Summary
Endovascular treatments of cerebral aneurysms with bare platinum coils have a higher rate of recurrence compared to surgical clipping. This may be related to failed vessel wall reconstruction since histological and scanning electron microscopy results following embolization failed to demonstrate neoendothelialization over the aneurysm neck. The present study tried to elucidate whether the use of modified coils resulted in a better rate of reconstructing the vessel wall over the aneurysm neck in experimental aneurysms.
Aneurysms were created in 20 rabbits by intraluminal elastase incubation of the common carotid artery. Five animals each were assigned to the following groups: untreated, bare platinum coils, bioactive coils with polyglycolic/polylactic acid coating, and hydrogel-coated platinum coils. After 12 months, angiography, histology and scanning electron microscopy was performed.
No neoendothelial layer was visualized in the bioactive and bare coil groups with a tendency to an increased layering of fibroblasts along the bioactive coils at the aneurysm fundus. However, at the aneurysm neck perfused clefts were present and although a thin fibrinous layer was present over some coils, no bridging neointimal or neoendothial layer was noted over different coils. Following loose Hydrogel coiling, a complete obliteration of the aneurysm was present with neoendothelialization present over different coil loops.
The study demonstrates that with surface coil modifications complete and stable aneurysm obliteration may become possible. A smooth and dense surface over the aneurysm neck may be necessary for endothelial cells to bridge the aneurysm neck and to lead to vessel wall reconstruction.
Key words: aneurysm, coiling, surface modification, Hydrogel-coated coils, bioactive coils
Introduction
Two principal methods can be used to treat cerebral arterial aneurysms: surgical clipping and endovascular therapies. In recent years, especially since the results of the International Subarachnoid Aneurysm Trial (ISAT) 1 were published, the endovascular treatment of cerebral aneurysms with coils has gained an increasing importance, not only in those aneurysms that are difficult to access surgically (i.e. those in the posterior circulation), but also in uncomplicated aneurysms of the anterior circulation. Along with the increasing number of aneurysms treated endovascularly came an increasingly refined armamentarium of endovascular techniques and devices to be used in the therapy of both ruptured and unruptured aneurysms. Complex shaped coils, balloon remodelling techniques and stents were established to treat those aneurysms that had a broad base and thereby rendered conventional coiling techniques rather hazardous because of the risk of coil herniation and subsequent thrombosis of the parent aneurysm 2. Despite these new techniques, the major argument that still spoke in favor of surgical ligation of the aneurysm instead of endovascular occlusion was that the surgical clip approximates two healthy vessel walls close to each other thereby excluding the weakened vessel wall, i.e. the aneurysm, from the circulation and reconstructing the normal vessel wall 3,4. Endovascular techniques, on the other hand, did not demonstrate a complete neoentholialization of the coil loops over the former aneurysm ostium. Although the aneurysm dome was completely occluded with fibrinous and collageneous extracellular matrix and granulation tissue, the aneurysm neck and clefts within the coiled aneurysm were still perfused, even in the angiographically completely occluded aneurysms 5. Of note, however, was, that some of the coil loops were in fact covered by a neoendothelium, however other coil loops lay bare within the vessel with fresh thrombus material present on their surface. Since these results were obtained at six months after treatment, the question as to whether this neoendothelialization was an ongoing process just taking more time compared to clipping remained unanswered 5.
Endovascular treatment with platinum coils is aimed at permanently occluding the aneurym lumen and is based on the following assumptions. Immediately after the coils are deployed in the aneurysm thrombocytes adhere to the coil loops due to changes in hemodynamics and flow velocity followed by fibrous organisation of the primary thrombus over the course of the following days 6. Mechanical stability is further increased over the course of the next weeks by collageneous extracellular matrix and granulation tissue in between the coil loops that might then build the ground for neoendothelial sprouting starting from the healthy vessel walls 7. Although these theoretical considerations seem appealing, experimental results were not always accordant but instead demonstrated that bare coils did not induce neoendothelial sprouting 8. This in turn fuelled strong interest in changing the propensities of the coil either by making them "bioactive" (i.e. inducing a biological response such as increased scarring from the host) or by providing a ground for better neoendothelialization by coating them with a Hydrogel layering. The so-called "Matrix-coil" (Boston Scientific, Fremont, Ca, USA) was the first bioactive coil system introduced to clinical practice 9,10. It is a platinum coil covered with a bioabsorbable polymer consisting of a polyglycolic/polylactic acid (PGLA) coating. PGLA produces a mild inflammatory reaction thought to enhance clot organization and maturation and to accelerate neointimal proliferation 11-14. The Hydrogel-coated platinum coil (HydroCoil; Microvention) consists of a Hydrogel polymer surface coating which has the property of volumetric swelling and results in a denser packed aneurysm 15. In addition, it seems to lower the rate of coil compaction and aneurysm recanalization 16-19. In an experimental bifurcation aneurysm model a significantly thicker neointimal proliferation in HydroCoil treated animals could be shown 20. Although some studies have evaluated the responses to those different coils in short term animal experiments 21, no such study investigated the long-term effects (i.e. longer than three months) using both angiographic, histologic and scanning electron microscopy data in the same animal model.
