Summary
Carotid artery stenosis due to arteriosclerosis increases the risk of cerebral ischemia via embolic phenomena or reduced blood flow. The changes in cerebral perfusion that may occur after treatment are not clearly understood. This study evaluated the changes in cerebral microcirculation following carotid angioplasty with stenting (CAS) under cerebral protection with filters using ultrafast gradient echo (GRE) perfusion weighted imaging (PWI) with magnetic resonance imaging (MRI). Prospectively, 21 cervical carotid stenosis patients, mean age 69.95 years, underwent MRI 12 h before and 72 h after CAS. PWI parameters were collected for statistical analysis: cerebral blood volume (CBV), mean transit time (MTT) and time to peak (TTP). Statistical analysis was applied to absolute parameters and to values normalized against those from the contralateral parenchyma. The main finding of this study was improved hemodynamics for the normalized data after CAS, shown by reduced MTT (p<0.001) and TTP (p=0.019) in the territory fed by the middle cerebral artery ipsilateral to the CAS. Absolute data showed increased blood volume in the cerebral hemispheres after CAS, which was more accentuated on the stent side (p=0.016) than the contralateral side (p=0.029). Early improvements in cerebral perfusion, mainly seen in the normalized data, were clearly demonstrated in the timing parameters - TTP & MTT - after CAS .
Key words: perfusion-weighted MRI, carotid angioplasty and stenting, carotid stenosis, neuroradiology, interventional radiology, digital subtraction angiography
Introduction
Currently, perfusion-weighted images by MRI are used to supply information regarding cerebral microcirculation using an exogenous paramagnetic contrast agent such as gadolinium chelate, a rare earth metal. Transient signal loss from contrast passage through the capillary bed is used to construct concentration time curves. These curves are decomposed by mathematical equations to obtain the relative hemodynamic values for the examined area, for example, regional cerebral blood volume (CBV) and regional cerebral blood flow (CBF), as well as timing parameters such as regional contrast mean transit time (MTT) and time to peak (TTP). These parameters are closely related. CBF, is directly proportional to CBV and inversely proportional to MTT (CBF≅CBV/ MTT), and is thus inversely proportional to TTP 1-4.
Cervical carotid artery stenosis frequently promotes ischemia by embolic phenomena, but can also induce ischemia via a reduction in blood flow 5,3. Cerebral perfusion failure can occur from reductions in blood flow from a stenosis or an occlusive vascular lesion 6. The main example of this mechanism is the atheromatous plaque, which attacks larger arteries, often in the carotid bulb 5.
Different imaging techniques have been used to evaluate cerebral microcirculation in carotid stenosis patients, including cervical and transcranial Doppler 7, perfusion by helicoidal computed tomography 8,4, intracarotid xenonium injection9, and perfusion by MRI 3,10-12 Although perfusion study evaluation by MRI is semi-quantitative, its advantages are that it is a non-invasive method of evaluating cerebral blood flow and is available in most large cities 13,6. Studies using PWI by MRI to evaluate the cerebral capillary bed in carotid artery stenosis patients have shown injury from reduced cerebral perfusion ipsilateral to the cervical carotid artery stenosis in timing parameters and CBV 14,1,6. Studies performing analysis before and after revascularization have seen improved cerebral perfusion after endarterectomy 15,16,11,12 and angioplasty with stent placement 3,7,8,10-12. Some authors have only evaluated flow parameters after carotid angioplasty with stenting (CAS) without detailing the relationship between CBV and MTT 11,12. Others have evaluated the impact of carotid artery stenosis treatment after CAS by PWI by MRI using PWI protocols with relatively long repetition time (TR) and have not found major improvements in timing parameters 3,10. As a result, our understanding of cerebral hemodynamic changes after CAS is still incomplete, particularly with regard to timing parameters. The aim of this work was to evaluate modifications in cerebral microcirculation after CAS under cerebral protection, using ultra-fast PWI by MRI.
Methods
Twenty-one patients with severe cervical carotid artery stenosis (≥60% occlusion) according to NASCET criteria 17 were referred for CAS between March 2005 and November 2006 at our interventional neuroradiology service and underwent a MRI and PWI study 12 h before and 72 h after angioplasty. Patients with significant stenosis or occlusion in the contralateral carotid artery or vertebral arteries or who had intracranial stenosis were excluded.
Mean patient age was 69.95 years (range 5487 years); 14 were male and seven female, and all had severe cervical carotid stenosis. The stenosis was on the right side in nine patients and on the left in 12 patients, with a mean stenosis degree of 77.48% (range 60-99%).
