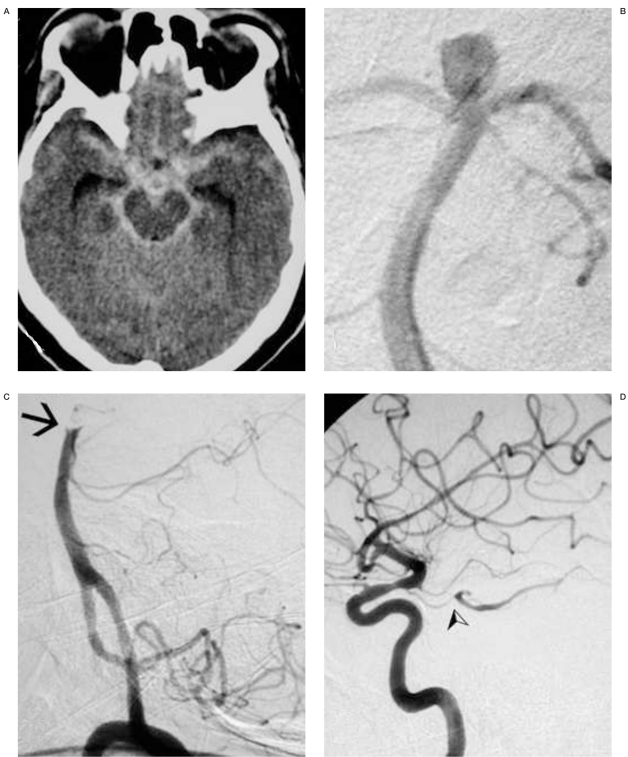
Figure 1.
A) Acute plain CT scan demonstrating diffuse subarachnoid hemorrhage and mild ventricular enlargement. B) Left vertebral DS-angiography, PA view, showing a saccular aneurysm at the basilar bifurcation. C) Left vertebral angiogram, lateral view: the tip of the basilar artery is occluded (arrow), the posterior cerebral arteries and the aneurysm are not filling. D) Right and left internal carotid angiograms demonstrate good flow within both posterior cerebral arteries through the posterior communicating arteries (arrow).
The left vertebral artery was catheterized without difficulties with a 5F Guider Softip® guiding catheter (Boston Scientific, Natick, MA, USA), over 0.035" steerable hydrophilic guide wire (Terumo, Tokyo, Japan). Once the guiding catheter had been properly positioned within the distal cervical vertebral artery (VA), the first contrast injection demonstrated complete thrombotic occlusion of the basilar bifurcation above the origin of the superior cerebellar arteries (SCA), and the disappearance of the aneurysm (Figure 1C).
Both posterior communicating arteries (PComA) provided good collateral circulation for the two posterior cerebral arteries (PCA) (Figure 1D). Considering the risks of thrombolysis in face of a recently ruptured aneurysm and the good collateral circulation, and the lack of mechanical thrombectomy devices at the time of intervention, no treatment aiming recanalization was performed. The patient was awakened and presented no neurological symptoms. Systemic full-dose heparin infusion was started and continued for 24 hours. The patient was stable and symptom-free.
A follow-up DSA six days later demonstrated a complete recanalization of the basilar artery (BA) without filling of the aneurysm. Simultaneous CT scan demonstrated contrast enhancement within the aneurysm suggesting subacut thrombus within the aneurysm sac. The DSA was repeated three months later with the same result (Figure 2). The patient did not consented to another DSA, so follow-up magnetic resonance (MR) imaging and 3D time of flight (TOF) MR-angiography (MRA) was performed at three, six and 20 months, and thereafter yearly for a further five years. On the 20 month follow-up MR-MRA a small regrowth was observed at the level of the former aneurysm neck that remained unchanged during the following five years (Figure 3).
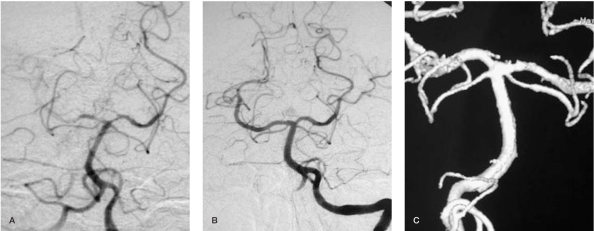
Figure 2.