The aim of the present study was to determine whether a complete obliteration of an aneurysm is achieved by bare, bioactive or Hydrogel-coated coils in the 12 month follow-up. This was achieved by histologically studying the vessel wall reaction close to the former aneurysm ostium following the presumption that an intact endothelial layer over the former aneurysm neck constitutes a complete healing response 5.
Methods
The animal protocol for our study was approved by the Institutional Animal Care and Use Committee of our University Hospital and was conducted according to current national regulations and guidelines for animal welfare (AZ: 50.203.2-AC 24, 24/01) and to the international principles of laboratory animal care. All procedures were performed under general anaesthesia that was induced by intramuscular injection of 0.2 ml /kg KG ketamin (10%) and 0.3 ml/kg KG medetomidin. Maintenance of anesthesia was achieved by 2.5% isoflurane inhalation. During all interventions, we gave heparin (100 units/kg) intravenously.
Aneurysm Creation, Treatment and Euthanization
Aneurysms were created in 20 New Zealand White rabbits (3.4-4.5 kg) as described previously 22,23: After surgical exposure of the right carotid common artery (CCA) the vessel was ligated distally and controlled proximally with 3-0 silk sutures. After arteriotomy, a 4 F vascular sheath (Radiofocus Introducer, Terumo, Japan) was introduced retrogradely to the midportion of the CCA. Under fluoroscopic guidance, a 2F Fogarty balloon (Pan Medical Ltd., Gloucestershire, UK) was advanced retrograde to the origin of the CCA and, once in position, inflated with iodinated contrast material. Occlusion of the vessel was verified by retrograde injection of contrast via the sheath. A microcatheter supported by a microguide was placed via the sheath directly above (distal to) the Fogarty balloon by which 20 units of porcine elastase (Sigma-Aldrich, St. Quentin-Fallavier, France) were administered and incubated within the thus separated right CCA lumen. After 20 minutes, the balloon was deflated, the sheath was removed and the vessel was ligated in the mid-portion. The skin was closed with a running suture. Aneurysms formed from the stump of the right CCA and were treated at least 14 days following aneurysm induction. Prior to treatment, animals were randomly assigned to each of four groups consisting of five animals each: untreated to verify stability of the aneurysm, treatment with bare Guglielmi detachable coils (GDC 10, Boston Scientific, Fremont CA, USA Standard, Soft and Ultrasoft Coils, 360° for framing followed by 2D for filling), treatment via bioactive coils (Matrix ® 10, Boston Scientific, Fremont, CA, USA) or treatment with Hydrogel-coated coils (HydroCoil ®, MicroVention, Inc., Aliso Viejo, CA, USA).
Endovascular treatment was carried out as described previously via a right transfemoral approach after microcatheter placement in the aneurysm. Coiling was performed with standard techniques using "road-mapping". Tight packing of the aneurysm was achieved by subsequently downsizing the coils and repositioning the microcatheter if needed. In no animals, coils of different types were mixed to ensure homogeneous groups. After the procedure, we performed a DSA of the aneurysm-bearing brachiocephalic trunk through the microcatheter to ensure complete occlusion of the aneurysm. Thereafter the catheter and sheath were removed, the femoral artery was ligated with a 3.0 silk suture.
After the observation period of 12 months, the final angiography was performed via a left transfemoral approach in all animals employing DSA via a 4F catheter that was advanced in the aortic arch where a 3D rotational angiography was performed (Siemens Axiom Artis, Erlangen, Germany) to evaluate possible recanalization of the aneurysm. Thereafter, the animals were euthanasized using intravenously injected barbiturates. The brachiocephalic trunk, the right subclavian artery up to the vertebral artery and the aneurysm were resected en bloc and placed directly into 4% paraformaldehyde phosphate buffered saline (PBS) solution for a rapid fixation.
Histology and Scanning Electron Microscopy
After fixation and macroscopic preparation of the vessels, the whole vessel segment (brachiocephalic trunk from the origin of the left common carotid artery to the origin of the right vertebral artery) was embedded in Technovit 7100 (Heraeus-Kulzer) thereby enabling slides with a thickness of 2 µm to be obtained by a rotation microtome. This technique allowed us to visualize both the soft tissue (with a special emphasis on a potential neo-endothelial layer) and the implanted metals on light microscopy. These slides were subsequently stained with hematoxylin-eosin (H&E) and Elastica van Gieson (EvG) stains for appraising the configuration of the arterial vessel wall. This technique was performed in three aneurysms of each of the three groups. The remaining two aneurysms were further investigated with scanning electron microscopy to obtain an "in-vessel-view" of the treated aneurysms: the brachiocephalic trunk was longitudinally incised directly opposite the treated aneurysm and carefully spread apart. After this procedure, the specimens were fixed in 3.9 % glutaraldehyde. They were dehydrated in a graded acetone series (30, 50, 70, 90, 3x100%) and critical-point-dried in carbon dioxide. The samples were fixed on scanning electron microscopy (SEM) stubs and sputter-coated with gold (SCD 030, Balzers Union, FL), then investigated in an ESEM XL 30 FEG (Environmental Scanning Electron Microscope XL 30 Field Emission Gun FEI Philips, Eindhoven, NL) in high vacuum mode with accelerating voltages of 2-10 kV.
