Summary
We present a case of delayed aggravation of initially-resolved symptoms in a patient after successful embolization of a T5 spinal dural arteriovenous (AV) fistula with N-butyl cyanoacr- ylate. The symptoms were attributed to venous thrombosis and resolved with systemic antico- agulation after five days of treatment. Although the most adequate treatment for preventing venous thrombosis after spinal dural AV fistula is not known, we describe this patient as a case for more aggressive prophylactic anticoagulation measures in the immediate post-embolization time period.
Key words: spinal dural arteriovenous fistula, embolization, anticoagulation, venous thrombosis
Illustrative Case
This 59-year-old woman with no significant medical history presented with complaints of low back pain, gait instability, and bilateral lower extremity paresthesias below the knee that had progressed in severity over one year. Two months before presentation, the patient noted intermittent episodes of urinary incontinence. On examination, she demonstrated no objective cranial nerve, motor, sensory, or cerebellar dysfunction or saddle anesthesia. She was able to ambulate unassisted with a normal gait.
Initial work-up included an outside institution contrast-enhanced MRI of the thoracic and lumbar region demonstrating T2 hypersignal from the mid thoracic cord to the conus medullaris, with perimedullary flow-voids along the posterior thoracic cord. Diagnostic angiography performed at an outside institution demonstrated a type I dural AV-fistula arising from the spinal branch of the left T5 intercostal artery with abnormally dilated perimedullary venous drainage. The patient underwent spinal angiography and embolization at our institution, which confirmed the previous findings (Figure 1A). There was a sub-occlusive dissection of the proximal left T5 intercostal artery related to the first angiographic procedure, and collateral vessels arising from the left T6 intercostal artery provided arterial feeders to the AV fistula via T6-T5 latero-corporeal anastomosis. (Figure 1A).
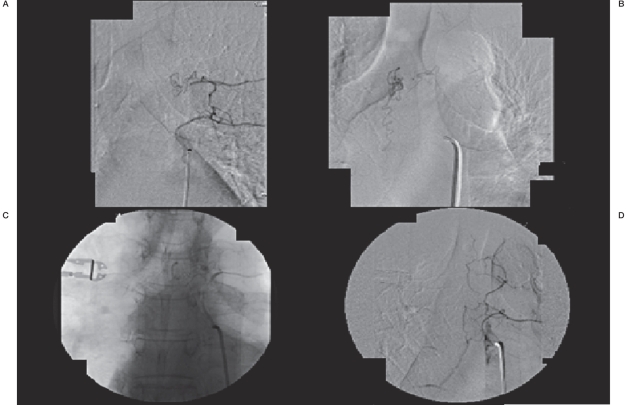
Figure 1.
A) Selective catheter spinal angiography through the left T6 radicular artery demonstrating a type I spinal dural AV fistula with arterial feeders arising from the left T5 level, and latero-corporeal anastomotic feeders from the left T6 artery to that level. B) Venous drainage from the fistula inferiorly through dilated perimedullary veins. C) Unsubtracted angiography demonstrating n-BCA glue embolic material filling the fistula to including the first centimeter of draining vein. D) Post-embolization spinal angiogram demonstrating anterograde flow through the thoracic intercostals arteries with no further filling of AV fistula.
It was decided to access the T5 spinal branch by traversing the T5 intercostal artery dissection. A Prowler 14 microcatheter (Cordis Endovascular Systems, Inc., Miami, FL, USA) was navigated through a coaxial assembly system over an Agility 14 microwire (Cordis Endovascular Systems, Inc.) through the left T5 intercostal artery past the area of dissection and into the spinal branch then the arterial feeder to the spinal AV fistula. Selective angiography through the microcatheter delineated nearwedge anterograde flow into the dural AV fistula with inferior venous drainage through dilated perimedullary veins (Figure 1B).