Left vertebral artery DSA and 3D reconstructed angiograms one week (A) and 3 months (B,C) after treatment attempt. Whilst the 1 week follow-up shows recanalization of the distal basilar artery without aneurysm filling, the 3 months follow-up images show a complete remodeling of the basilar bifurcation.
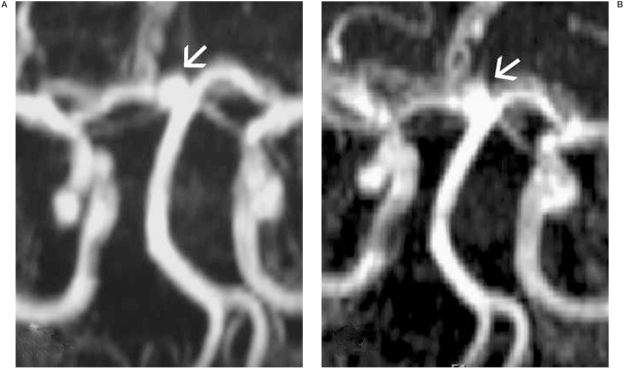
Figure 3.
TOF MRA performed 20 months (A) and 7 years (B) after the event, demonstrating a small, approximately 2 x 3 mm aneurysm regrowth at the level of the former neck, which proved to be stable during the follow-up.
Discussion
This case report describes a rare event of endogenous thrombosis of a small, ruptured basilar bifurcation aneurysm that showed persistence over the long-term follow-up. Complete spontaneous thrombosis of intracranial aneurysms is uncommon. The term spontaneous thrombosis is used in the literature when thrombotic occlusion of an aneurysm occurs without invasive interventions, like open or endovascular surgery. Giant intracranial aneurysms have a high tendency to show partial clot formation1, with complete thrombosis - with or without the concomitant occlusion of the parent vessel - occurring in up to 20%2.
The spontaneous complete occlusion of ruptured, non giant intracranial aneurysms is rarer3-8. Previously estimated to occur with an incidence of 1-2%2, there are no exact data regarding frequency. The phenomenon has been related to hypotension, aneurysm morphology (large or giant aneurysms with small orifice showing a higher tendency for blood stagnation and consequent thrombosis), local vessel wall injury or vasospasm. Aneurysm thrombosis was formerly facilitated by administration of antifibrinolytic agents4,9-11. It may also be accompanied by parent artery occlusion causing ischaemic stroke12-16. It was also suggested to occur more often in dissecting type of lesions 16-18.
The persistence of endogenous occlusion in ruptured aneurysms and recurrence over time is not well documented in the literature, with several case illustrations reporting different follow-up periods. Nakau et Al reported on a ruptured and later on spontaneously thrombosed superior cerebellar artery aneurysm without recurrence at nine months 18. De Luca et Al presented a case of a ruptured posterior inferior cerebellar artery aneurysm that did not show recurrence eight years after spontaneous thrombosis 3. Sobel et Al described the spontaneous disappearance of a ruptured posterior communicating aneurysm with a follow-up of five months5. Hamilton et Al presented a ruptured small anterior communicating aneurysm that disappeared at the two year follow-up 10. Kumar et Al demonstrated a posterior cerebral artery aneurysm thrombosis, with persistence over two months 8. Davila et Al reported on an anterior communicating ruptured aneurysm thrombosis, with persistence over one year6. Although these data may suggest that once thrombosed, these aneurysms do not recur, recurrence of initially thrombosed aneurysms, with or without consequent rupture has also been described 19-22.
As occlusion of the aneurysm and that of the parent artery occurred shortly following diagnostic angiography in our case, thrombosis may not be considered fully spontaneous and might be related to a thromboembolic complication of the diagnostic study. The most probable mechanism for this event could be thrombus formation during or after catheterization of the vertebral artery leading to occlusion of the aneurysm and the distal basilar artery. The thrombus occluding the basilar artery underwent a thrombolytic process with complete dissolution during a period of six days, while the aneurysm remained occluded. The intra-aneurysmal clot probably underwent a healing process before endogenous thrombolysis took place.
