Summary
Isolated posterior spinal artery aneurysms are rare vascular lesions. We describe the case of a 43-year-old man presenting with spinal subarachnoid hemorrhage after a minor trauma who was found to have a dissecting aneurysm of a posterior spinal artery originating from the right T4 level. Endovascular treatment was not contemplated because of the small size of the feeding artery, whereas surgical resection was deemed more appropriate because of the posterolateral perimedullary location that was well appreciated on CT angiography. After surgical resection of the aneurysm the patient had a complete neurological recovery. In comparison to anterior spinal artery aneurysms whose pathogenesis is diverse, posterior spinal aneurysms are most often secondary to a dissection and represent false or spurious aneurysms. Although the definite diagnosis still requires spinal angiography, MRI and CT may better delineate the relationship of the aneurysm to the spinal cord in order to determine the best treatment method. Prompt treatment is recommended as they have high rebleeding and mortality rates.
Key words: spinal aneurysm, posterior spinal artery, spinal subarachnoid hemorrhage, arterial dissection
Introduction
Subarachnoid hemorrhage (SAH) attributed to a spinal origin is present in less than 1% of all cases with SAH 1. The most frequent etiologies are rupture from spinal cord arteriovenous malformations (ScAVMs) followed by bleeding from intraspinal tumors. Spinal aneurysms are an extremely rare entity, found in only one of the more than 3,000 spinal angiograms reviewed by Djindjian et al. 2,3. Spinal aneurysms are typically reported in association with lesions that increase the blood flow through the spinal arteries. This may be present in spinal arteriovenous malformations (both of the glomerular and fistulous type) 4-7, less commonly in dural arteriovenous fistulas 8 and in patients with coarctation of the aorta 9-11, bilateral vertebral artery occlusion 12 or Moya-moya disease 13 in whom the anterior spinal artery (ASA) serves as collateral supply. If a spinal artery aneurysm is not associated with the aforementioned conditions it is referred to as an "isolated spinal aneurysm". We describe a case of spinal SAH caused by rupture of an isolated posterior spinal aneurysm.
Case Report
Clinical Findings
Four days prior to admission, a 43-year-old man felt a sudden stabbing pain in the upper thoracic spine during skiing immediately after an axial compression trauma. Plain films were normal, the pain ceased within hours and the patient returned home. Three days later, on the day of admission to an outside hospital, the patient had a sudden and acute onset of thunderclap headaches. While cranial CT including cranial CT angiography was normal, CSF studies revealed a subarachnoid hemorrhage. Spinal MRI confirmed the diagnosis of subarachnoid hemorrhage that was most pronounced in the upper thoracic region. No pathological vessels were seen after contrast enhancement. The patient was transferred to our hospital. On admission, the patient had a positive stiff neck and Lasègue sign, severe headaches and thoracolumbar pain that radiated into both knees. There were no sensory disturbances, bowel or bladder disturbances or motor weaknesses.
Imaging
MRI of the spine including a temporal resolved MR angiography was repeated and demonstrated T1 and FLAIR hyperintensities around the cord, most pronounced in the upper thoracic region indicative of a subarachnoid hemorrhage. The cord was normal without any edema. No pathological vessels were noticed. At T3 level, there was a circumscribed area of T2 hypointensity at the dorsolateral aspect of the cord, which showed nodular enhancement following contrast administration. Contrast-enhanced, temporally resolved MR angiography did not show any early draining veins. Spinal digital subtraction angiography revealed a false aneurysm originating from a dorsolateral radiculopial artery from the right Th4 segmental artery located superficial to the cord on the right dorsolateral paramedian part of the cord. The aneurysm demonstrated slow filling with stagnation of the contrast to the venous phase. The feeding artery was not dilated and the small diameter of the artery prohibited an endovascular approach with secure exclusion of the aneurysm proper. Filling of the aneurysm was also noted after injection into the anterior spinal artery system via radiculopial collaterals of the vasocorona. Therefore, proximal parent vessel occlusion of the feeding artery was not contemplated. A CT angiography was performed to exactly localize the aneurysm in relation to the cord. ECG-triggered multislice computed tomography was performed using a 64 Multislice dual source CT Scanner (Siemens Somatom Definition). Contrast enhancement was obtained by intravenous application of 100 ml of an iodinated contrast agent (Ultravist© 300 mg of Iodine /ml, Schering, Berlin, Germany). Scan parameters were as follows: tube voltage 120 kV and 200 mAs, pitch 0.7 resulting in a slice thickness of 0.65 mm. By using different convolution kernels, images were calculated and analysed on window settings for bone, lung, and soft tissue. Additionally, multiplanar and 3D reconstructions were calculated. The CT scan showed the dimensions of the aneurysm as well as its exact location , and a surgical approach to occlude the aneurysm was chosen.