Next, under subtracted fluoroscopy, n-BCA (TruFill, Codman, Boston MA) diluted within a one-to-three ratio with Ethiodol (Savage, Melville, NY, USA) was slowly injected through the microcatheter until filling of the fistula and first centimeter of the pial draining vein (Figure 1C). Post-injection angiogram through the bilateral T4, T5 and T6 intercostal arteries showed no further filling of the fistula or the abnormal perimedullary veins (Figure 1D).
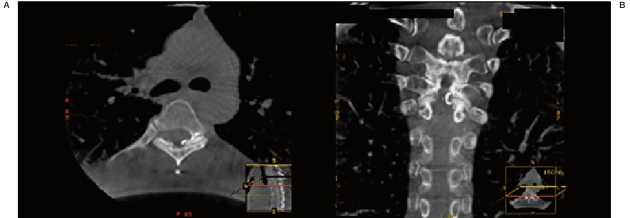
An intra-operative CT scan was performed after embolization. Axial (Figure 2A) and reconstructed (Figure 2B) images demonstrated n-BCA glue embolic material at the level of the dural nerve root sleeve into the first centimeter of the pial vein draining the fistula. The patient received a one-time prophylactic dose of 300 mg aspirin and was placed on 5000 Units of heparin SQ BID.
Figure 2.
Axial (A) and reconstructed coronal (B) CT scan images demonstrating intradural location of embolic n-BCA glue to the level of the AV fistula draining vein.
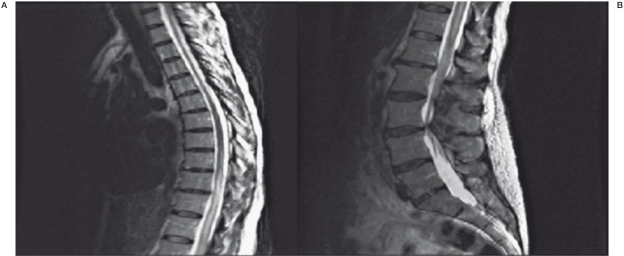
Post-operatively, the patient awoke neurologically non-focal with a normal cranial nerve, motor, sensory, and cerebellar examination. She still noted stable mild bilateral paresthesias in her lower extremities. She was able to ambulate and void without incontinence, and was discharged home on post-operative day #1. The patient returned to the Emergency Room the evening of post-operative day #1 after awaking from a nap and noting bilateral lower extremity weakness and multiple episodes of urinary incontinence. She was unable to ambulate on her own. She was noted on examination to have marked bilateral lower extremity paraparesis (proximal musculature affected more severely than distal musculature) with upgoing toes bilaterally. MRI of the thoracic and lumbar region demonstrated unchanged intramedullary T2 hypersignal from T9 to the conus medullaris, primarily involving the central gray matter (Figure 3). There was an ill-defined linear susceptibility artifact within the canal at T5, consistent with embolic material. There was no evidence of acute infarct or hemorrhage at that level, or the presence of persistent abnormal flow-voids to suggest residual AV fistula.
Figure 3.
Post-embolization sagittal T2-weighted sequence MRI of the thoracic and lumbar spine demonstrating continued intramedullary hyperintensity that spans from T9 to the conus medullaris without evidence of acute hemorrhage.
The patient was determined to be suffering from venous ischemia related to propagation of thrombus into the normal draining venous system. Systemic anticoagulation with heparin was initiated for PTT goal 1.5-2 times normal. She displayed a marked improvement in her neurologic examination by the second day of therapeutic anticoagulation and was transitioned to Coumadin for an INR goal of 2-3. She was discharged home ambulating and neurologically intact without incontinence five days after readmission. One month after embolization, she was back to work, still needed a cane to walk on irregular surfaces, and had no incontinence. She will be anticoagulated with Coumadin for six months post-operatively.
Discussion
Spinal dural arteriovenous (AV) fistulas are rare lesions that occur in the dura along the nerve root sleeve. They are classically supplied by a dural branch of the radicular artery and drain into dilated pial perimedullary veins 1.