An animal study characterized thrombus organization and aneurysm healing on an experimental swine aneurysm model 23. The healing process already started at day 3, and at one and three months the aneurysms became shrunken, containing solid connective tissue with large amounts of collagen. The results of this study are in accordance with our findings. At the three month follow-up angiogram, no signs of aneurysm remnant or recurrence were seen, suggesting a complete vessel wall remodeling. We hypothesize that at this stage the endothelium was probably completely covering the former aneurysm orifice. The simultaneous thrombosis of the parent artery (the basilar bifurcation) may have facilitated fast and complete thrombus organization inside the aneurysm. Later, the recanalization process of the parent artery may have accelerated the neointimal growth over the free surface of the intraaneurysmal clot. These effects provided a „secure" period for the thrombus organization and healing process to be completed, preventing from spontaneous thrombolysis and recanalization.
As the aneurysm did not show any signs of recurrence up until 20 months we assume that endogenous thrombus formation was subsequently followed by shrinkage of the aneurysm and healing of the basilar bifurcation, however we have no obvious data (ie. thin sliced MR study of the region) to prove this theory. Hence the small recurrence observed on the 20 month MR-follow up may be a de novo aneurysm initiation, but also the recanalization of the previously thrombosed and shrunken sac. Whatever the exact pathomechanism may be, the original aneurysm-inducing factors - including vascular anatomy, local hemodynamics, and systemic risk factors the patient had before SAH-remained unchanged.
We assume that those factors, which induced aneurysm formation and rupture before, were acting continuously on the basilar bifurcation after aneurysm healing, and started to regenerate the same process.
Conclusions
We presented a very rare case of endogenous thrombosis of a ruptured basilar tip aneurysm that showed a healing-like process at the beginning, and later on a small regrowth, which remained stable during the long-term follow-up. Unchanged biological and hemodynamic characteristics however, may pose an elevated risk of aneurysm recurrence over time, making long-term imaging follow-up, and in case of progression, occlusion of the aneurysm necessary for the patient.
References
- 1.Whittle IR, Dorsch NW, Besser M. Spontaneous thrombosis in giant intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1982;45(11):1040–1047. doi: 10.1136/jnnp.45.11.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownlee RD, Tranmer BI, et al. Spontaneous thrombosis of an unruptured anterior communicating artery aneurysm. An unusual cause of ischemic stroke. Stroke. 1995;26(10):1945–1949. doi: 10.1161/01.str.26.10.1945. [DOI] [PubMed] [Google Scholar]
- 3.De Luca GP, Volpin L, et al. Spontaneous healing of cerebral aneurysm at the beginning of left posterior inferior cerebellar artery (PICA). Case report. J Neurosurg Sci. 1998;42(2):115–118. [PubMed] [Google Scholar]
- 4.Maiuri F, Iaconetta G, et al. Spontaneous cure of a ruptured intracranial aneurysm. Acta Neurol (Napoli) 1993;15(2):106–113. [PubMed] [Google Scholar]
- 5.Sobel DF, Dalessio D, et al. Cerebral aneurysm thrombosis, shrinkage, then disappearance after subarachnoid hemorrhage. Surg Neurol. 1996;45(2):133–137. doi: 10.1016/s0090-3019(96)80005-x. [DOI] [PubMed] [Google Scholar]
- 6.Davila S, Oliver B, et al. Spontaneous thrombosis of an intracranial aneurysm. Surg Neurol. 1984;22(1):29–32. doi: 10.1016/0090-3019(84)90223-4. [DOI] [PubMed] [Google Scholar]