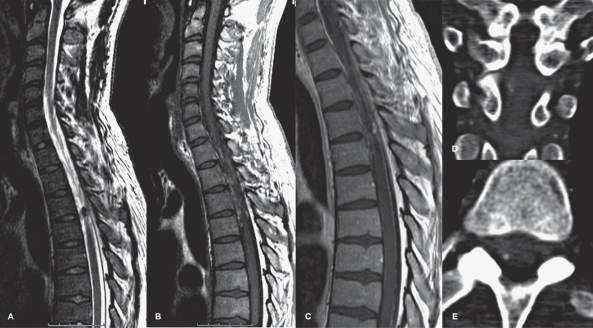
Figure 1.
Sagittal T2W(A), T1W(B) and T1W post Gd (C) MR of the spine demonstrating T1 hyperintensities around the cord from T1 to T4 levels, representing spinal SAH. A circumscribed area of T2 hypointensity at the dorsolateral aspect of the cord at T3 level is noted, with nodular enhancement following contrast administration. CT angiography in coronal reformatted (D) and axial (E) views reveals the exact location of the aneurysm at the right dorsolateral aspect of the cord.
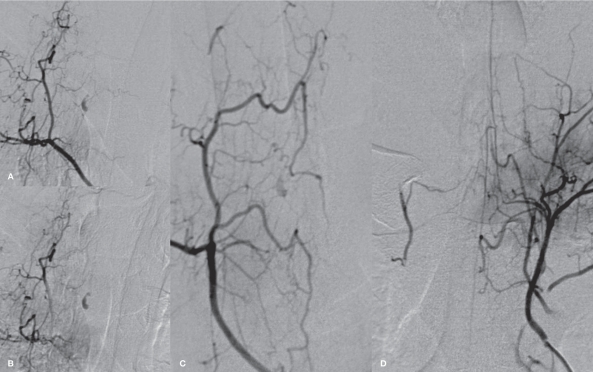
Figure 2.
Right supreme intercostal spinal DSA in arterial (A) and late venous (B) phases revealing a fusiform lesion, which shows slow filling and stagnation of the contrast to the venous phase, originating from a dorsolateral radiculopial artery from the right T4 segmental artery compatible with a false aneurysm. Oblique view (C) better demonstrates the small radiculopial artery supplying the aneurysm. Faint filling of the aneurysm after injection into the anterior spinal artery system from the contralateral side (D) via the vasocorona collaterals is observed.
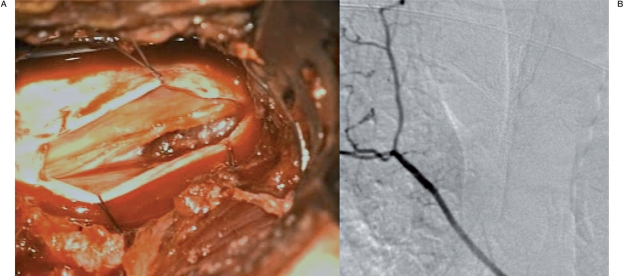
Figure 3.
Intraoperative view after right T3 hemilaminectomy (A) revealing the fusiform aneurysm at the dorsolateral aspect of the cord. Post operative angiogram of the right supreme intercostal artery (B) demonstrating disappearance of the previously seen aneurysm.
Surgical Approach
After hemilaminectomy of Th3 and opening of the dura mater employing a microsurgical technique, the typical findings after subarachnoid hemorrhage were found. The arachnoid layer was dissected and the bean-shaped posterolateral aneurysm was identified. The feeding radiculopial artery was identified using microdoppler below the aneurysm and followed to the dorsolateral aspect of the cord. The partially thrombosed aneurysm was coagulated and removed after the feeding radiculopial artery was closed. Electrophysiological spinal monitoring during the operation was performed and remained unchanged in normal pre and postoperative amplitudes.
