Summary
The advent of Onyx has provided a new method for neurointerventional therapists to treat brain AVMs. Although some retrospective studies have reported complications for AVM embolization with Onyx, periprocedural bleeding complications with Onyx embolization have not yet been described in detail. The aim of this retrospective study was to analyze the factors of Onyx-related bleeding complications and to find a way to avoid and manage these complications.
From January 2003, patients with AVMs recruited in our institution started to be treated by Onyx embolization. From January 2007 to July 2009, 143 consecutive interventions were performed in 126 patients using flow-independent microcatheters and Onyx as embolic agents. Seven patients encountered bleeding complications (5.4% per patients and 4.7% per procedures) during or after the endovascular procedures. Among them, five bleeding episodes occurred during procedures, the other two after procedures. Details of the seven patients' clinical presentations, imaging presentations, speculative reasons and management of these complications were recorded. Follow-up data, including postoperative course, clinical symptoms and duration of follow-up were documented.
The five active bleedings discovered in procedures were managed in time, and the patients recovered without any new neurological symptoms compared with preoperation. However, of the two bleeding episodes that occurred after interventional procedures, one was detected half an hour later: the patient was remained comatose two months later after resection of right occipital hematoma; the other who encountered intraventricular and midbrain hemorrhage was treated conservatively and suffered Parinaud syndrome and hemianesthesia.
Conclusion: Periprocedural bleeding of AVMs embolization is considered a severe and devastating complication. The clinical course and prognosis of bleeding mostly depends on prompt detection and management. Interventional embolization is an effective method to manage bleeding during procedures, and the detection of risk factors and imaging signs of bleeding is extremely important.
Key words: arteriovenous malformation, bleeding, complications, embolization, Onyx
Introduction
Hemorrhages are the most frequent and devastating complications of the endovascular treatment of cerebral AVM1, but to our knowledge, the factors responsible for bleeding complications in endovascular treatment and how to manage these complications are not well established in the literature. In addition, the reasons for bleeding were not illustrated in detail in these articles. Intracranial bleeding during or after therapeutic endovascular procedures produces an acute clinical patient deterioration if it is ignored or not managed in time. This article retrospectively describe seven cases of periprocedural hemorrhagic complications in treating intracranial AVMs with Onyx embolization from January 2007 to July 2009 in our department. The morphological architecture of AVMs, bleeding modality, management, clinical outcome and the speculative bleeding mechanisms are described in detail. The purpose of this single-center study was to analyze determinants of Onyx-related bleeding complications and how to manage these complications.
Material and Methods
From January 2007 to July 2009,143 consecutive interventions were performed in 126 patients using flow-independent microcatheters and Onyx as embolic agents. Seven patients encountered bleeding complications (5.4% per patients and 4.7% per procedures). Clinical records were examined to determine patient demographics, chief complaint and imaging characteristics before embolization. In addition, details of the primary endovascular therapy and subsequent interventions were recorded. Follow-up data, including the general state, postoperative course, and duration of follow-up were documented.
Interventional Procedure
All AVMs embolizations in our department were carried out under general anaesthesia. In each procedure, transfemoral access was achieved with a 6F valved introducer sheath. A 6F guiding catheter (Cordis Corp, NJ, USA) was inserted via the sheath with continuous heparinized high-pressure irrigation and placed in the internal carotid or the vertebral artery. A marathon floating microcatheter guided by a mirage-008 microguidewire (Micro Therapeutics, Inc., Irvine, CA. USA) was used to navigate into the nidus through the feeding artery under roadmap.
The microcatheter was advanced intranidally in a wedged position if possible, or as close as possible to the nidus. If necessary, this was also done with the aid of guidewires, but the tip of the wire never extended beyond the microcatheter tip when approaching the nidus. Each embolization was preceded by superselective angiogram, all in two planes, and then we used 0.23 ml dimethyl sulfoxide (DMSO) to flush the lumen of the marathon microcatheter in about 40 seconds to prevent microcatheter adhesion. Embolization was performed in all cases with Onyx-18 (Micro Therapeutics, Inc., Irvine, CA, USA) under biplane blank roadmapping. When the Onyx refluxed or did not penetrate into the ideal place, we stopped injection and waited a few minutes, an angiogram might be given if necessary. After completion of the embolization, a control angiography was generally performed in two planes.
