Summary
We describe a case of a persistent primitive trigeminal artery (PPTA) coexistent with a clival chordoma. During surgery of the tumor, the partially incorporated PPTA was inadvertently traumatized and ruptured. The operation was discontinued and the PPTA was endovascularly occluded permitting further safe resection of the tumor.
Key words: primitive persistent trigeminal artery, chordoma, endovascular occlusion
Introduction
Clival chordomas are difficult lesions to treat. The transnasal approach offers a less invasive access to these tumors. Preoperative MR and CT may provide details on th exact borders of the lesion, and involved or invaded adjacent structures. Co-existence of skull base chordoma and persistent primitive trigeminal artery (PPTA) has never been reported in the literature. We describe a case of a clival chordoma associated with a PPTA partially incorporated in the tumor, which was ruptured during surgery.
Case Report
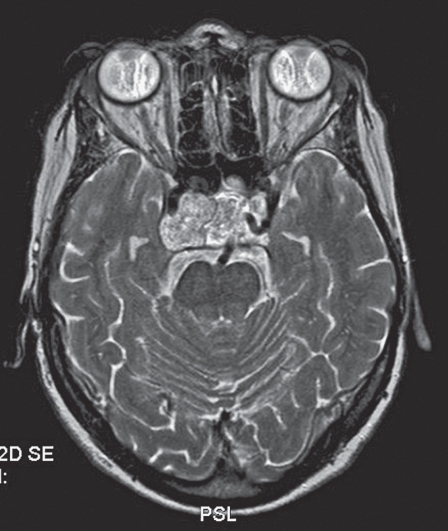
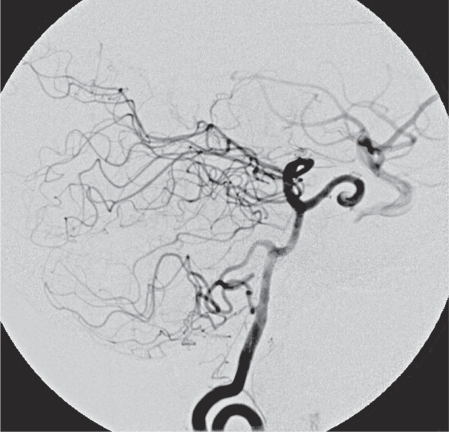
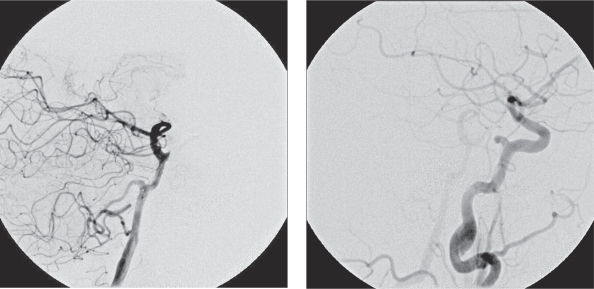
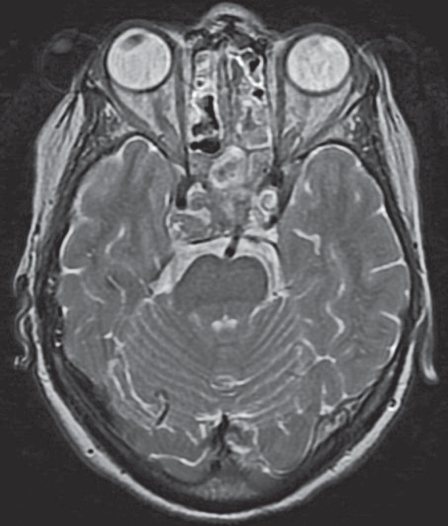
A 64-year-old woman presented with right VIth nerve paresis. Brain MR and CT revealed a mass within the sphenoid sinus and sella infiltrating the clivus. The diagnosis was chordoma, which was confirmed by biopsy. In contact with the tumor and partially incorporated a PPTA was identified on the left side (Figure 1). Surgical resection requiring a combined neurosurgical and ENT operation was recommended. The patient underwent surgery via the transnasal approach. During surgery sudden massive bleeding occurred and accidental laceration of the PPTA was suspected. The operation was suspended until an urgent angiogram could confirm the suspected cause of bleeding. A four vessel digital angiogram was urgently performed. In the angiogram a tiny contrast spot beyond the outline of the inferior wall of PPTA was hardly visible (Figure 2). The basilar and carotid arteries proximal and distal to the PPTA were both well-developed. Additionally, no arterial branches of the PPTA supplying the brain parenchyma were opacified and therefore its endovascular occlusion was decided. A microcatheter Tracker 10 carried over a Transend 10 microwire were used for catheterization of the PPTA through the left vertebral artery. Three platinum coils were delivered covering the segment where the laceration point was located. Vertebral and left carotid injection confirmed the complete occlusion of the PPTA (Figure 3). The patient was brought back to the operating room and resection of the tumor was resumed. Clinical examination and CT/MR scan postoperatively did not show any adverse event (Figure 4).