Results
All rabbits tolerated the surgical and endovascular treatment procedures well. The aneurysms resembled those on human cerebral arteries in size, configuration and neck morphology. There were no complications such as haemorrhage, thrombosis, coil dislocation or rupture of an aneurysm, no animal was lost during the follow-up period of 12 months. Aneurysm sizes ranged in width from 2.3 mm to 5.6 mm (mean 3.6 mm, standard deviation 1.3 mm) and in height from 2.5 mm to 6.9 mm (mean 5.3 mm, standard deviation 2.8 mm). Between the three groups there were no significant differences concerning the size of the aneurysm.
Untreated Group
In the untreated group, there was no spontaneous thrombosis of aneurysms, all five aneurysms remained patent for the investigated time of 12 months.
Neither was there a tendency to grow over time with the size of the aneurysm, both in height, width, and aneurysm neck size remaining stable. On gross pathological inspection, the distal portion of the CCA, from the ligated portion of the vessel downward was completely obliterated whereas the portion of the former vessel close to the aneurysm dome showed thrombosis. Histologically, a loss of the internal elastic lamina and a thrombosed dome was demonstrated in all animals.
Bare Coils
In the bare coil group successful delivery of the coils and dense packing of the aneurysm was possible in four out of five cases. Postembolization DSA revealed complete occlusion with no contrast medium visible within the aneurysm within these four aneurysms, there was no coil dislocation into the parent artery present.
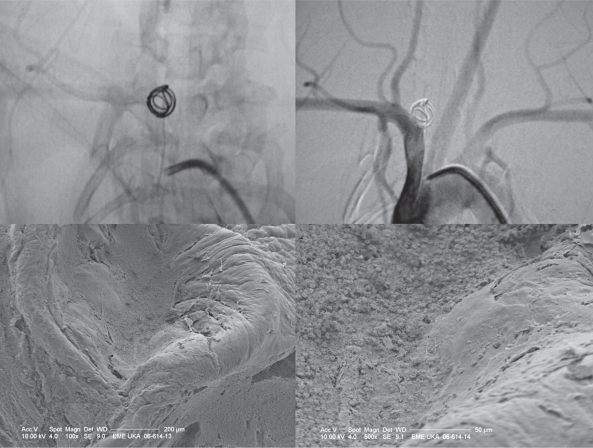
In one animal, a small residual aneurysm neck was present (a so-called "dog-ear"), that could not be filled since all coils aimed at filling this part of the aneurysm repeatedly protruded into the parent artery and were therefore not detached. After 12 months, we found that only two of the four aneurysms that were initially completely occluded according to angiographic criteria remained stable, in the other animals, coil compaction was visible with contrast filling of the aneurysm neck. Further compaction of coils was also noted in the aneurysm that was initially not completely occluded. Here, contrast material was visible not only within the aneurysm neck but also in the midportion of the respective aneurysms. On histological examination, a neoendothelial layer over those coils that were close to the aneurysm ostium was not present, although in some intraaneurysmal regions especially close to the aneurysm dome, fibrosis between the coil loops (i.e. organised thrombus) could be visualized (Figure 1, upper row). Fresh thrombus as a sign for still perfused aneurysm parts was seen not only at the aneurysm neck but also around some of the coil loops, even in those aneurysms that were angiographically completely obliterated. On scanning electron microscopy, these findings were further underlined: only some of the coil loops were completely endothelialized, some coil loops demonstrated a thin fibrinous layer, however, most of the coil loops close to the former aneurysm neck were uncoated and lay in direct contact to the flowing blood with some fresh thrombus material close to coils present in all cases (Figure 2). A complete obliteration (i.e. neoendothelialization over the aneurysm ostium) was not present in any aneurysm treated with bare coils.
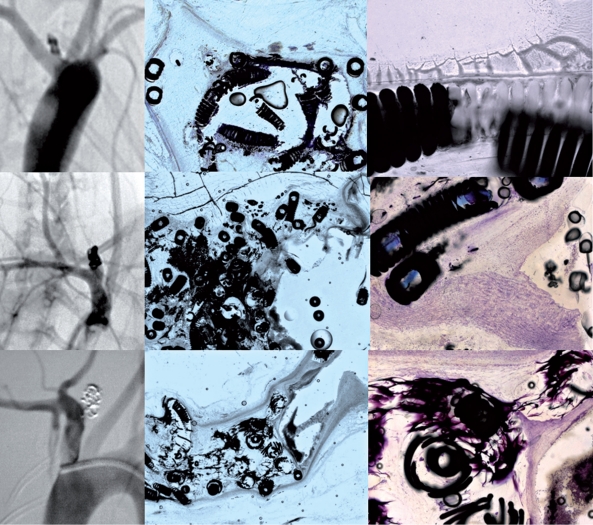
Figure 1.