The patients were then monitored for 24 hours, and a strict upper systolic blood pressure limit of 140 mm Hg was maintained. Beyond the heparinized flush of the guiding catheter, no additional heparin was given, so no reversal of heparin effect was necessary. The bleeding of all patients was identified by CT scan. AVM morphology and embolization-induced morphologic changes, focusing on venous drainage, and intranidus aneurysms were all analyzed in detail.
Follow-up
Follow-up included clinical and radiological assessments with cerebral angiography; Outcome was graded using the Glasgow outcome scale (GOS) score.
Results
Details of the seven patients are presented in Table 1, the bleeding modality included intraventricular hemorrhage (IVH), subarachnoid hemorrhage (SAH), intracranial hematoma (ICH). One case of bleeding occurred after retraction of the catheter; three were detected by venous embolization in the angiograms during the procedure, two were detected by intranidal aneurysm rupture - We did not find any risk factors in the angiogram of one patient, so we postulated the reason of bleeding was hemodynamic changes.
Table 1.
AVM characteristics and interventional procedures of the seven patients.
| Patient No./ Sex/ Age |
Presentation | AVM Location |
Feeding Artery |
Draining vein |
Bleeding detection |
Bleeding modality |
Final embolization result (%) |
Outcome (GOS, follow-up months) |
Bleeding management |
Reasons for bleeding |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/F/31 | Apopsychia, 5 years after NBCA embolization |
Right fr. temp. Lobe |
MCA cortex branches and lateral lent.str.A |
Vein of Trolard and sup. sylvian vein |
Extra-vascular effusion of contrast agent during angiogram |
Hematoma and SAH |
90 | Good (5.12) |
Onyx embolization |
Retraction of microcatheter |
|
| 2/M/15 | Intracranial hematoma |
Right fr. temp, lobe |
Two branches of fr.-par. ascend. A |
Vein of Trolard |
Extravascular effusion of contrast agent; stagnation in the parenchyma |
Hematoma, broke into lateral ventricle during manipulation |
100 | Good (5,6) |
Onyx embolization |
Draining vein occlusion; venous aneurysm rupture |
|
| 3/F/49 | Intracranial hematoma, 6 months after radiosurgery |
Right par. occip. lobe |
ACA and LPChA |
Vein of Galen |
Ventricular cast mould of contrast agent |
Hematoma and IVH |
100 | Good (5,6) |
Onyx embolization; lumbar puncture |
Draining vein occlusion; intranidal aneurysm rupture |
|
| 4/F/19 | Generalized epilepsy |
Left par. lobe |
ACA, cortex branches of MCA, left lateral lent. str. A |
Vein of Trolard |
Extra-vascular effusion of contrast agent and stagnation in the cerebral sulcus |
SAH | 100 | Good (5,10) |
Onyx embolization |
Intranidal aneurysm rupture during superselective angiograms |
|
| 5/F/42 | IVH 18 years ago, dizziness 4 years |
Right Parahippo gyrus; uncus |
Branches of PCA |
Aplastic Vein of Galen |
Ventricular cast mould of contrast agent |
IVH | 100 | Fair (4,6) |
Onyx embolization; EVD |
Draining vein occlusion |
|
| 6/M/22 | IVH | Quad.cist.; midbrain |
LPChA; MPChA |
Vein of Galen |
Coma 2 days after procedure CT scan |
IVH | 95 | Fair (4,3) |
Conservative treatment |
Intranidal aneurysm rupture |
|
| 7/M/37 | Vision ambiguity |
Left occip. lobe |
PCA cortex branches |
Occip. vein; vein of Galen |
Coma 30 min after procedure; CT scan |
Hematoma | 50 | Comatose (2,2) |
Craniotomy | Hemodynamic change of feeding artery |
|
| Note: IVH, intraventricular hemorrhage: GOS, Glasgow Outcome Scale: EVD, extraventricular drainage: MCA, middle cerebral artery: ACA, anterior cerebral artery: SAH, subarachnoid hemorrhage: Fr, frontal: Temp, temporal: Occip, occipital: Lent.str.a, lenticulostriate artery: PChA, posterior chor oidal artery: Sup, superior: Quad.cist., quadrigeminal cistern: V, vein. | |||||||||||
Among the seven patients, five bleedings were detected in angiograms or fluoroscopy during the procedure and managed in time with Onyx-18, all of them had recovered without new neurological symptoms when they discharged, in spite of the severe manifestations of CT scan. One of the patients who presented with IVH had extraventricular drainage, another one had lumbar puncture, and the others were treated conservatively. Two were detected after the procedure, the patient in whom occipital hematoma was detected half an hour later experienced emergency craniotomy resection of the hematoma and remained comatose two months later. The other patient who encountered intraventricular and midbrain hemorrhage about 30 hours after the procedure was treated conservatively and suffered Parinaud syndrome and hemianesthesia, the Parinaud syndrome gradually recovered two months later, but the hemianesthesia was not remarkably improved.