All MRI examinations employed the same technique, using a high-field system (1.5T, LX Horizon®, General Electric Healthcare, Milwaukee, WI, USA) in a commercially available birdcage transmit/receive quadrature. All patients were submitted to a complete conventional encephalic study by MRI to exclude infarcts or other pathologies in the regions of interest (ROI). Gadolinium injection (Magnevist; Schering, Berlin, Germany) was standardized in a forearm vein with 20 ml (3 ml/s) by power injector and a delay of 10 s, followed by 30 ml of a saline push. MRI parameters for PWI were: a GRE (gradient-echo) EPI sequence; axial plane; matrix 64×64; 1 NEX; FOV 30 cm; thickness 10 mm; gap 1 mm; TE 50 ms; TR 1000 ms; single shot; flip angle 75°; 64 phases per location; interleaved phase acquisition order.
The location of the ROI was determined in the area adjacent to the lateral ventricles (Figure 1). This region was chosen from the tissue supplied by the middle cerebral artery (MCA) to be a reliable representation of flow from a unilateral carotid artery, as opposed to the tissue supplied by the anterior cerebral arteries, which can receive flow from either of the carotid arteries. The ROI was manually marked to avoid infarct areas and the sylvian fissure. In cases of cerebral infarct in this region, the level of analysis ascended to the lateral portions of the corona radiata or centrum semiovale region. After demarcation of one side of the ROI, an axis of symmetry was traced on the midline and a mirror image of the ROI was reflected onto the contralateral hemisphere, thus constraining these areas to have the same dimensions and topography in both hemispheres.
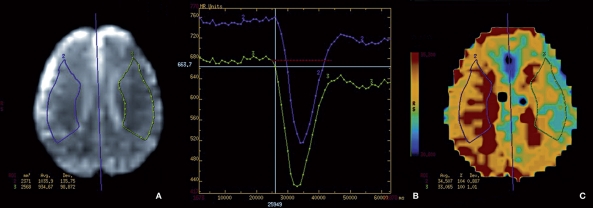
Figure 1.
Example of ROI placement in a 54-year-old man with right carotid artery stenosis estimated at 70% by DSA. Initially the ROI representative of the right MCA (A) was traced manually (area traced in purple), followed by a central line of symmetry and the area mirrored on the left hemisphere (area drawn in green). The timing graph (B) shows the TTP curve (purple) for the area representing the right MCA with a delay compared to the green curve representing the left MCA. The PWI map in TTP (C) demonstrates the evident timing delay in the purple demarked area.
ROIs were traced first for the pre-CAS PWI studies. Immediately following angioplasty, the ROI was traced for the post-CAS PWI to reproduce the pre-CAS ROI as closely as possible in shape and axial plane.
The following regional perfusion parameters were collected for statistical analysis: cerebral blood volume (CBV), mean transit time (MTT) and time to peak (TTP). As PWI parameters are influenced by physiological factors, normalization was performed using values from the contralateral territory, according to Wilkinson 3. Thus relative (normalized) measurements of MTT and TTP were obtained from the differences between hemispheres:
| dMTT=MTTstent–MTTcontralateral; |
| dTTP=TTPstent–TTPcontralateral. |
Relative CBV measurements were calculated as the ratio between the two hemispheres:
| rCBV=CBVstent/CBVcontralateral. |
PWI evaluation was performed by two radiologists blinded to clinical and CAS data, using Functool 2 (General Electric Medical Systems, Milwaukee, USA) in an advanced workstation from the same manufacturer·
All CASs were performed by conventional carotid angioplasty in the angiography suite and included transfemoral access under local anesthesia and sedation, full heparinization (70 U/kg) with use of a self-expanding stent (Wallstent; Boston Scientific, Natick, MA, USA) protected by a filter (EPI; Boston Scientific, Natick, MA, USA) following dilation by a 6 mm balloon (Ultrasoft; Boston Scientific, Natick, MA, USA)· Stent selection was based on the artery and stenosis dimensions· The procedure was successful in all cases, which resulted in less than 30% residual stenosis at the end of CAS· The final control angiograms after CAS did not show any evidence of intracranial macroembolism. All patients were treated with 75 mg clopidogrel and 100 mg aspirin daily for at least five days. No adverse periprocedural events occurred in this series.
This was a prospective trial with no control group, and data were analyzed by the parametric Student's t-test for paired data using Statistical Package for the Social Sciences Version 15 for Windows (SPSS, Chicago, IL, USA). Values were considered statistically significant when p<0.05. The study was approved by the Ethics Committee, and all patients gave written informed consent.