Angiographic and histologic results one year after coiling of experimental aneurysms with bare coils (upper row), Matrix ® coils (middle row) and HydroCoils ® (lower row). Angiography demonstrates complete occlusion in all three animals despite a rather loose packing in the animal treated with Hydrogel-coated coils. Histology (middle row) demonstrates in the bare coil group that the aneurysm fundus was completely occluded with fibrinous and collageneous extracellular matrix and granulation tissue, however, surrounding the coil loops at the aneurysm orificium (right row) there is only a thin layer of fibrinous tissue and no neoendothelium present. The Matrix Coils did not show a complete neoendothelial layer bridging the aneurysm neck, however, as demonstrated in the right image of the middle row, a beginning layering of neointima and neoendothelium was present over the aneurysm neck. It was only in the Hydrogel-coated coils that a complete neoendothelialization was present with a neointima spanning the coil loops at the aneurysm neck leading to a complete exclusion of the aneurysm form the circulation.
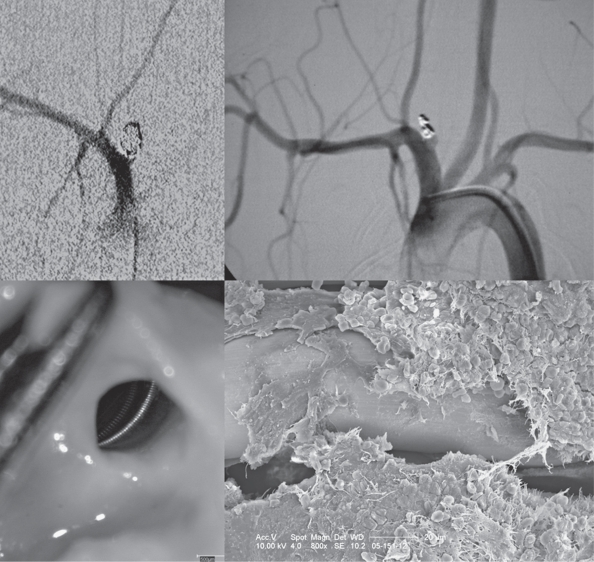
Figure 2.
Bare coils: angiography and scanning electron microscopy: The upper row shows an aneurysm following treatment with bare coils and following the observational period of six months. Depsite angiographic occlusion, the in-vessel view that was used for subsequent electron microscopy demonstrates an open aneurysm orificium with bare coil loops being in contact with the flowing blood. Fresh erythrocytes are present over the coil loops and there is only a thin layering of fibrinous tissue surrounding the loops with no significant neoendothelium.
Bioactive Coils
Delivery and detachment of the bioactive coils was possible and resulted in a complete occlusion of the aneurysm in all five aneurysms. After 12 months, two animals still had a complete occlusion following angiographic criteria, even though the packing was not very dense. In two additional animals a clear compaction of the coils was noted with evidence for a residual aneurysm at its neck.
In the fifth animal contrast material was present at the aneurysm neck and between loose coils at the aneurysm fundus suggesting and a major recanalization. On histological examination, no complete neoendothelial layer over the aneurysm neck was present, although in a single animal a beginning layering of neoendoethelium was present in coil loops close to the neck (Figure 1, middle row). This neoendothelium however did not span across different coil loops. Within the aneurysm fundus and close to the coil loops fibrosis and organised thrombus was seen including multilayer of fibroblasts attached to the coils. A similar appearance was never seen in the bare coil group. However, as in the bare coil group, erythrocytes as a sign for still perfused aneurysm parts were seen surrounding the coil loops at the aneurysm neck and within clefts visible inbetween the coils, even in those aneurysms that were angiographically completely obliterated. Scanning electron microscopy demonstrated endothelialization of single coil loops, however, no spanning of tissue from one coil loop to the other. Most coil loops demonstrated covering by a thin fibrinous layer, but again clefts and uncoated coil loops were also present at the former neck (Figure 3).
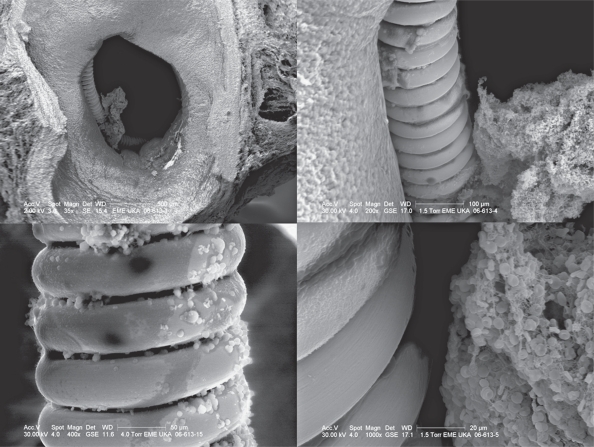
Figure 3.
Scanning electron microscopy of an aneurysm treated with Matrix ® coils. The aneurysm ostium is still open and coil loops are seen that are not covered by endothelium or neointima. Fresh clot is present within the clefts of the aneurysm.