Discussion
Treatment decisions for patients with brain arteriovenous malformations (AVMs) are based on natural-course risk estimates weighed against outcome data from invasive intervention 5,8. Thanks to the improvement of endovascular materials and experience, endovascular therapy has become increasingly important in the management of cerebral AVMs. The main goals of AVM embolization are nidus reduction before surgery or radiosurgery, curative embolization, and palliative embolization3,25,27-28. Since the pioneers in the early 1960s reported using methyl methacrylate to embolize the feeding pedicle to treat AVMs, a considerable evolution of microcatheter tools, embolization materials and techniques has proved the success of embolization. Onyx is a new embolization material that has gradually been adopted for AVM embolization during the past decade, and some authors have already reported their initial experiences in the treatment of AVMs with Onyx 11-12,5-16,19,21,23,25-28. As mentioned in these articles, Onyx is a nonadhesive agent with controllable endovascular behavior, which allows more precise nidus penetration creating a solid cast, the overall initial complete obliteration rate of intracranial AVMs with Onyx is relatively high compared with that of other embolic agents 7,28.
Despite improvements in endovascular techniques and embolic agents, the complication profile of brain AVM embolization is of concern and not well described2-4. Several causes have been proposed in the literature to explain acute periembolization hemorrhage, such as inappropriate venous occlusion of a partially embolized AVM, increased pressure in feeding arteries as a result of embolization, normal perfusion pressure breakthrough, hyperemia of normal brain or redistribution of cerebral blood flow into adjacent regions, venous thrombosis secondary to stasis caused by substantial obliteration of the AVM, inflammatory reaction or mural necrosis induced by the embolic material, ischemic softening of tissue around an abnormal blood vessel that bled under pressure, and intranidal rupture of an aneurysm1,2. Over-all, the rate of periprocedural bleeding complications reported in the literature ranged between 2% and 16.7% after endovascular use of Onyx 7,11,12,15-16,24-27. But most of the reasons for bleeding were lack of objective evidence and the extensional mechanisms were not fully illustrated. This article discusses the mechanisms of bleeding and attempts to manage these devastating complications.
Mechanisms of Bleeding
Vessel perforation caused by devices
Vessel perforation during microcatheter placement used to be significant cause of periembolization hemorrhage 4. However, because of the manufacturer's improvements to the microcatheter and the microguidewire, with some experience in handling the devices, perforations are extremely rare.
The microcatheter and the guidewire should coordinate, when the floating microcatheter and the guidewire are near the nidus, the guidewire tip should not exceed the microcatheter tip, otherwise, the sharp tip of the guidewire may destroy the fragile vascular wall of the AVM. Because the tip of the marathon microcatheter is very soft, in the seven cases we reported, none of the bleedings occurred during microcatheter navigation.
Catheter adhesion
Onyx is a nonadhesive embolic agent with lavalike patterns. The microcatheter tip is not easily glued within the vessel, and thus it is possible to interrupt the injection and analyze the actual Onyx casting11,13,27. However, gluing catheter incidents still occur in some cases 24,27. In our experience, when the Onyx embolizes the nidus to a certain degree, the Onyx will no longer penetrate ahead because the pressure ahead might be higher than backward. In order to push the Onyx to continue to disperse into the nidus, we have to let the Onyx reflux a little with the catheter tip berried in the Onyx, the goal was to form an attenuated cast of Onyx around the tip of the microcatheter over a short distance.