Results
Comparisons of absolute (non-normalized) and relative (normalized) PWI data are summarized in Tables 1 and 2 , respectively.
Table 1.
Absolute data - Mean and Standard Deviation for CBV, MTT, and TTP for the CAS treated and contralateral sides (n=21 patients)
| PWI parameter | Mean | Standard deviation | p |
|---|---|---|---|
| CBV | |||
| STENT side Pre-CAS | 854.6 | 462.4 | |
| STENT side Post-CAS | 1172.6 | 738.2 | 0.016 |
| CL side Pre-CAS | 886.2 | 436.6 | |
| CL side Post-CAS | 1195.8 | 755.8 | 0.029 |
| MTT | |||
| STENT side Pre-CAS | 568.0 | 476.9 | |
| STENT side Post-CAS | 695.2 | 665.1 | 0.182 |
| CL side Pre-CAS | 568.1 | 488.7 | |
| CL side Post-CAS | 698.6 | 672.2 | 0.179 |
| TTP | |||
| STENT side Pre-CAS | 36.47 | 4.21 | |
| STENT side Post-CAS | 37.99 | 5.05 | 0.200 |
| CL side Pre-CAS | 36.35 | 4.35 | |
| CL side Post-CAS | 38.47 | 5.62 | 0.105 |
| Note: CAS indicates carotid angioplasty with stent; CL, carotid territory contralateral to the stent; STENT carotid territory ipsilateral \to the stent. Student t test for paired data. | |||
Table 2.
Relative data by normalization - Mean and Standard Deviation for rCBV, dMTT, and dTTP (n=21 patients)
| PWI parameter | Mean | Standard Deviation |
P | |
|---|---|---|---|---|
| rCBV | Before CAS | 0.96 | 0.14 | 0.940 |
| After CAS | 0.97 | 0.10 | ||
| dMTT | Before CAS | −0.06 | 14.73 | <0.001 |
| After CAS | −3.46 | 14.65 | ||
| dTTP | Before CAS | 0.12 | 1.00 | 0.019 |
| After CAS | −0.47 | 1.08 | ||
| Note: CAS indicates carotid angioplasty and stenting;. dMTT indicates MTT difference; dTTP, TTP difference; rCBV, CBV ratio. Paired t test. | ||||
Absolute data show reduced CBV in the stenosis side before CAS compared to the contralateral side pre-CAS. Paradoxically, blood volume increased in both cerebral hemispheres after CAS, but this was more accentuated in the stent side (p=0.016) than the contralateral side (p=0.029). There was a delay in TTP in the territory ipsilateral to the carotid stenosis before CAS. After CAS, ipsilateral TTP became earlier than the contralateral territory.
Relative PWI data acquired before CAS produced rCBV, dMTT, and dTTP values that showed a small reduction in CBV, a discrete delay in TTP, and slightly shorter MTT in the middle cerebral artery (MCA) territory ipsilateral to the stenosis. After CAS, cerebral hemodynamics were improved, as measured by dMTT and dTTP; dMTT was reduced from -0.06 before CAS to -3.46 after CAS (p<0.001), by a reduction in MTT on the angioplasty side and dTTP dropped from 0.12 s before CAS to -0.47s after CAS (p=0.019), demonstrating a much earlier contrasting time to peak after CAS in the ipsilateral MCA territory. After CAS, rCBV suffered a slight increase from a mean of 0.96 before angioplasty to 0.97 after CAS (p=0.940). The mean variation results for rCBV, dMTT, and dTTP are exemplified by the patient shown in Figure 2 , who presented with a much earlier time to peak after CAS on the MCA territory ipsilateral to the stent.
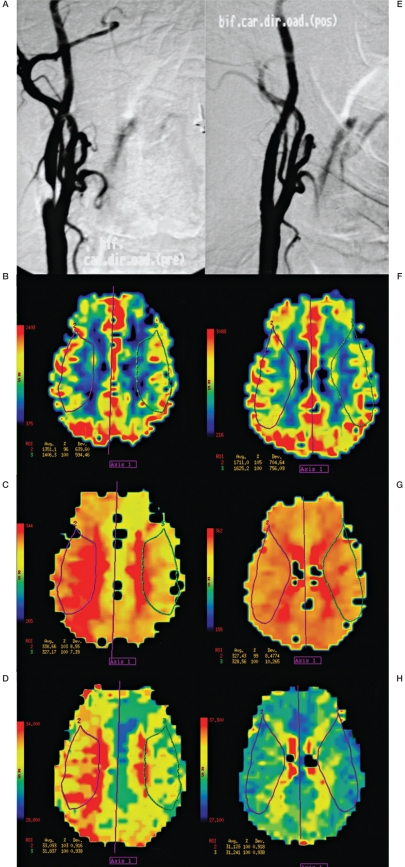
Figure 2.