Hydrogel-Coated Coils
In the Hydrogel coilgroup, successful delivery of the coils and packing of the aneurysm was possible in four out of five cases. In one rabbit, following deployment of the first coil the microcatheter dislodged from the aneurysm and could not be reinserted into the aneurysm despite numerous attempts resulting in an extremely loose packing of the aneurysm. Postembolization DSA revealed complete occlusion of all five aneurysm despite a rather loose packing. After 12 months, we found stable occlusion in four of the five aneurysms with one single aneurysm demonstrating a small neck remnant ("dog-ear") (Figure 4). Even in the aneurysm that was loosely packed with only a single coil a complete obliteration of the aneurysm was noted (Figure 5).
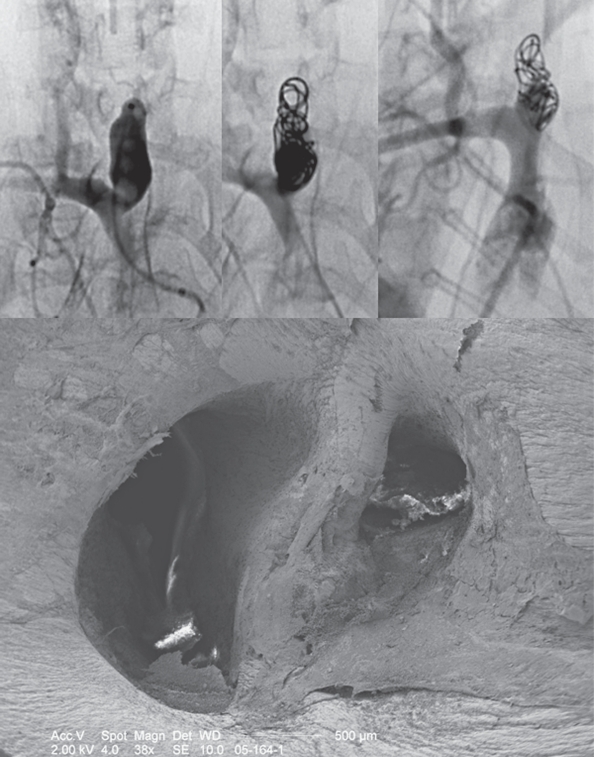
Figure 4.
Angiography and scanning electron microscopy of the HydroCoilt-treated aneurysm with a small "dog-ear" remnant. The upper row shows the aneurysm prior to treatment and following treatment and the follow-up result after 12 months. Coiling was rather loose, however, following the intervention, trapped contrast material was present surrounding the coil loops and further treatment was therefore not performed. On follow-up a stable coil package is seen, however, there is some contrast filling visible in the aneurysm neck. Scanning electron microscopy of this animal demonstrated that the coil loops were completely endothelialized and that the dog-ear itself was also covered with a neointima.
Figure 5.
Angiography and scanning electron microscopy of a loosely packed aneurysm treated with a single HydroCoil ®. Twelve months after the intervention a complete exclusion of the aneurysm is seen despite very loose packing. Scanning electron microscopy was focused on the former aneurysm neck and demonstrates a complete covering of the neck with neointima and neoendothelium with only a slight protrusion into the parent vessel.
Histology demonstrated neoendothelial covering spanning over different coil loops in all animals (Figure 1, lower row). A complete covering of the aneurysm neck was present in four animals and there was a dense fibrosis present within the aneurysm fundus. Following the Hydrogel expansion, the loops were very tight adhering to each other thereby creating a smooth surface at the aneurysm neck. On scanning electron microscopy, a complete neoendothelial covering was found that completely excluded the aneurysm from the flow and lead to a true vessel wall healing. The neoendothelialization was even present in the single animal with a dog ear remnant (Figure 4).
Discussion
This study employed an animal model to investigate the long-term results of different coils in relation to the obliteration rate of the aneurysm on the one hand, and to the vessel wall response, especially the neoendothelialization of the former aneurysm neck on the other. In addition to bare platinum coils we utilized two second generation coil systems with different surface properties: The Matrix coil consisting of a PGLA bioreactive surface coating and the HydroCoil having a polymer on its surface that enlarges under physiologic conditions and creates a smooth surface that is supposed to have better properties of neoendothelial invasion.