Histopathology revealed a blood clot consisting of mainly lytic erythrocytes, scattered leucocytes and fibrin which was in part surrounded by loosely textured bundles of collagen fibers without elastic material in between. In these parts no muscular tunica media could be identified. However, focally frayed remnants of an arterial wall with an internal elastic lamina were seen. Sparse hemosiderin deposits could be detected between the collagen lamellae as well as slight proliferation of fibroblasts mainly at the border of the coagulum. No endothelial lining was noted between the blood clot at the luminal surface and the fibrous connective tissue of varying thickness. Signs of inflammation were absent.
Postoperatively, the patient had no new neurological deficits, especially no new sensory deficits, the thoracolumbar pain and the headaches slowly decreased. Control digital subtraction angiography revealed no residual filling of the aneurysm and normal filling of the anterior spinal artery axis. The patient was discharged from the hospital after four days.
Discussion
Ever since Babonneix and Wediez reported the first case of an isolated anterior spinal artery aneurysm that was presumably caused by syphilis in 1930 11, 32 cases of isolated spinal aneurysms have been reported in the literature, of these only nine cases (including the patient reported here), were located at the posterior spinal (radiculopial) artery (PSA) 2,14-18, two were located at a radicular artery 18,20, in two cases the exact location was not specified 11,21, while in the remaining 20 cases the aneurysm arose from the anterior spinal (radiculomedullary) artery 2,11,18,22-36 (Table 1).
Table 1.
Previously reported cases of isolated spinal aneurysms (posterior spinal artery aneurysms are shaded in white)
| Authors (Year) | Age/ Sex |
Underlying condition |
Presentation | Location/Level | Associated findings/ Pathology |
Treatment |
|---|---|---|---|---|---|---|
| Babonneix & Wediez11 (1930) |
56/NA | Syphilis | NA | NA | ||
| Echols & Hol- come11 (1941) |
30/F | Paraparesis | ASA (T6) | |||
| Henson & Croft16 (1956) |
51/M | SAH | PSA (C1) | None → died | ||
| Kinal & Sejanovich21 (1957) |
41/F | Paraparesis | Unidentified intramedullary a (C7) |
Sx resection | ||
| Hopkins et al.25 (1966) |
27/M | Rt hemiparesis | ASA (C4) | |||
| Leech et al.27 (1976) |
25/F | Paraparesis | ASA (T7) | Sx resection | ||
| Garcia et al.24 (1979) |
34/F | Pregnancy | SAH | ASA (T6) | None → died | |
| Thomson32 (1980) |
66/F | Quadriparesis | ASA (C1-2) | Sx clipping | ||
| Vincent34 (1981) |
30/F | SAH | ASA (C1) | Sx clipping | ||
| Moore et al.28 (1982) |
30/F | SAH | ASA (C1) | Sx clipping | ||
| Kito et al.26 (1983) |
37/F | Pseudo- xanthoma elasticum |
SAH | ASA (T10) | Conservative | |
| Smith et al.30 (1986) |
29/M | SAH | ASA (T12) | Sx clipping of feeding a |
||
| Saunders et al.29 (1987) |
44/F | FMD | SAH | ASA (T1) | Sx resection | |
| Goto et al.14 (1988) |
53/M | SAH | PSA (LSA C2) | Sx resection | ||
| el Mahdi et al.23 (1989) |