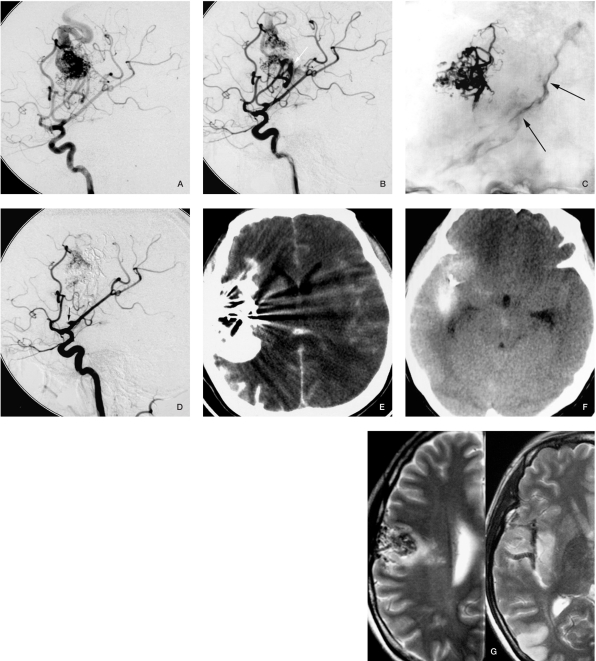
The injection procedure was then interrupted while waiting a few minutes for precipitation to create a second penetration to the nidus11. But this technique might lead to difficulty of microcatheter retraction. The difficulty in removing the catheter depends on the tortuosity of the catheterized vessels, the duration of the precipitation, the distance of the reflux, and the amount of experience of the investigator manipulating the catheter27. We did not find any absolute criteria in the literature as to how long the precipitation or how long the reflux of Onyx would be safe for the patient, but in our experience, the time of injection should not exceed 30 minutes, and the reflux of Onyx should not exceed 20 mm during AVM embolization (that may be different to DAVFs). Retention of the microcatheter is unlikely to occur, even in cases with extended reflux, due to the nonadhesive features of Onyx. Nevertheless, when trapped, it is better to leave the microcatheter in place to prevent potential vessel rupture24. In our case 1 (Figure 1), the bleeding after microcatheter retrieval was because of the tortuosity of the catheterized vessel. We used another marathon microcatheter and Onyx to embolize the torn vessel, but that catheter also became trapped and was left in a branch of the MCA in place without any clinical symptoms.
Figure 1.
Case 1. A) Angiogram before embolization. B) Angiogram after catheter retrieval. C) Contrast medium in the central sulcus could be seen during fluoroscopy (arrow). D) The whole vessel was occluded, only the stump of the vessel could be seen (arrow). E) CT scan immediately after embolization. F) CT scan the day after embolization shows the contrast agent was absorbed very quickly. G) MRI 3 months later.
Draining vein occlusion
Several studies have reported that exclusive deep venous drainage is a definite risk factor of AVM bleeding, either in endovascular embolization or microsurgical resection2,3,5,16,20. Although venous occlusion may be a goal when attempting intranidal embolization to achieve complete occlusion, continued inflow into the malformation with impaired outflow increases the risk of rupture and hemorrhage 5,16,18. Venous congestion in the adjacent brain might progress by delayed thrombosis in the draining vein, leading ultimately to venous bleeding. With regard to delayed postembolization hemorrhage, some reports 11,23,24 have postulated that delayed vein occlusion might occur because of sluggish flow rather than by direct occlusion of the vein by embolic material. Protection of the draining vein is extremely important during embolization, but it may be hard to manipulate during the procedure, once we find the AVMs' draining vein is stagnation.
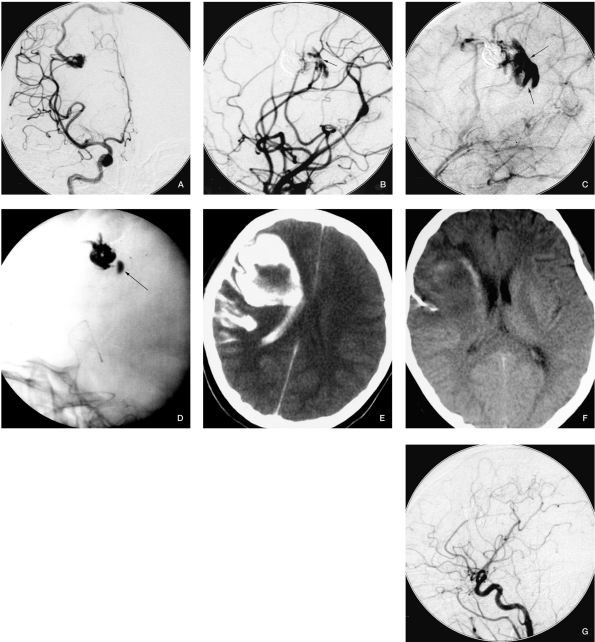
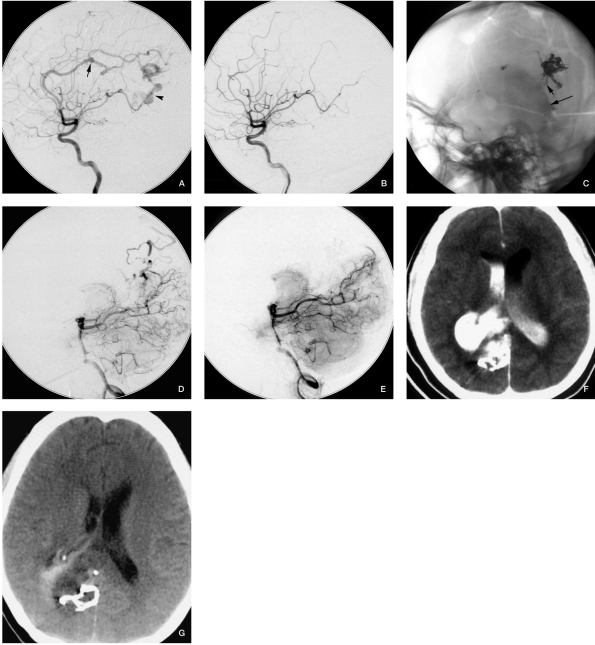
The bleeding of our cases 2, 3 and 5 (Figures 2, 3 and 5 respectively) are thought to have been induced by venous occlusion and AVM remnant.