Angiography and perfusion data from a 75-year-old woman with severe right carotid artery stenosis by digital angiography (A). PWI before CAS: CBV of the right MCA territory is 4% less than contralateral value (B), MTT shows a 3% delay (C) and TTP a 3% delay (D). Angiography after angioplasty with stent showing the absence of residual stenosis (E). PWI after CAS in the same axial section revealing improvement in all three perfusion parameters by MRI: right side CBV is 5% higher than contralateral value (F), MTT 1% quicker (G), and TTP without delay (H).
Discussion
In contrast to previous studies 3,10,12, we have improved timing resolution to evaluate timing parameters (MTT and TTP) more accurately. For this we used a PWI protocol with a gradient echo T2*-weighted technique and TR of 1000 ms to improve temporal differentiation for the mathematical curves describing our timing parameters. The flip angle was discretely adjusted to 75° to reduce image contamination. We normalized data to the contralateral parenchyma to avoid contamination from cross-circulation in the circle of Willis and any influence from physiological factors.
We considered normalized (interhemispheric) data more reliable, as did other authors 1,18,3,4, due to a diminished influence from physiological oscillations, collateral arterial pathways (via the circle of Willis or leptomeningeal and ophthalmic collaterals), stenosis/occlusion in the contralateral carotid artery, hydration status, changes in cardiac output, and non-homogenous magnetic field. Although absolute quantification can have advantages over relative measurements due to the multiple factors cited above and others still unknown, true absolute quantification is difficult. Therefore, it is preferable to use normalized values to evaluate cerebral perfusion by MRI 3.
The main finding in our patients was an improvement in hemodynamics after CAS by normalized data, clearly shown by the reduction in dMTT (p<0.001) and dTTP (p=0.019) in the territory fed by the MCA ipsilateral to the CAS. Increases in rCBV after CAS in our series were minor and not significant. Wilkinson et al. also found no significant differences in rCBV between territories before and after carotid stenosis treatment by CAS. They also demonstrated a significant reduction in MTT by MRI 3 h after CAS 3.
Teng et al. demonstrated TTP to be a valuable tool for estimating hemodynamic changes in carotid stenosis cases and with a good correlation to MTT 14.
Moftakhar et al. performed a comparative visual analysis of perfusion maps using a rainbow color scale, then a study of qualitative analysis 19. Mapping studies may be appropriate for illustrating tendencies, but their applicability and reproducibility without numerical data extracted from ROIs are reduced. We considered visual analyses by maps more susceptible to large inter- and intra-observer variations and therefore preferred to compare numerical data from ROIs normalized against the contralateral territory.
Martin et al. analyzed MR data with PWI and Qflow (a cardiovascular flow analysis software) in 12 patients and observed a marked increase in cerebral flow in the carotid artery that underwent CAS. The increase in flow was more pronounced in arteries with more severe preoperative stenosis. In their observations, there was no change in flow in the contralateral carotid artery or posterior circulation after CAS. They deduced that flow, as quantified by the Qflow technique, increased after angioplasty and that some time was necessary for this flow to normalize 10. Our data show a discrete, although not significant, improvement in CBV after revascularization. Martin et al. used MRI perfusion studies with EPI technique and a TR of 2000 ms, defined ROIs over a wide territory, and included anterior cerebral artery territories as well as MCA territories 10 . They did not observe any significant change in CBV, as we did in our study, and noted only modest improvement in MTT and TTP. Our study focused on areas representative of the middle cerebral artery only, to avoid perfusion data from the anterior cerebral arteries, which can be fed by either of the carotid arteries, thus altering the expected changes in perfusion. We considered the MCA territory represents ipsilateral carotid artery flow more reliably. Our study normalized stent territory data against data from the contralateral hemisphere, which made pre- and post-intervention comparisons easier. Furthermore, we used a much shorter TR, which enabled us to better evaluate timing parameters, which improved markedly after CAS.
It is possible that complex self-regulatory mechanisms could become active in severe stenoses that could include changes in microcirculation, vasodilatation or direct action from the central nervous system. It is also theoretically possible that before CAS, the microcirculation is in a state of vasodilatation induced by the presence of a severe long-term proximal stenosis, and that after CAS, the microcirculation is no longer dilated, thus reducing the blood volume in the ipsilateral territory.