17/F | LBP, sciatica | T12 | |||
| Handa et al.15 (1992) |
3/F | Quadriparesis | PSA (LSA C2, LVA) |
Dissection possible (patho) |
Sx resection (post.) |
|
| Bahar et al.22 (1993) |
40/M | Behcet's disease | SAH | ASA (RM - C5-6, RVA br) |
Dissection of RVA |
Conservative, F/U; complete resolution |
| Rengachary et al.11 (1993) |
50/F | SAH | ASA (RM - Rt T12) |
Arteritis (patho) |
Sx resection | |
| Mohsenipour et al.20 (1994) |
59/F | SAH | Radicular A (Rt T8) |
Saccular (patho) |
Sx Clipping | |
| Vishteh et al.35 (1997) |
30/M | SAH | ASA (RM - Lt T12) |
Dissection (fusiform) |
Sx wrapping | |
| Taniura & Watanebe31 (2000) |
54/F | Tetraplegia fol- lowing angiog- raphy |
ASA (RM - C5 LVA) |
Dissection (Idiopathic) |
Sx wrapping | |
| Yahiro et al.36 (2004) |
71/F | Post endoscope | SAH | ASA (RM - Lt T5) |
Dissection (patho) |
Sx resection |
| Berlis et al.2 (2005) |
62/F | - | SAH, paraparesis |
PSA (RP Rt T5) |
Sx Clipping → cured |
|
| 48/M | Systemic candidiosis |
SAH, paraplegia | ASA, T12 | Occlusion of ASA (? Dissection) |
Conservative → stable |
|
| 69/F | SAH, paraplegia |
ASA (RM Lt L1) |
? Dissection | Conservative, F/U → com- plete resolution |
||
| Massand et al.18 (2005) |
30/M | Paraparesis | ASA (RM Lt T11) |
Dissection | Sx wrapping | |
| 69/M | SAH | PSA (RP Lt L1) |
Dissection (patho) |
Sx resection → cured |
||
| 54/M | SAH | PSA (RP Lt T12) |
Dissection (patho) |
Sx resection → cured |
||
| 73/M | SAH | Radicular A (Lt T6) |
Sx reconstruction |
|||
| Kocak et al.17 (2006) |
54/F | SAH | PSA (LSA C2, LVA) |
Rebled → died | ||
| Nemecek et al.19 (2006) |
55/M | Tetraplegia (SDH, IMH) |
PSA (RP Lt T12) |
Dissection (patho) |
Sx resection | |
| Toyota et al.33 (2007) |
65/F | RA | SAH | ASA (RM C2 LVA) |
Dissecting aneurysm LVA |
Emb coils (sacrifice) |
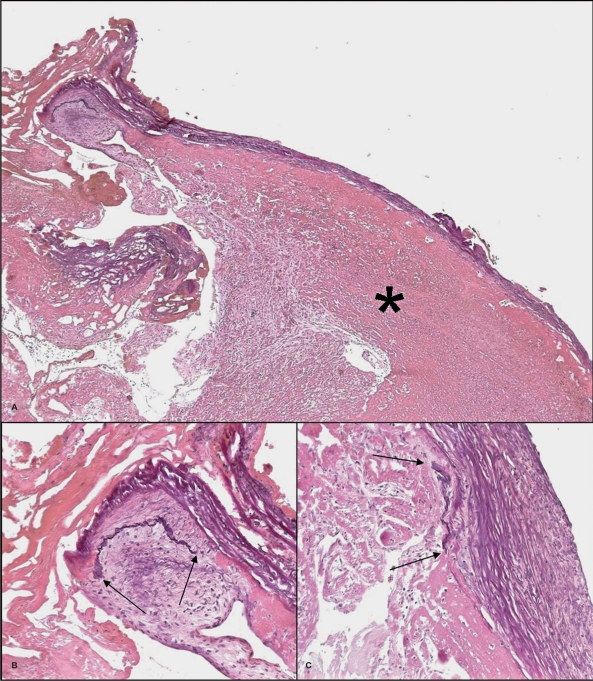
Figure 4.
Microscopic overview (A) of a part of the endoluminal thrombus (asterisk) covered by bundles of collagen fibers (Elastica-van Gieson stain, x50). Focal remnants of an arterial wall are seen (B and C). The elastic lamina (black) stops at the arrows where a pad of intimal thickening is seen (Elastica-van Gieson stain, x200).
In the 20 cases located on the anterior axis, an underlying disease or state possibly leading to infection/inflammation or vascular wall weakness with development of an aneurysm was identified in five cases, including, pseudoxantho- ma elasticum 26, fibromuscular dysplasia (FMD) 29, Behcet's disease 22, systemic candidiosis and rheumatoid arthritis 36, while in the PSA group, none had any identifiable underlying disease.
Of the nine cases located at the posterior spinal axis, there was a slight male predominance (6/3), with an average age of 49 years. With the exception for one three-year-old patient reported by Handa et al. 15 in 1992, all patients were in their fourth to sixth decades. Spinal SAH was the most common presenting symptom. Four cases were located at the upper cervical level (between C1-2), four cases at the thoracic level (T4,T5,2-T12) and one case at L1.