Figure 2.
Case 2. A) Angiogram before embolization. B) Contrast agent extravasation after occluding 1 feeding pedicle. C) The draining vein cannot be seen even in venous phase, and the contrast agent stagnation in the hematoma confirms draining vein occlusion. D) Contrast agent stagnation during fluoroscopy. E) CT scan immediately after embolization. F) CT scan when the patient was discharged. G) Follow-up angiography six months later, shows completely occlusion of the AVM.
Figure 3.
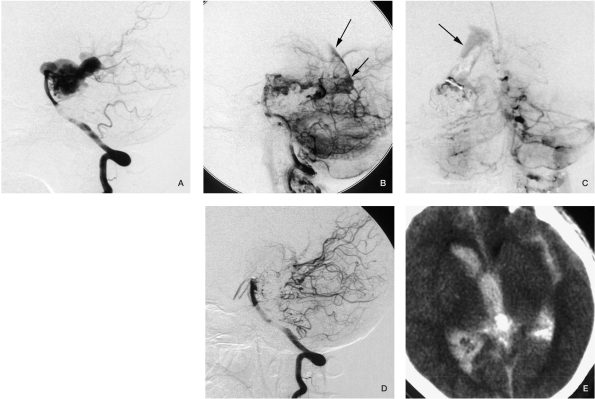
Case 3. A) Angiogram from the carotid artery before embolization: note a hemodynamic-related aneurysm and the draining vein. We embolized the AVM and the aneurysm through RICA-AcomA-LICA. B,C) The angiogram and fluoroscopy after embolization through LACA, internal carotid artery injection showed the AVM was no longer filled, and the aneurysm was embolized as well, but we can see contrast agent in the lateral ventricle and the hematoma (arrow). D) The venous phase of the vertebral injection before embolization through the cortical branch of the PCA no longer shows the draining vein, but there was an AVM remnant. E) Vertebral injection after embolization through the medial posterior choroidal artery. F,G) CT scan postprocedure before discharge.
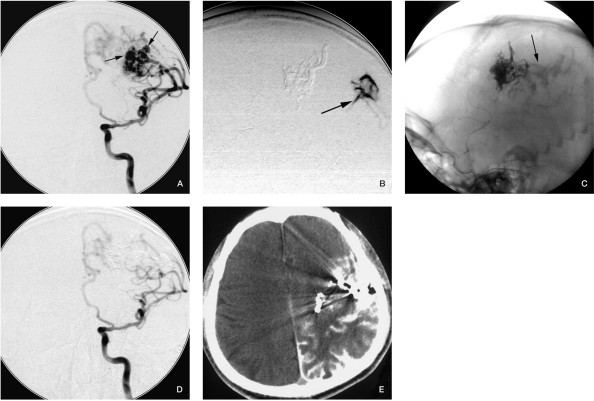
Figure 4.
Case 4. A) A-P position carotid angiogram before embolization shows intranidal aneurysms. B) Superselective angiogram before Onyx injection displays contrast medium extravasation. C) The contrast medium diffused into the subarachnoid space during fluoroscopy. D) Angiogram after the procedure, the intranidal aneuryms disappeared. E) CT scan immediately after embolization appears to show severe SAH, but the patient did not have any symptoms.