Dural AV Fistulas most commonly present in the thoracolumbar region in male patients older than 50 years of age. Although unknown, their etiology is felt to be due to vascular shunts that develop secondary to either environmental or congenital causes of venous outflow obstruction 2. Treatment can be achieved by surgery or embolization. The goal of both techniques is to permanently close the pial vein draining the fistula. This is accomplished by surgically coagulating and dividing the vein as it enters the dura, or endovascularly filling the initial centimeter of the vein with a liquid embolic material. Particulate or liquid embolization that does not fill the vein has a high risk of recurrence 3-6.
Recanalization rates are lower when postembolization CT scans have demonstrated embolic material to be intradural as opposed to extradural, indicating greater success with more distal locations 7. This relates to the fact that although multiple collateral arterial feeders may supply a spinal dural AV fistula, venous drainage is unique to that particular shunt. Today, most experts define permanent cure without risk for anastomotic recurrence by this radiographic standard 8-11. However, this efficacy goal needs to be balanced with the risk that too distal a location of embolic material can cause perimedullary venous outflow thrombosis and obstruction to an already compromised drainage system. This is why only the initial segment of the pial vein should be embolized, and perimedullary veins should be respected. Foix-Alajouanine syndrome, commonly referred to the acute deterioration in patients with spinal dural AV fistulas, may also include a syndrome of reversible venous congestion 12. Post-embolization venous thrombosis represents a variant mechanism of the same reversible ischemia that typifies the disease presentation.
The differential of post-embolization venous thrombosis must be considered in any patient who demonstrates subsequent aggravation of symptoms after treatment of a spinal dural AV fistula. Although fistulous recurrence or spinal cord hemorrhage can present with the same features, this patient's presentation within 24 of hours of intervention suggested thrombosis as the etiology, which was confirmed by a lack of prominent flow-voids or hemorrhage on re-admission MRI and by progressive improvement in symptoms with systemic anticoagulation.
Niimi et al. 8 reported two of 49 cases of spinal dural AV fistula embolization that developed venous thrombosis and ischemia within one month of treatment. These patients were treated with systemic heparinization to maintain PTT 1.5-2 times normal, the same protocol utilized for our patient until bridging to Coumadin. Ohta et al. 13 describe a case of postembolization venous thrombosis in a patient seven days after discontinuation of a prophylactic antithrombin agent and substitution with aspirin monotherapy. Upon resolution of symptoms with dual-therapy anti-platelet agents, the patient developed subsequent thrombosis after a return to single anti-platelet therapy.
The best method for treating venous thrombosis is anticoagulation rather than platelet antagonists, specifically the use of heparin/vitamin K antagonists 14. Stam 14 recommends a six-month treatment with vitamin K antagonists for a target INR 2.5 for cerebral venous thrombosis, suggesting that warfarinzation may be an adequate choice for spinal venous thrombosis as well, forming the basis for management in our patient.
In the case of our patient, immediate postembolization intra-operative CT scan demonstrated efficacious intradural placement of n-BCA glue at the fistulous connection without further migration into large draining perimedullary veins. However, despite radiographically ideal placement of embolic material, the patient developed progressive venous ischemia secondary to thrombosis while on anti-platelet monotherapy and standard post-operative dose of prophylactic subcutaneous heparin. We suggest that after treatment of spinal AV fistulas with embolization and possibly by surgery, patients may be at risk for propagation of thrombus into the normal venous system, and represent a subset of post-operative patients with a relative hypercoagulable state. Anti-platelet monotherapy and standard subcutaneous doses of heparin-derived substances may not afford adequate protection in a subset of patients who receive this intervention. We advocate that more aggressive post-intervention prophylaxis with systemic heparin anticoagulation while bridging to vitamin K antagonists for should be employed for longer-term protection against a pro-thrombotic cascade. Given the relative rarity of this disease, however, a large cohort in which to prospectively test this theory may be difficult to establish.