Dissection of the arterial wall was the most common cause of the posterior spinal aneu- rysms, proven by histopathology in five cases 15,18,19. Classically, an interruption of the tunica media was seen, the wall of the aneurysm was composed of fibro-collagenous tissue without endothelial lining, testifying for its nature as a spurious aneurysm with a false sac. In one case where surgery was delayed, the aneurysm could not be identified which may have been secondary to a complete healing of the dissection 2. Whereas in the literature no trauma was reported preceding the subarachnoid hemorrhage, one may speculate whether in the present case the axial compression trauma could have led to the dissection.
The diagnosis of spinal aneurysms can be difficult and delayed due to its rarity. In cases where a subarachnoid hemorrhage is proven by CSF studies, a spinal origin should be suspected when the initial cranial CT is negative for SAH or when the blood is localized mainly in the posterior fossa and the cerebral angiography is negative. In our practice, we perform T1 and FLAIR weighted sequences to verify the spinal SAH, T2-weighted sequences to look for per- imedullary flow voids that may point to the most common origin of spinal SAH (i.e. arteri- ovenous shunts), dynamic contrast enhanced MRAs and post contrast scans. MR findings of spinal aneurysms demonstrate, as seen in our case, a T2 hypointensity (that can be due to a mural hematoma, stagnating blood or a flow void) with a contrast enhancing area that - as in our case - represents the contrast stagnation within the false aneurysm. Contrast-enhanced CT of the spine may be helpful to better visualize the exact location of the perfused part of the aneurysm in relation to the cord. Most posterior spinal artery aneurysms are located close to the bend of the supplying radiculopial artery where it reaches the cord surface. They are often irregular in shape with contrast stagnation within the sac on the late venous phase, suggesting its dissecting nature. This finding is slightly different from dissecting aneurysms of the anterior spinal artery, which tend to be more often fusiform.
The treatment of posterior spinal aneurysms is mainly surgical due to the dorsolateral and superficial location of the aneurysms. Whereas the endovascular route may be too small to be safely reached by present microcatheters, the surgical resection through a posterior approach via a hemilaminectomy usually is able to identify and remove the aneuryms. Preservation of the parent artery is usually not possible due to the dissecting nature and no true neck; therefore surgical resection is typically performed, which is different from aneurysms of the anterior spinal artery or the radiculomedullary arteries that may have devastating neurological complications due to the major cord supply if disrupted and therefore have been proposed to be observed 2. Of the six surgically treated PSA aneu- rysm cases 14,15,18,19, all were cured on follow-up studies with good clinical outcomes. Although one case spontaneously healed 2, there were two cases that had early rebleeding and subsequently died 11,17, therefore prompt surgical treatment is recommended. Endovascular treatment with embolization using either coils or glue is possible however may be technically challenging due to the small size of the radiculopial arteries on which the aneurysms are located.
Conclusion
Isolated posterior spinal aneurysms are rare lesions caused by dissection of the arterial wall. Spinal angiographic findings of a saccular out- pouching at the bend of a radiculopial artery on the cord surface leads to final diagnosis, but the relationship to the spinal cord surface may be better visualized with MRI/CT. Surgical resection still remains the standard treatment of these lesions with a very good outcome, In our opinion, treatment should not be delayed due to the high mortality rate associated with early rebleeding.