Figure 5.
Case 5. A) Vertebral angiogram before embolization. B) Angiogram after embolization shows the contrast agent diffused into the lateral ventricle. C) A-P angiogram after embolization: the arrow shows the contrast in the lateral ventricle. D) CT scan immediately after embolization appears to show severe IVH so we gave the patient extraventricular drainage post procedure. E) Angiogram follow-up six months later.
Pressure changing during superselective angiogram
Because we must identify whether the tip of the microcatheter is in an ideal position, superselective angiograms are necessary before Onyx injection. But the strength we use during superselective injection should not be too great, especially if the tip of the microcatheter is in intranidal aneurysms, otherwise the AVMs nidus may rupture due to abruptly increased pressure. In addition, because the AVM angioarchitecture is not clear before embolization, the intranidal aneurysms may be hard to detect. Bleeding in our case 4 (Figure 4) may have been caused by this mechanism.
Intranidal aneurysm rupture
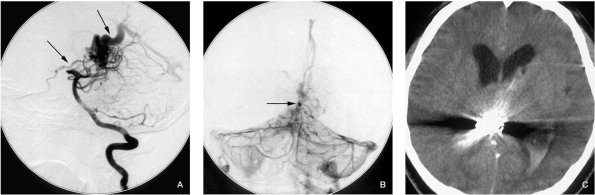
In a combined prospective and retrospective series of 632 patients with AVM, a significant association with hemorrhagic AVM presentation was found for intranidal but not for feeding artery aneurysms 16. Furthermore, intranidal aneurysms are also risk factors of periprocedural bleeding. Bleeding in our case 6 (Figure 6) was because an intranidal aneurysm that we did not discover during the procedure, although the aneurysm was very small, because it was in the posterior wall of the third ventricle and midbrain, the symptoms were severe.
Figure 6.
Case 6. A) Lateral position vertebral injection before embolization, both the two main draining veins can be seen. B) Vertebral injection, venous phase, contrast medium is stagnant in a small intranidal aneurysm. This was not noted during the procedure. C) The patient experienced a sudden coma two days after the procedure, CT scan showed midbrain hematoma and IVH. The patient suffered Parinaud syndrome and hemianesthesia, the Parinaud syndrome gradually recovered 2 months later, but the hemianesthesia was not remarkably improved.
Inflow-outflow imbalance
The small size of AVMs increases the risk of a first (incident) hemorrhage and implies a subsequent hemorrhage5,8,21. Partial embolization of AVMs might be dangerous, especially in cases with small AVMs and the blood supplies are simple, as in our cases 2 and 7. According to our experience, if the AVM involves a large area of the brain, and is supplied by multiple branches, staged embolization is preferable as completely embolization in one procedure is often dangerous. But if the AVMs are small or supplied by a small number of branches, we should embolize the AVMS as completely as possible. Duong et Al6 reported that high arterial input pressure and venous outflow restriction (exclusively deep venous drainage) were the most powerful risk predictors for hemorrhagic AVM presentation.
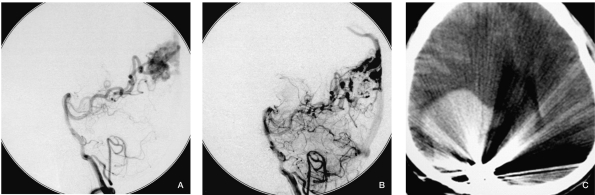
We think the bleeding of case 7 (Figure 7) might be due to this factor. We did partial embolization of the AVM in the occipital lobe for fear of hemiablepsia, there were no risk factors detected in the angiogram, and the draining veins were protected well after the procedure, but the patient experienced severe headache half an hour later, then quickly became comatose, CT scan showed large a hematoma in the occipital lobe. The patient experienced craniotomy and remained comatose during two months of follow-up.
Figure 7.
Case 7. A) Vertebral injection before embolization. B) Vertebral injection after embolization showing the AVM was partially embolized; the draining vein was well protected. C) The patient complained of a sudden severe headache half an hour later, then became comatose. The CT scan showed a large hematoma in the right occipital lobe.
Other factors
The Onyx cast may cover the remnant nidus of the AVMs or even the intranidal aneurysm during angiogram, so multi-angle projections are necessary to avoid missing some bleeding risk factors.