Bare coils are thought to induce thrombocyte adhesion to the coil loops due to changes in hemodynamics and flow velocity followed by fibrous organisation of the primary thrombus 6,24,and increasing mechanical stability by collageneous extracellular matrix and granulation tissue inbetween the coil loops. However, the newly developed thrombus which mainly consists of erythrocytes captured within thin fibrin strands may not be able to withstand the arterial blood pressure and the presence of fibrinolytic agents in the circulating blood. Moreover, physiological thrombus retraction, the physical effects of the so-called water-hammer effect on the coil material and the possible presence of old thrombus within the aneurysm dome into which coil loops may migrate can lead to a delayed aneurysm regrowth 8,25,26,27. Recently, histological and scanning electron microscopy re-sults following embolization with bare coils were compared against those obtained after surgical clipping of experimental aneurysms after six months of follow-up. In this study, following coiling, the aneurysm dome was occluded with fibrinous and collageneous material while the aneurysm neck remained perfused. Coil loops lay bare within the vessel with fresh thrombus material on their surface, a neoendothelialization was not present while, after clipping, a thin layer of endothelial lining bridging the two attached vessel walls was present thereby completely obliterating the aneurysm and reconstructing the vessel wall. In the present experiment we were similarly not able to demonstrate a complete neoendothelialization of the bare coil loops after the longer observational period of 12 months. While the aneurysm fundus was completely occluded with fibrinous and collageneous extracellular matrix and granulation tissue, the aneurysm neck and clefts within the coiled aneurysm were still perfused, even in the angiographically completely occluded aneurysms. A vessel wall reconstruction by a bridging neoendothelium over the coil loops at the aneurysm was not visible after 12 months.
In the group of animals treated with bioactive coils, similar results were obtained: after 12 months the aneurysms treated by bioactive coils demonstrated in nearly all cases a perfused neck and bare loops that were not completely covered by endothelium. Although some coil loops demonstrated a multilayer of fibroblasts within the aneurysm fundus, the amount of scarring tissue or fibroblasts at the aneurysm neck was negligible. There were no signs for a healing response present at the aneurysm neck. Coils with fresh thrombus layers protruded into the parent vessel lumen while clefts surrounding the coils that were still perfused suggesting for an incomplete obliteration of the aneurysms. Neoendothelium was present on single coil loops but did not span over different coils and therefore did not lead to a complete exclusion of the aneurysm from the circulation. In the literature, experimental results concerning occlusion rates and fibrous reaction of bioactive coils remain controversial. Murayama et al. report an increased short term inflammatory reaction combined with thickening of the aneurysm wall and enhanced tissue filling compared to bare platinum coils which is levelled out after a three and six month period 10,28. On the other hand these bioactive coils showed a significantly higher rate of coil compaction even in aneurysms with a high inflammatory response compared to bare platinum and Hydrogel coils 21. These results were confirmed in a canine bifurcation aneurysm model that demonstrated significant coil compaction and aneurysmal recanalization despite an increased inflammatory and cellular reaction around the coil loops and at the aneurysm neck 29.
However, these data and the present experiment were performed in animal models with its concomitant drawbacks and may not necessarily hold true for the use of these coils in humans. Different authors have found that the utilization of Matrix coils leads to decreased or equal recanalization rates compared to bare GDCs 30,31. In addition, autoptically and surgically removed aneurysms treated with Matrix coils showed no short-term cellular reaction within eight days but in one case the aneurysm was removed after six months and it was filled with connective tissue. In contrast, aneurysms treated by bare coils generally lacked this biological response even after longer follow-up periods 14. A potential explanation for these differences may be that animal results were not obtained in the subarachnoid space but rather in the soft tissues of the neck, thereby providing a potentially different environment for the invasion of fibroblasts.
In the group of animals treated with Hydrogel-coated coils, we were able to demonstrate a high rate of complete occlusions, even though packing density was rather loose. In all animals, neoendothelial sprouting was visible covering the Hydrogel coating and spanning over multiple coil loops. These findings are comparable to those previously reported 20 and may be due to either a denser aneurysm package due to swelling of the Hydrogel layer or to an accelerated cellular response. The major mechanism put forward in previous studies is that of an increased packing density that can be achieved with Hydrogel-coated coils. Once inside the aneurysm sac, the Hydrogel expands over approximately 20 minutes to fill additional space. In different experimental aneurysm models, the Hydrogelcoated coil treatment resulted in statistically significant increases in volumetric filling and the amount area occupied by embolic material in the aneurysm sac compared to platinum coils 32. It was put forward that it was primarily this increased filling of the aneurysm sac that led to stable angiographic and histologic occlusion of the aneurysm sac 15,20. In the present study, the filling of the aneurysm was rather loose, however, the neoendothelial formation across different coil loops that may have been due to their tighter adhesion seemed to result in the stable occlusion of the aneurysm. In addition, this neoendothelialization was even present, when a dog ear remnant was noted on angiography. In animal studies it has been shown, that the porous structure of the Hydrogel material may assist the migration of endothelial cells over the coil surface, which may contribute to a lower risk of recanalization and a more active tissue response. Using HydroCoils in humans resulted in significantly increased initial occlusion rates while the risk of periprocedural complications such as thromboembolic events or aneurysm rupture was reported to be as low or lower compared to bare GDCs 17,33. On midterm follow-up, occlusion rates seemed to be similar to those found in bare GDC treated patients or higher 16 which is in keeping with our histological and scanning electron microscopy findings. However, a major unwanted side-effect of the present Hydrogel-coated coils seems to be the development of delayed aseptic meningitis with consecutive hydrocephalus. These observations were independently reported by different groups in patients with unruptured aneurysms who underwent endovascular embolization using Hydrogel-coated coils alone or in combination with other coil systems 16,34,35. Whether the higher rate of occlusions outweighs the potential risk of developing hydrocephalus is an ongoing matter of debate to which this study cannot contribute.