References
- 1.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–18. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 2.Berlis A, Scheufler KM, Schmahl C, et al. Solitary spinal artery aneurysms as a rare source of spinal sub- arachnoid hemorrhage: potential etiology and treatment strategy. Am J Neuroradiol. 2005;26:405–10. [PMC free article] [PubMed] [Google Scholar]
- 3.Djindjian R. Angiography of the spinal cord. Surg Neurol. 1974;2:179–85. [PubMed] [Google Scholar]
- 4.Konan AV, Raymond J, Roy D. Transarterial embolization of aneurysms associated with spinal cord arteriovenous malformations. Report of four cases. J Neurosurg. 1999;90:148–54. doi: 10.3171/spi.1999.90.1.0148. [DOI] [PubMed] [Google Scholar]
- 5.Lavoie P, Raymond J, Roy D, et al. Selective treatment of an anterior spinal artery aneurysm with endosaccular coil therapy. Case report. J Neurosurg Spine. 2007;6:460–4. doi: 10.3171/spi.2007.6.5.460. [DOI] [PubMed] [Google Scholar]
- 6.Matsui T, Taniguchi T, Saitoh T, et al. Hematomyelia caused by ruptured intramedullary spinal artery aneurysm associated with extramedullary spinal arteriovenous fistula--case report. Neurol Med Chir (Tokyo) 2007;47:233–6. doi: 10.2176/nmc.47.233. [DOI] [PubMed] [Google Scholar]
- 7.Ohmori Y, Hamada J, Morioka M, et al. Spinal aneurysm arising from the feeding pedicle of a thoracic perimedullary arteriovenous fistula: case report. Surg Neurol. 2005;64:468–70. doi: 10.1016/j.surneu.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Malek AM, Halbach VV, Phatouros CC, et al. Spinal dural arteriovenous fistula with an associated feeding artery aneurysm: case report. Neurosurgery. 1999;44:877–80. doi: 10.1097/00006123-199904000-00114. [DOI] [PubMed] [Google Scholar]
- 9.Hino H, Maruyama H, Inomata H. [A case of spinal artery aneurysm presenting transverse myelopathy associated with coarctation of the aorta] Rinsho Shinkeigaku. 1989;29:1009–12. [PubMed] [Google Scholar]
- 10.Jiarakongmun P, Chewit P, Pongpech S. Ruptured anterior spinal artery aneurysm associated with coarctation of aorta. Intervent Neuroradiol. 2002;8:285–92. doi: 10.1177/159101990200800308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rengachary SS, Duke DA, Tsai FY, et al. Spinal arterial aneurysm: case report. Neurosurgery. 1993;33:125–9. doi: 10.1227/00006123-199307000-00020. discussion 9-30. [DOI] [PubMed] [Google Scholar]
- 12.Kawamura S, Yoshida T, Nonoyama Y, et al. Ruptured anterior spinal artery aneurysm: a case report. Surg Neurol. 1999;51:608–12. doi: 10.1016/s0090-3019(98)00114-1. [DOI] [PubMed] [Google Scholar]
- 13.Walz DM, Woldenberg RF, Setton A. Pseudoaneurysm of the anterior spinal artery in a patient with Moyamoya: an unusual cause of subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2006;27:1576–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Goto Y, Kamijyo Y, Yonekawa Y, et al. Ruptured aneurysm of the posterior spinal artery of the upper cervical spinal cord: case report. Neurosurgery. 1988;22:558–60. doi: 10.1227/00006123-198803000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Handa T, Suzuki Y, Saito K, et al. Isolated intramedullary spinal artery aneurysm presenting with quadriplegia. Case report. J Neurosurg. 1992;77:148–50. doi: 10.3171/jns.1992.77.1.0148. [DOI] [PubMed] [Google Scholar]
- 16.Henson RA, Croft PB. Spontaneous spinal subarachnoid haemorrhage. Q J Med. 1956;25:53–66. [PubMed] [Google Scholar]
- 17.Kocak A, Ates O, Cayli SR, et al. Isolated posterior spinal artery aneurysm. Br J Neurosurg. 2006;20:241–4. doi: 10.1080/02688690600852704. [DOI] [PubMed] [Google Scholar]
- 18.Massand MG, Wallace RC, Gonzalez LF, et al. Subarachnoid hemorrhage due to isolated spinal artery aneurysm in four patients. Am J Neuroradiol. 2005;26:2415–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Nemecek AN, Sviri G, Hevner R, et al. Dissecting aneurysm of the thoracic posterior spinal artery. Case illustration. J Neurosurg Spine. 2006;5:555. doi: 10.3171/spi.2006.5.6.555. [DOI] [PubMed] [Google Scholar]