Management of Bleeding
Emergency craniotomy to evacuate the hematoma and possibly resection of the residual nidus will help minimize or avoid neurological morbidity9,14. Iwama et Al9 reported the results of emergency craniotomy for intraparenchymal massive hematoma after embolization of supratentorial AVMs. In their series, 605 patients with a brain AVM underwent 1066 embolization sessions. Hemorrhage during or after the endovascular procedure was observed in 24 (4%) patients. Fourteen patients (2.3%) had massive intraparenchymal hematomas and deteriorated to a comatose state or death. Among the 12 (2%) patients who underwent craniotomy, four recovered with a good outcome, five remained moderately disabled, one patient remained in a persistent vegetative state and two patients died. Therefore, detection of the bleeding in time is very important. During the procedures, the signs of bleeding in the angiogram may be hard to detect, such as extravasation of contrast medium during subtraction angiography, contrast medium stagnation in the subarachnoid space, ventricular system or brain parenchyma, but if we are more careful and experienced, bleeding can be detected. Acute systemic hypertension, lowering of the heart rate and pupil dilatation develop a few seconds later, but these signs are more notable in patients who experience SAH. Once these signs are detected, bleeding might occur. If the bleeding occurs during the procedure, we should prevent the bleeding by an interventional method at first, for interventional procedures are faster than a craniotomy procedure. When the bleeding stops, then craniotomy should be considered if necessary.
In addition, the CT scan may exaggerate the intracranial bleeding after an interventional procedure. During the procedure, we did several angiograms to certify whether the bleeding stopped, so there must be lots of contrast medium dispersed into the subarachnoid space with blood. The contrast medium has a good diffuse ability, so it can easily diffuse in the subarachnoid space, in addition, contrast medium shows high density like blood in the CT scan, therefore the SAH seen in the CT scan could be more extensive than the actual condition. According to our experience, the contrast medium can be absorbed much faster than blood. We thought this is because it is a micromolecule substance which could be easily absorbed by the arachnoid granulations within several hours and participates in the CSF circulation, i.e., patients with severe imaging signs in the CT scan could have relatively mild clinical symptoms. The intracranial pressure will decline rapidly with a quick absorption of contrast medium.
Conclusions
Periprocedural bleeding of AVMs embolization is considered a devastating complication. Patients' clinical course and prognosis mostly depend on prompt detection and management of bleeding. Interventional embolization is an effective method to prevent bleeding during procedures, and detection of risk factors and imaging signs of bleeding is extremely important.
References
- 1.Biondi A, Le Jean L, et al. Fatal hemorrhagic complication following endovascular treatment of a cerebral arteriovenous malformation. Case report and review of the literature. J Neuroradiol. 2006;33(2):96–104. doi: 10.1016/s0150-9861(06)77238-8. [DOI] [PubMed] [Google Scholar]
- 2.Hartmann A, Stapf C, et al. Determinants of neurological outcome after surgery for brain arteriovenous malformation. Stroke. 2000;31:2361–2364. doi: 10.1161/01.str.31.10.2361. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann A, Pile-Spellman J, et al. Risk of endovascular treatment of brain arteriovenous malformations. Stroke. 2002;33:1816–1820. doi: 10.1161/01.str.0000020123.80940.b2. [DOI] [PubMed] [Google Scholar]
- 4.Ledezma CJ, Hoh BL, et al. Complications of cerebral arteriovenous malformation embolization: multivariate analysis of predictive factors. Neurosurgery. 2006;58(4):602–611. doi: 10.1227/01.NEU.0000204103.91793.77. discussion 602-611. [DOI] [PubMed] [Google Scholar]
- 5.Stapf C, Mast H, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66:1350–1355. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- 6.Duong DH, Young WL, et al. Feeding artery pressure and venous drainage pattern are primary determinants of hemorrhage from cerebral arteriovenous malformations. Stroke. 1998;29:1167–1176. doi: 10.1161/01.str.29.6.1167. [DOI] [PubMed] [Google Scholar]
- 7.Velat GJ, Reavey-Cantwell JF, et al. Comparison of Nbutyl cyanoacrylate and onyx for the embolization of intracranial arteriovenous malformations: analysis of fluoroscopy and procedure times. Neurosurgery. 2008;63(1 Sup 1):ONS73–78. doi: 10.1227/01.neu.0000335015.83616.12. discussion ONS78-80. [DOI] [PubMed] [Google Scholar]