Limitations of our study include the small number of aneurysms per group. In addition no quantitative measurements of neoendothelium or fibrous responses can be inferred from twodimensional structures, in slices cut from the center of the aneurysm.
In addition, these measurements suffer from a postmortem loss of vascular tone. Still our results indicate that a complete healing response with neoendothelial lining is possible when using surface modified coils. It is interesting to note, that similar results of a neoendothelium bridge have been found following clipping of aneurysms. Here, the clip blades tightened the opposing healthy vessel walls to each other and a thin layer of endothelial lining was able to bridge the two attached vessel walls. Within the present study, we found the same results for the smooth surface of the Hydrogel-coated coils, whereas in none of the other coils was bridging of the endothelial lining seen in between coil loops. This may indicate, that to allow for a complete reconstruction of the vessel wall, a smooth surface and a minimal distance between structures to be bridged has to be present.
References
- 1.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 2.Krings T, Hans FJ, Moller-Hartmann W, et al. Treatment of experimentally induced aneurysms with stents. Neurosurgery. 2005;56:1347–1359. doi: 10.1227/01.neu.0000159887.03290.d1. discussion 1360. [DOI] [PubMed] [Google Scholar]
- 3.Ebina K, Iwabuchi T, Suzuki S. Histological change in permanently clipped or ligated cerebral arterial wall. Part I. An experimental study in dogs. Acta Neurochir (Wien) 1982;65:253–276. doi: 10.1007/BF01405851. [DOI] [PubMed] [Google Scholar]
- 4.Ebina K, Iwabuchi T, Suzuki S. Histological change in permanently clipped or ligated cerebral arterial wall. Part II: Autopsy cases of aneurysmal neck clipping. Acta Neurochir (Wien) 1982;66:23–42. doi: 10.1007/BF01809301. [DOI] [PubMed] [Google Scholar]
- 5.Krings T, Busch C, Sellhaus B, et al. Long-term histological and scanning electron microscopy results of endovascular and operative treatments of experimentally induced aneurysms in the rabbit. Neurosurgery. 2006;59:911–923. doi: 10.1227/01.NEU.0000232841.08876.DA. discussion 923-914. [DOI] [PubMed] [Google Scholar]
- 6.Byrne JV, Hope JK, Hubbard N, et al. The nature of thrombosis induced by platinum and tungsten coils in saccular aneurysms. Am J Neuroradiol. 1997;18:29–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999;91(2):84–2. doi: 10.3171/jns.1999.91.2.0284. [DOI] [PubMed] [Google Scholar]
- 8.Reul J, Weis J, Spetzger U, et al. Long-term angiographic and histopathologic findings in experimental aneurysms of the carotid bifurcation embolized with platinum and tungsten coils. Am J Neuroradiol. 1997;18:35–42. [PMC free article] [PubMed] [Google Scholar]
- 9.Fujiwara NH, Kallmes DF. Healing response in elastase-induced rabbit aneurysms after embolization with a new platinum coil system. Am J Neuroradiol. 2002;23:1137–1144. [PMC free article] [PubMed] [Google Scholar]
- 10.Murayama Y, Tateshima S, Gonzalez NR, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: long-term experimental study. Stroke. 2003;34:2031–2037. doi: 10.1161/01.STR.0000083394.33633.C2. [DOI] [PubMed] [Google Scholar]
- 11.Fiorella D, Albuquerque FC, McDougall CG. Durability of aneurysm embolization with matrix detachable coils. Neurosurgery. 2006;58:51–59. doi: 10.1227/01.neu.0000194190.45595.9e. discussion 51-59. [DOI] [PubMed] [Google Scholar]
- 12.Mitra D, Herwadkar A, Soh C, et al. A Follow-up of intracranial aneurysms treated with matrix detachable coils: a single-center experience. Am J Neuroradiol. 2007;28:362–367. [PMC free article] [PubMed] [Google Scholar]
- 13.Niimi Y, Song J, Madrid M, et al. Endosaccular treatment of intracranial aneurysms using matrix coils: early experience and midterm follow-up. Stroke. 2006;37:1028–1032. doi: 10.1161/01.STR.0000206459.73897.a3. [DOI] [PubMed] [Google Scholar]
- 14.Szikora I, Seifert P, Hanzely Z, et al. J, Nyary I. Histopathologic evaluation of aneurysms treated with Guglielmi detachable coils or matrix detachable microcoils. Am J Neuroradiol. 2006;27:283–288. [PMC free article] [PubMed] [Google Scholar]
- 15.Kallmes DF, Fujiwara NH. New expandable hydrogelplatinum coil hybrid device for aneurysm embolization. Am J Neuroradiol. 2002;23:1580–1588. [PMC free article] [PubMed] [Google Scholar]