- 20.Mohsenipour I, Ortler M, Twerdy K, et al. Isolated aneurysm of a spinal radicular artery presenting as spinal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1994;57:767–8. doi: 10.1136/jnnp.57.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinal ME, Sejanovich C. Spinal cord compression by an intramedullary aneurysm; case report and review of the literature. J Neurosurg. 1957;14:561–5. doi: 10.3171/jns.1957.14.5.0561. [DOI] [PubMed] [Google Scholar]
- 22.Bahar S, Coban O, Gurvit IH, et al. Spontaneous dissection of the extracranial vertebral artery with spinal subarachnoid haemorrhage in a patient with Behcet's disease. Neuroradiology. 1993;35:352–4. doi: 10.1007/BF00588368. [DOI] [PubMed] [Google Scholar]
- 23.el Mahdi MA, Rudwan MA, Khaffaji SM, et al. A giant spinal aneurysm with cord and root compression. J Neurol Neurosurg Psychiatry. 1989;52:532–5. doi: 10.1136/jnnp.52.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia CA, Dulcey S, Dulcey J. Ruptured aneurysm of the spinal artery of Adamkiewicz during pregnancy. Neurology. 1979;29:394–8. doi: 10.1212/wnl.29.3.394. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins CA, Wilkie FL, Voris DC. Extramedullary aneurysm of the spinal cord. Case report. J Neurosurg. 1966;24:1021–3. doi: 10.3171/jns.1966.24.6.1021. [DOI] [PubMed] [Google Scholar]
- 26.Kito K, Kobayashi N, Mori N, et al. Ruptured aneurysm of the anterior spinal artery associated with pseudoxanthoma elasticum. Case report. J Neurosurg. 1983;58:126–8. doi: 10.3171/jns.1983.58.1.0126. [DOI] [PubMed] [Google Scholar]
- 27.Leech PJ, Stokes BA, ApSimon T, et al. Unruptured aneurysm of the anterior spinal artery presenting as para- paresis. Case report. J Neurosurg. 1976;45:331–3. doi: 10.3171/jns.1976.45.3.0331. [DOI] [PubMed] [Google Scholar]
- 28.Moore DW, Hunt WE, Zimmerman JE. Ruptured anterior spinal artery aneurysm: repair via a posterior approach. Neurosurgery. 1982;10:626–30. doi: 10.1227/00006123-198205000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Saunders FW, Birchard D, Willmer J. Spinal artery aneurysm. Surg Neurol. 1987;27:269–72. doi: 10.1016/0090-3019(87)90041-3. [DOI] [PubMed] [Google Scholar]
- 30.Smith BS, Penka CF, Erickson LS, et al. Subarachnoid hemorrhage due to anterior spinal artery aneurysm. Neurosurgery. 1986;18:217–9. doi: 10.1227/00006123-198602000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Taniura S, Watanebe T. A ruptured dissecting aneurysm of the anterior radiculomedullary artery caused by vertebral angiography. Neuroradiology. 2000;42:539–42. doi: 10.1007/s002340000322. [DOI] [PubMed] [Google Scholar]
- 32.Thomson RL. Aneurysm in the cervical spinal canal. Med J Aust. 1980;1:220–2. [PubMed] [Google Scholar]
- 33.Toyota S, Wakayama A, Fujimoto Y, et al. Dissecting aneurysm of the radiculomedullary artery originating from extracranial vertebral artery dissection in a patient with rheumatoid cervical spine disease: an unusual cause of subarachnoid hemorrhage. Case report. J Neurosurg Spine. 2007;7:660–3. doi: 10.3171/SPI-07/12/660. [DOI] [PubMed] [Google Scholar]
- 34.Vincent FM. Anterior spinal artery aneurysm presenting as a subarachnoid hemorrhage. Stroke. 1981;12:230–2. doi: 10.1161/01.str.12.2.230. [DOI] [PubMed] [Google Scholar]
- 35.Vishteh AG, Brown AP, Spetzler RF. Aneurysm of the intradural artery of Adamkiewicz treated with muslin wrapping: technical case report. Neurosurgery. 1997;40:207–9. doi: 10.1097/00006123-199701000-00047. [DOI] [PubMed] [Google Scholar]
- 36.Yahiro T, Hirakawa K, Iwaasa M, et al. Pseudoaneurysm of the thoracic radiculomedullary artery with sub- arachnoid hemorrhage. Case report. J Neurosurg. 2004;100:312–5. doi: 10.3171/spi.2004.100.3.0312. [DOI] [PubMed] [Google Scholar]