In a study of normalized cerebral perfusion data in patients with carotid artery stenosis, Bozzao et al. found that prolonged MTT on the stenotic side was due to hemodynamic compensation between the cerebral hemispheres 20. Given the hemodynamic parameter equation, we are forced to consider the following possibility. Theoretically, as there was a reduction in timing parameters after CAS without a reduction in CBV, a state of cerebral hyperflow would exist, leading to possible complications such as the dangerous hyperperfusion syndrome 3,7,21. However, this was not seen in our series.
Fukuda et al., using PWI and single photon emission computed tomography, demonstrated that increased CBV is predictive for hyperperfusion syndrome after endarterectomy 22. The technique of studying CBF by intracarotid xenon injection or photon emission tomography found that patients with a two-fold increased CBF after endarterectomy were at greater risk of developing hyperperfusion syndrome 9.
We considered the vasodilatation and selfregulation rationales to be valid, because equilibrium needs to be dynamically maintained and patients have their cerebral blood equilibrium preserved. Otherwise, all patients would show signs of cerebral ischemia from low flow as MTT is reduced after CAS.
Absolute data taken before CAS from our study, in agreement with Kluytmans et al. 1 revealed a reduced CBV on the stenosis side and not a compensatory increase in CBV consistent with the action of vasodilatation. Therefore, we believe that the main benefit of carotid stenosis treatment is the prevention of embolic infarcts, rather than restoration of cerebral blood flow. Our absolute data demonstrate an increase in cerebral hemispheric blood volume after CAS, which was more accentuated on the angioplasty side (p=0.016) than the contralateral side (p=0.029). This bilateral increase made the improvement in rCBV on the stent side less marked than in the relative analysis (normalized).
Similarly, Laar et al., found an increase in CBF on the carotid stenosis side after CAS or CEA, making their CBF similar to the control group 12. In agreement with our observed bilateral increase in CBV, Ko et al. found a bilateral increase in CBF after unilateral CEA using intra-arterial xenon. They concluded that the treatment of carotid stenosis does not explain this fact, based solely on hemodynamics 21.
According to Niesen et al., an understanding of hemodynamic changes after CAS is still limited. Other unknown factors must play an important role in perfusion changes before and after carotid stenosis treatment 7.
In our absolute data, we noted a TTP delay in the MCA territory ipsilateral to the carotid artery before CAS compared to the contralateral side. This was also observed by Gauvrit et al. in carotid artery stenosis patients 23. There was significant improvement in TTP after CAS in our study, which was apparent much earlier in the ipsilateral side than in the contralateral territory.
Other techniques can also evaluate encephalic perfusion, such as transcranial Doppler, which has the advantage of being non-invasive, but these are generally limited to analysis of only one vessel, usually the middle cerebral artery ipsilateral to the carotid stenosis. Doppler studies can also be impaired if a cardiac arrhythmia is present, which can make the velocity on the spectral trace unreliable 24. Perfusion by tomography has undergone important technical refinements, but its application is still limited to small sections or only one section per examination. Evaluation by intracarotid xenonium has been reserved for research. MRI with a PWI protocol is available in most large cities and can non-invasively evaluate the brain with a safe paramagnetic contrast agent, while avoiding ionizing radiation and retaining the advantages of good spatial resolution and excellent correlation with single photon emission computed tomography 25,26.
There were certain limitations to our study. Studying perfusion by MRI is always performed with patients at rest in the supine position. This position is excellent for cerebral blood flow due to the absence of gravitational action between the heart and brain. We believe that this could in part explain the observation of relatively few PWI deficits in our carotid stenosis patients. On the other hand, standing or seated patients would have gravity acting against cerebral perfusion, which could make this perfusion deficit more obvious in absolute data.
Although improvements in cerebral perfusion parameters have been demonstrated in this study and in previous investigations, these findings require clinical correlation, which can be obtained with cognitive evaluation questionnaires used to evaluate various types of dementia. A short study by Moftakhar et al. of 20 cases of cervical carotid angioplasty and anterior and posterior circulation intracranial angioplasties evaluated hemodynamic status by MRI 19. They found improved cognition scores in 79% of patients following angioplasty. Of the patients with carotid stenoses (cervical and intracranial), 11 out of 14 (79%) presented with perfusion abnormalities before treatment, and exhibited improvements in cerebral perfusion by MRI after angioplasty. They concluded that an improvement in perfusion parameters by MRI was predictive of improved cognition after angioplasty 19. Turk et al. evaluated patients with >50% stenosis and found a significant improvement in cognitive scores after CAS 27. Studies with a larger series are needed to confirm this tendency.