- 16.Berenstein A, Song JK, Niimi Y, et al. Treatment of cerebral aneurysms with hydrogel-coated platinum coils (HydroCoil): early single-center experience. Am J Neuroradiol. 2006;27:1834–1840. [PMC free article] [PubMed] [Google Scholar]
- 17.Cloft HJ. HydroCoil for Endovascular Aneurysm Occlusion (HEAL) study: 3-6 month angiographic follow-up results. Am J Neuroradiol. 2007;28:152–154. [PMC free article] [PubMed] [Google Scholar]
- 18.Deshaies EM, Adamo MA, Boulos AS. A prospective single-center analysis of the safety and efficacy of the hydrocoil embolization system for the treatment of intracranial aneurysms. J Neurosurg. 2007;106:226–233. doi: 10.3171/jns.2007.106.2.226. [DOI] [PubMed] [Google Scholar]
- 19.White PM, Lewis SC, Nahser H, et al. HydroCoil Endovascular Aneurysm Occlusion and Packing Study (HELPS trial): procedural safety and operator-assessed efficacy results. Am J Neuroradiol. 2008;29:217–223. doi: 10.3174/ajnr.A0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshino Y, Niimi Y, Song JK, et al. Endovascular treatment of intracranial aneurysms: comparative evaluation in a terminal bifurcation aneurysm model in dogs. J Neurosurg. 2004;101:996–1003. doi: 10.3171/jns.2004.101.6.0996. [DOI] [PubMed] [Google Scholar]
- 21.Ding YH, Dai D, Lewis DA, et al. Angiographic and histologic analysis of experimental aneurysms embolized with platinum coils, Matrix, and HydroCoil. Am J Neuroradiol. 2005;26:1757–1763. [PMC free article] [PubMed] [Google Scholar]
- 22.Altes TA, Cloft HJ, Short JG, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. Am J Roentgenol. 2000;174:349–354. doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 23.Krings T, Moller-Hartmann W, Hans FJ, et al. A refined method for creating saccular aneurysms in the rabbit. Neuroradiology. 2003;45:423–429. doi: 10.1007/s00234-003-0976-2. 26. [DOI] [PubMed] [Google Scholar]
- 24.Tong FC, Cloft HJ, Dion JE. Endovascular treatment of intracranial aneurysms with Guglielmi Detachable Coils: emphasis on new techniques. J Clin Neurosci. 2000;7:244–253. doi: 10.1054/jocn.1999.0211. [DOI] [PubMed] [Google Scholar]
- 25.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212:348–356. doi: 10.1148/radiology.212.2.r99jl47348. [DOI] [PubMed] [Google Scholar]
- 26.Murayama Y, Nien YL, Duckwiler G, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 27.Rufenacht DA, Mandai S, Levrier O. Endovascular treatment of intracranial aneurysms. Am J Neuroradiol. 1996;17:1658–1660. [PMC free article] [PubMed] [Google Scholar]
- 28.Murayama Y, Vinuela F, Ishii A, et al. Initial clinical experience with matrix detachable coils for the treatment of intracranial aneurysms. J Neurosurg. 2006;105:192–199. doi: 10.3171/jns.2006.105.2.192. [DOI] [PubMed] [Google Scholar]
- 29.Song JK, Niimi Y, Yoshino Y, et al. Assessment of Matrix coils in a canine model of a large bifurcation aneurysm. Neuroradiology. 2007;49:231–235. doi: 10.1007/s00234-006-0177-x. [DOI] [PubMed] [Google Scholar]
- 30.Pierot L, Leclerc X, Bonafe A, et al. Endovascular treatment of intracranial aneurysms with matrix detachable coils: midterm anatomic follow-up from a prospective multicenter registry. Am J Neuroradiol. 2008;29:57–61. doi: 10.3174/ajnr.A0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taschner CA, Leclerc X, Rachdi H, et al. Matrix detachable coils for the endovascular treatment of intracranial aneurysms: analysis of early angiographic and clinical outcomes. Stroke. 2005;36:2176–2180. doi: 10.1161/01.STR.0000181770.14869.ce. [DOI] [PubMed] [Google Scholar]
- 32.Cruise GM, Shum JC, Plenk H. Hydrogel-coated and platinum coils for intracranial aneurysm embolization compared in three experimental models using computerized angiographic and histologic morphometry. J Mater Chem. 2007;17:3965–3973. [Google Scholar]
- 33.Cloft HJ. HydroCoil for Endovascular Aneurysm Occlusion (HEAL) study: periprocedural results. Am J Neuroradiol. 2006;27:289–292. [PMC free article] [PubMed] [Google Scholar]
- 34.Im SH, Han MH, Kwon BJ, et al. Aseptic meningitis after embolization of cerebral aneurysms using hydrogel-coated coils: report of three cases. Am J Neuroradiol. 2007;28:511–512. [PMC free article] [PubMed] [Google Scholar]
- 35.Meyers PM, Lavine SD, Fitzsimmons BF, et al. Chemical meningitis after cerebral aneurysm treatment using two second-generation aneurysm coils: report of two cases. Neurosurgery. 2004;55:1222. doi: 10.1227/01.neu.0000140987.71791.df. [DOI] [PubMed] [Google Scholar]