- 8.Mast H, Young WL, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformatio. Lancet 11: 1997;350(9084):1065–1068. doi: 10.1016/s0140-6736(97)05390-7. [DOI] [PubMed] [Google Scholar]
- 9.Iwama T, Yoshimura K, et al. Emergency craniotomy for intraparenchymal massive hematoma after embolization of supratentorial arteriovenous malformations. Neurosurgery. 2003;53:1251–1258. doi: 10.1227/01.neu.0000093198.98170.d4. discussion 1258-1260. [DOI] [PubMed] [Google Scholar]
- 10.Heidenreich JO, Hartlieb S, et al. Bleeding complications after endovascular therapy of cerebral arteriovenous malformations. Am J Neuroradiol. 2006;27:313–316. [PMC free article] [PubMed] [Google Scholar]
- 11.Jahan R, Murayama Y, et al. Embolization of arteriovenous malformations with Onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48(5):984–995. doi: 10.1097/00006123-200105000-00003. discussion 995-997. [DOI] [PubMed] [Google Scholar]
- 12.Mounayer C, Hammami N, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. Am J Neuroradiol. 2007;28:518–523. [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaraman MV, Marcellus ML, et al. Neurologic complications of arteriovenous malformation embolization using liquid embolic agents. Am J Neuroradiol. 2008;29:242–246. doi: 10.3174/ajnr.A0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carvi y Nievas MN, Haas E, Hollerhage H-G. Severe intracranial bleedings during endovascular procedures: outcome of surgically treated patients. Neurol Res. 2007;29:81–90. doi: 10.1179/174313206X152492. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Higueras A, Rossi Lopez R, Quinones Taria D. Endovascular treatment of cerebral AVM: our experience with Onyx. Interventional Neuroradiology. 2005;11:141–157. doi: 10.1177/15910199050110S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierot L, Januel AC, et al. Endovascular treatment of brain arteriovenous malformations using Onyx: preliminary results of a prospective multicenter study. Interventional Neuroradiology. 2005;11:159–164. doi: 10.1177/15910199050110S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedlander RM. Arteriovenous malformations of the brain. N Engl J Med. 2007;356:2704–2712. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 18.Redekop G, TerBrugge K, et al. Arterial aneurysms associated with cerebral arteriovenous malformation: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998;89:539–546. doi: 10.3171/jns.1998.89.4.0539. [DOI] [PubMed] [Google Scholar]
- 19.Al-Shahi R, Warlow C. Arteriovenous malformations of the brain: ready to randomise? J Neurol Neurosurg Psychiatry. 2005;76:1327–1329. doi: 10.1136/jnnp.2004.036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song DL, Leng B, et al. Clinical experience of 70 cases of cerebral arteriovenous malformations embolization with Onyx, a novel liquid embolic agent [in Chinese] Zhonghua Wai Ke Za Zhi. 2007;45:223–225. [PubMed] [Google Scholar]
- 21.The Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adult. N Engl J Med 10: 1999;340(23):1812–1818. doi: 10.1056/NEJM199906103402307. [DOI] [PubMed] [Google Scholar]
- 22.Tevah J, Huete I. Endovascular treatment of cerebral AVMs with a new material: Onyx. Interventional Neuroradiology. 2005;11:165–170. doi: 10.1177/15910199050110S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor CL, Dutton K, et al. Complications of preoperative embolization of cerebral arteriovenous malformations. J Neurosurg. 2004;100(5):810–812. doi: 10.3171/jns.2004.100.5.0810. [DOI] [PubMed] [Google Scholar]
- 24.Panagiotopoulos V, Gizewski E, et al. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx) Am J Neuroradiol. 2008;30:99–106. doi: 10.3174/ajnr.A1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsaridis V, Papagiannaki C, Aimar E. Curative embolization of cerebral arteriovenous malformations (AVMs) with Onyx in 101 patients. Neuroradiology. 2008;50:589–597. doi: 10.1007/s00234-008-0382-x. [DOI] [PubMed] [Google Scholar]
- 26.van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. Am J Neuroradiol. 2007;28:172–177. [PMC free article] [PubMed] [Google Scholar]
- 27.Weber W, Kis B, et al. Endovascular treatment of intracranial arteriovenous malformations with Onyx: Technical aspects. Am J Neuroradiol. 2007;28:371–177. [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace RC, Flom RA, et al. The safety and effectiveness of brain arteriovenous malformation embolization using acrylic and particles: The experiences of a single institution. Neurosurgery. 1995;37(4):606–618. doi: 10.1227/00006123-199510000-00002. [DOI] [PubMed] [Google Scholar]