For us, as for other authors 3,7,21, an understanding of the interhemispherical asymmetry of perfusion by MRI in patients is far from complete, highlighting the need for future studies in this area.
Conclusion
Early improvement in cerebral perfusion in patients submitted to angioplasty with stenting was clearly demonstrated by MRI. Improvement was seen primarily in the timing parameters.
Abbreviations
CAS - carotid angioplasty and stenting
CBF - regional cerebral blood flow
CBV - regional cerebral blood volume
CEA - carotid endarterectomy
CL - territory contralateral to CAS
DSA - digital subtraction angiography
GRE - gradient-echo sequence
MCA - middle cerebral artery
MRI - magnetic resonance imaging
MTT - regional mean transit time
PWI - perfusion weighted images
ROI - region of interest
SPECT - single photon emission computed tomography
TR - repetition time
TTP - regional time to peak
Acknowledgments
This study was funded by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo). The authors thank Eduardo Figueiredo of GE Medical Systems, Milwaukee, USA for his assistance in the preparation and execution of the investigation protocol and for building the PWI graphs of this article.
Appendix.
Individual perfusion data before and after carotid angioplasty with stent (CAS)
| Patient | Caroti CAS |
Degr sten* |
Before CAS | After CAS | Before CAS | After CAS | Before CAS | After CAS | ||||||
| Number | (side) | (%) | CBV Stent side |
CBV CL side |
CBV Stent side |
CBV CL side |
MTT Stent side |
MTT CL side |
MTT Stent side |
MTT CL side |
TTP Stent side |
TTP CL side |
TTP Stent side |
TTP CL side |
| 1 | L | 70 | 324.18 | 340.45 | 355.69 | 360.92 | 1849 | 1868.7 | 2222.9 | 2255.5 | 35.325 | 35.733 | 41.447 | 41.995 |
| 2 | L | 70 | 400.22 | 379.99 | 242.48 | 259.06 | 1464.7 | 1503.2 | 1648.5 | 1690.5 | 28.412 | 28.716 | 29.389 | 29.802 |
| 3 | R | 95-99 | 653.99 | 922.22 | 341.99 | 376.35 | 288.79 | 268.45 | 289.29 | 287.1 | 32.299 | 30.198 | 40.321 | 39.400 |
| 4 | L | 90 | 809.74 | 867.18 | 589.98 | 679.57 | 390.73 | 391.85 | 476.37 | 481.25 | 37.300 | 38.308 | 51.591 | 55.083 |
| 5 | R | 70 | 846.74 | 882.83 | 1532.5 | 1412.3 | 374.74 | 370.54 | 405.27 | 402.81 | 35.652 | 36.104 | 39.288 | 39.300 |
| 6 | L | 95-99 | 1092.7 | 1020.3 | 1588 | 1983.7 | 404.95 | 420.67 | 402.14 | 422.04 | 38.244 | 40.342 | 37.675 | 40.396 |
| 7 | R | 80 | 911.87 | 874.52 | 102.47 | 138.8 | 375.1 | 366.48 | 2287.9 | 2280.8 | 36.124 | 35.223 | 42.639 | 44.425 |
| 8 | R | 95-99 | 1351.1 | 1406.3 | 1711 | 1625.2 | 338.66 | 327.17 | 327.33 | 328.56 | 33.093 | 31.837 | 31.125 | 31.241 |
| 9 | R | 70 | 773.59 | 741.2 | 863.23 | 763.47 | 382.53 | 384.72 | 422.69 | 421.61 | 33.859 | 34.403 | 40.892 | 40.862 |
| 10 | L | 80 | 1284.8 | 1316.3 | 1334 | 1345.9 | 347.91 | 337.24 | 363.78 | 331.98 | 32.851 | 33.977 | 32.675 | 32.527 |
| 11 | L | 70 | 761.2 | 778.06 | 820.94 | 809.08 | 448.18 | 446.36 | 427.24 | 431.69 | 41.087 | 41.003 | 39.009 | 39.797 |
| 12 | L | 70 | 972.75 | 1333.1 | 1454.1 | 1651.2 | 425.79 | 413.26 | 335.73 | 333.28 | 42.801 | 42.661 | 32.003 | 31.842 |
| 13 | R | 80 | 690.33 | 525.39 | 853.05 | 882.88 | 424.37 | 432.97 | 407.54 | 414.95 | 42.248 | 41.656 | 44.588 | 44.068 |
| 14 | L | 80 | 1022.8 | 1102.4 | 1320.1 | 1291.8 | 343.9 | 332.74 | 381.11 | 377.67 | 33.421 | 32.546 | 36.642 | 36.522 |
| 15 | L | 70 | 466.49 | 537.48 | 1639.2 | 1488.4 | 465.58 | 467.93 | 388.93 | 390.04 | 43.719 | 44.220 | 39.190 | 39.030 |
| 16 | L | 70 | 1269.8 | 1431.8 | 1906.9 | 1704.3 | 400.59 | 399.69 | 368.62 | 369.27 | 38.815 | 38.779 | 35.503 | 35.439 |
| 17 | L | 70 | 862.6 | 855.59 | 884.03 | 888.46 | 373.93 | 362.28 | 382.94 | 381.97 | 31.826 | 31.983 | 34.156 | 34.408 |