- 7.Edner G, Forster DM, et al. Spontaneous healing of intracranial aneurysms after subarachnoid hemorrhage. Case report. J Neurosurg. 1978;48(3):450–454. doi: 10.3171/jns.1978.48.3.0450. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Rao VR, et al. Disappearance of a cerebral aneurysm--an unusual angiographic event. Clin Neurol Neurosurg. 1991;93(2):151–153. doi: 10.1016/0303-8467(91)90058-w. [DOI] [PubMed] [Google Scholar]
- 9.Fodstad H, Liliequist B. Spontaneous thrombosis of ruptured intracranial aneurysms during treatment with tranexamic acid (AMCA). Report of three cases. Acta Neurochir (Wien) 1979;49(3-4):129–144. doi: 10.1007/BF01808955. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton MG, Dold ON. Spontaneous disappearance of an intracranial aneurysm after subarachnoid hemorrhage. Can J Neurol Sci. 1992;19(3):389–391. [PubMed] [Google Scholar]
- 11.Scott RM, Garrido E. Spontaneous thrombosis of an intracranial aneurysm during treatment with epsilon aminocaproic acid. Surg Neurol. 1977;7(1):21–23. [PubMed] [Google Scholar]
- 12.Cohen JE, Itshayek E, et al. Spontaneous thrombosis of cerebral aneurysms presenting with ischemic stroke. J Neurol Sci. 2007;254(1-2):95–98. doi: 10.1016/j.jns.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Tsutsumi M, Kazekawa K, et al. Spontaneous thrombosis of a giant intracavernous internal carotid artery aneurysm and ipsilateral internal carotid artery occlusion. Radiat Med. 2002;20(5):261–263. [PubMed] [Google Scholar]
- 14.Dehdashti AR, de Tribolet N. Giant intracavernous aneurysm thrombosis by spontaneous carotid occlusion. Cerebrovasc Dis. 2003;15(4):301–302. doi: 10.1159/000069494. [DOI] [PubMed] [Google Scholar]
- 15.Kurokawa R, Kuroshima Y, et al. Spontaneous thrombosis of intracavernous internal carotid artery aneurysm and parent artery occlusion in patients with positive balloon test occlusion--two case reports. Neurol Med Chir (Tokyo) 2001;41(9):436–441. doi: 10.2176/nmc.41.436. [DOI] [PubMed] [Google Scholar]
- 16.Maeda K, Usui M, et al. Spontaneous occlusion of a giant basilar tip aneurysm and a basilar artery due to the dissection of both structures: case report. Surg Neurol. 1997;48(6):606–609. doi: 10.1016/s0090-3019(96)00544-7. [DOI] [PubMed] [Google Scholar]
- 17.Loevner LA, Ting TY, et al. Spontaneous thrombosis of a basilar artery traumatic aneurysm in a child. Am J Neuroradiol. 1998;19(2):386–388. [PMC free article] [PubMed] [Google Scholar]
- 18.Nakau H, Nagatani H, et al. Acute disappearance of ruptured aneurysm located near the origin of the superior cerebellar artery - case report. Neurol Med Chir (Tokyo) 2007;47(10):468–470. doi: 10.2176/nmc.47.468. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson JL, Lane JI, et al. Spontaneous thrombosis of posterior cerebral artery aneurysm with angiographic reappearance. Case report. J Neurosurg. 1993;79(3):434–437. doi: 10.3171/jns.1993.79.3.0434. [DOI] [PubMed] [Google Scholar]
- 20.Khurana VG, Wijdicks EF, et al. Acute deterioration from thrombosis and rerupture of a giant intracranial aneurysm. Neurology. 1999;52(8):1697–1699. doi: 10.1212/wnl.52.8.1697. [DOI] [PubMed] [Google Scholar]
- 21.Motohashi O, Kameyama M, et al. A distal anterior cerebral artery aneurysm in infant: disappearance and reappearance of the aneurysm. J Clin Neurosci. 2004;11(1):86–88. doi: 10.1016/j.jocn.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Alves JV, Andersson T, et al. Subarachnoid haemorrhage from a large cerebral aneurysm visible only on repeat angiography. Interventional Neuroradiology. 2005;11:59–62. doi: 10.1177/159101990501100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D, Yuki I, et al. Thrombus organization and healing in the swine experimental aneurysm model. Part I. A histological and molecular analysis. J Neurosurg. 2007;107(1):94–108. doi: 10.3171/JNS-07/07/0094. [DOI] [PubMed] [Google Scholar]