References
- 1.Merland JJ, Riche MC, Chiras J. Intraspinal extramedullary arteriovenous fistulae draining into the medullary veins. J Neuroradiol. 1980;7:221–231. [PubMed] [Google Scholar]
- 2.Jellema K, Tijssen CC, van Ginj J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006;129:3150–3164. doi: 10.1093/brain/awl220. [DOI] [PubMed] [Google Scholar]
- 3.Gobin YP. Classification and endovascular treatment of spinal of spinal cord arteriovenous malformations and fistulas. J Stroke Cerebrovasc Dis. 1997;6:282–286. doi: 10.1016/s1052-3057(97)80031-6. [DOI] [PubMed] [Google Scholar]
- 4.Hall WA, Oldfield EH, Doppman JL. Recanalization of spinal cord arteriovenous malformations following embolization. J Neurosurg. 1989;70:712–714. doi: 10.3171/jns.1989.70.5.0714. [DOI] [PubMed] [Google Scholar]
- 5.Morgan MK, Marsh WR. Management of spinal dural arteriovenous malformations. J Neurosurg. 1989;70:832–835. doi: 10.3171/jns.1989.70.6.0832. [DOI] [PubMed] [Google Scholar]
- 6.Nichols DA, Rufenacht DA, Jack CR, et al. Embolization of spinal dural arteriovenous fistula with polyvinyl alcohol particles: Experience in 14 patients. Am J Neuroradiol. 1991;134:933–940. [PMC free article] [PubMed] [Google Scholar]
- 7.Guillevin R, Vallee JN, Cormier E, et al. N-butyl 2-cyanoacrylate embolization of spinal dural arteriovenous fistulae: CT evaluation, technical features, and outcome prognosis in 26 cases. Am J Neuroradiol. 2005;26:929–935. [PMC free article] [PubMed] [Google Scholar]
- 8.Niimi Y, Berenstein A, Setton A, et al. Embolization of spinal dural arteriovenous fistulae: results and follow-up. Neurosurgery. 1997;40:675–683. doi: 10.1097/00006123-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Tacconi L, Lopez Izquierdo BC, Simon L. Outcome and prognostic factors in the surgical treatment of spinal dural arteriovenous fistulas: a long-term study. Neurosurgery. 1997;11:298–305. doi: 10.1080/02688699746078. [DOI] [PubMed] [Google Scholar]
- 10.Behrens S, Thron A. Long-term follow-up and outcome in patients treated for spinal dural arteriovenous fistula. J Neurol. 1999;246:181–185. doi: 10.1007/s004150050331. [DOI] [PubMed] [Google Scholar]
- 11.Symon L, Kuyama H, Kendall B. Dural arteriovenous malformations of the spine: clinical features and surgical results in 55 cases. J Neurosurg. 1984;60:238–247. doi: 10.3171/jns.1984.60.2.0238. [DOI] [PubMed] [Google Scholar]
- 12.Criscuolo GR, Oldfield EH, Doppman JL. Reversible acute and subacute myelopathy in patients with dural arteriovenous fistulas. Foix-Alajouanine syndrome reconsidered. J Neurosurg. 1989;70:354–359. doi: 10.3171/jns.1989.70.3.0354. [DOI] [PubMed] [Google Scholar]
- 13.Ohta T, Gomi M, Oowaki H, et al. Chronic venous congestion following embolization of spinal dural arteriovenous fistula. J Neurosurg Spine. 2008;9:186–190. doi: 10.3171/SPI/2008/9/8/186. [DOI] [PubMed] [Google Scholar]
- 14.Stam J. Thrombosis of the cerebral veins and sinuses. NEJM. 2005;352:1791–1798. doi: 10.1056/NEJMra042354. [DOI] [PubMed] [Google Scholar]