| 18 | R | 70 | 331.93 | 363.05 | 1755.4 | 1888.9 | 245.7 | 237.53 | 358.41 | 352.85 | 33.565 | 32.309 | 34.909 | 34.322 |
| 19 | R | 80 | 2319 | 2027.9 | 3165.3 | 3245.7 | 362.39 | 362.74 | 398.57 | 401.63 | 35.790 | 35.722 | 38.589 | 39.497 |
| 20 | R | 80 | 493.07 | 460.93 | 1783.2 | 1913.9 | 461.4 | 448.66 | 420.88 | 425.08 | 43.029 | 41.245 | 40.500 | 40.928 |
| 21 | L | 80 | 308.54 | 443.36 | 380.53 | 401.22 | 1759 | 1786.1 | 1882.6 | 1890.9 | 36.332 | 36.309 | 35.706 | 36.896 |
| Note: CAS, carotid angioplasty with stent; CBV, regional cerebral blood volume; CL, territory contralateral to CAS; R, right side; L, left side; MTT, regional mean transit time; TTP, regional time to peak. * Degree of stenosis according to angiography analysis in percentage points. The subocclusive stenosis situation (value = 95-99%) was taken as 99% for statistical calculations. | ||||||||||||||
References
- 1.Kluytmans M, van der Grond J, Eikelboom BC, et al. Long-term hemodynamic effects of carotid endarterectomy. Stroke. 1998;29:1567–1572. doi: 10.1161/01.str.29.8.1567. [DOI] [PubMed] [Google Scholar]
- 2.Apruzzese A, Silvestrini M, Floris R, et al. Cerebral hemodynamics in asymptomatic patients with internal carotid artery occlusion: a dynamic susceptibility contrast MR and transcranial Doppler study. Am J Neuroradiol. 2001;22:1062–1067. [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson ID, Griffiths PD, Hoggard N, et al. Shortterm changes in cerebral microhemodynamics after carotid stenting. Am J Neuroradiol. 2003;24:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- 4.Waaijer A, Leeuwen MS van, Osch MJP van, et al. Changes in cerebral perfusion after revascularization of symptomatic carotid artery stenosis: CT measurement. Radiology. 2007;245(2):541–548. doi: 10.1148/radiol.2451061493. [DOI] [PubMed] [Google Scholar]
- 5.Mohr JP, Albers GW, Amarenco P, et al. Etiology of stroke. Stroke. 1997;28:1501–1506. doi: 10.1161/01.str.28.7.1501. [DOI] [PubMed] [Google Scholar]
- 6.Bokkers RPH, Laar PJ, Vem KCC, et al. Arterial spinlabeling MR imaging measurements of timing parameters in patients with a carotid artery occlusion. Am J Neuroradiol. 2008;29:1698–170. doi: 10.3174/ajnr.A1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niesen W-D, Rosenkranz M, Eckert B, et al. Hemodynamic changes of the cerebral circulation after stentprotected carotid angioplasty. Am J Neuroradiol. 2004;25:1162–1167. [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts HC, Dillon WP, Smith WS. Dynamic CT perfusion to assess the effect of carotid revascularization in chronic cerebral ischemia. Am J Neuroradiol. 2000;21:421–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Bando K, Satoh K, Matsubara S, et al. Hyperperfusion phenomenon after percutaneous transluminal angioplasty for atherosclerotic stenosis of the intracranial vertebral artery. J Neurosurg. 2001;94:826–830. doi: 10.3171/jns.2001.94.5.0826. [DOI] [PubMed] [Google Scholar]
- 10.Martin AJ, Saloner DA, Roberts TPL, et al. Carotid stent delivery in an XMR suite: immediate assessment of the physiologic impact of extracranial revascularization. Am J Neuroradiol. 2005;26:531–537. [PMC free article] [PubMed] [Google Scholar]
- 11.Laar PJ, Grond J, Moll FL, et al. Hemodynamic effect of carotid stenting and carotid endarterectomy. J Vasc Surg. 2006;44:73–78. doi: 10.1016/j.jvs.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Laar PJV, Hendrikse J, Mali WPThM, et al. Altered flow territories after carotid stenting and carotid endarterectomy. J Vasc Surg. 2007;45:1155–1161. doi: 10.1016/j.jvs.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 13.Doerfler A, Eckstein HH, Eichbaum M, et al. Perfusion-weighted magnetic resonance imaging in patients with carotid artery disease before and after carotid endarterectomy. J Vasc Surg. 2001;34:587–593. doi: 10.1067/mva.2001.118588. [DOI] [PubMed] [Google Scholar]
- 14.Teng MM, Cheng HC, Kao YH, et al. MR perfusion studies of brain for patients with unilateral carotid stenosis or occlusion: evaluation of maps of time to peak and percentage of baseline at peak. J Comput Assist Tomogr. 2001;25:121–125. doi: 10.1097/00004728-200101000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Rutgers DR, Klijn CLM, Kappelle LJ, et al. Sustained bilateral hemodynamic benefit of contralateral carotid endarterectomy in patients with symptomatic internal carotid artery occlusion. Stroke. 2001;32:728–734. doi: 10.1161/01.str.32.3.728. [DOI] [PubMed] [Google Scholar]
- 16.Soinne L, Helenius J, Tatlisumak T, et al. Cerebral hemodynamics in asymptomatic and symptomatic patients with high-grade carotid stenosis undergoing carotid endarterectomy. Stroke. 2003;34:1655–1661. doi: 10.1161/01.STR.0000075605.36068.D9. [DOI] [PubMed] [Google Scholar]
- 17.North American Symptomatic Carotid Endarterectomy Trial Contributors. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 18.Maeda M, Yuh WT, Ueda T, et al. Severe occlusive carotid artery disease: hemodynamic assessment by MR perfusion imaging in symptomatic patients. Am J Neuroradiol. 1999;20:43–51. [PubMed] [Google Scholar]
- 19.Moftakhar R, Turk AS, Niemann DB, et al. Effects of carotid or vertebrobasilar stent placement on cerebral perfusion and cognition. Am J Neuroradiol. 2005;26:1772–1780. [PMC free article] [PubMed] [Google Scholar]
- 20.Bozzao A, Floris R, Gaudiello F, et al. Hemodynamic modifications in patients with symptomatic unilateral stenosis of the internal carotid artery: evaluation with MR imaging perfusion sequences. Am J Neuroradiol. 2002;23:1342–1345. [PMC free article] [PubMed] [Google Scholar]
- 21.Ko NU, Achrol AS, Chopra M, et al. Cerebral blood flow changes after endovascular treatment of cerebrovascular stenoses. Am J Neuroradiol. 2005;26:538–542. [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda T, Ogasawara K, Kobayashi M, et al. Prediction of cerebral hyperperfusion after carotid endarterectomy using cerebral blood volume measured by perfusion-weighted MR imaging compared with singlephoton emission CT. Am J Neuroradiol. 2007;28:737–742. [PMC free article] [PubMed] [Google Scholar]
- 23.Gauvrit JY, Delmaire C, Henon H, et al. Diffusion/ perfusion-weighted magnetic resonance imaging after carotid angioplasty and stenting. J Neurol. 2004;251:1060–1067. doi: 10.1007/s00415-004-0373-8. [DOI] [PubMed] [Google Scholar]
- 24.Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke. 1992;23:171–174. doi: 10.1161/01.str.23.2.171. [DOI] [PubMed] [Google Scholar]
- 25.Kucharczyk J, Vexler ZS, Roberts TP, et al. Echo-planar perfusionsensitive MR-imaging of acute cerebral ischemia. Radiology. 1993;188:711–717. doi: 10.1148/radiology.188.3.8351338. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi K, Murase K, Miki H, et al. Measurement of cerebral hemodynamics with perfusion-weighted MR imaging: comparison with pre- and post-acetazolamide 133Xe-SPECT in occlusive carotid disease. Am J Neuroradiol. 2001;22:248–54. [PMC free article] [PubMed] [Google Scholar]
- 27.Turk AS, Chaudry I, Haughton VM, et al. Effect of Carotid Artery Stenting on Cognitive Function in Patients With Carotid Artery Stenosis: Preliminary Results. Am J Neuroradiol. 2008;29:265–268. doi: 10.3174/ajnr.A0828. [DOI] [PMC free article] [PubMed] [Google Scholar]