Figure 1.
Brain MR image showing the PPTA in relation to the tumor.
Figure 2.
Vertebral injection showing the PPTA and its laceration point.
Figure 3.
Endovascular coil occlusion of the lacerated PPTA segment. A) Vertebral injection. B) Carotid injection.
Figure 4.
Post-operative MR image at the level of the pons, without infarction.
Discussion
Chordomas are relatively rare neoplasms arising from embryonic notochordal remnants and comprise less than 1% of intracranial neoplasms. Twenty-five to 40% of chordomas occur in the spheno-occipital or skull base region. These tumors occur predominately in the fourth and fifth decades of life and show a slight male predominance.
The first anatomic description of the persistence of a primitive trigeminal artery was provided by Quain 1 in 1844.
In 1959 Saltzman 2 provided the first angiographic classification of the PPTA based on the degree of filling of the distal basilar artery and its inverse relationship with the visualization of the posterior communicating artery.
Primitive trigeminal artery is the most common of the carotid-basilar persistent embryonic communications and it has been observed in 0.1 to 0.6% of cerebral angiograms. PPTA is associated with various vascular abnormalities such as brain aneurysms and AVMs 3-6 but rarely with tumors such as meningioma, pituitary adenoma, astrocytoma and hemangioblastoma 7,8. No previous report of co-existence of a PPTA with a clival chordoma either incorporating it or not has been found in the literature.
Occlusion of the PPTA
Occlusion of the PPTA is allowed only when: 1) Neither the blood supply to the carotid nor to the basilar system is crucially dependent on its contribution 9,10 and 2) No branches to the brain parenchyma arise from the occluded segment 11-15.
In our case neither the supply to the carotid nor to the basilar was dependent on the PPTA.
In 1990, Inoue et al. 11 described a cadaver specimen with the dorsal meningeal, tentorial and inferior hypophyseal arteries, usually branches of the MHT, arising from the PPTA as individual branches.
Khodadad 12 reported pontine branches arising from the trunk of the PPTA in brains of four, six, and eight month old fetuses. He suggested that the PPTAs might become of functional and clinical significance if they persisted.
Ohshiro et al. 13 presented a case of a PPTA arising from the inferolateral aspect of the ICA, which had two branches from its cisternal portion. One branch sent a feeding artery to the left trigeminal nerve root and a perforating artery to the pons. The other branch perforated directly into the pons.
Salas et al. 14 described a PPTA that gave rise to four pontine perforating arteries. In both of these cases, the PPTA arose again from the inferolateral aspect of the intracavernous carotid artery. In the same report, as was seen in a similar pattern reported by Ohshiro et al., a classification of PTA variations was proposed: a lateral petrosal variation and a medial sphenoid variation. It appears that the lateral variation can give rise to branches supplying the brainstem.
Suttner et al. 15 reported an anatomic specimen with a medial-origin variation of PPTA providing inferior hypophyseal and dorsal meningeal branches. Concluding, they suggested that the inferolateral origin might represent the true PPTA whereas a superolateral origin presented by Parkinson and Shields 16 and medial origin may represent a different carotid-basilar anastomosis from the PPTA, and the MHT may be the remnant of this vessel.
As described by Lasjaunias and Berenstein 17, these branches to the brain parenchyma or elsewhere can arise from the PPTA in cases of annexation of their territory. Based on their analysis, we can assume that each particular pattern of origin of the PPTA in relation to the ICA wall (medial, inferolateral or superolateral) determines the branches that can be annexed, with the inferolateral origin being more connected to significant branches (to the parenchyma or cranial nerves) and the medial or superolateral connected mainly to dural branches.
Therefore as Suttner et al. 15 noticed, when considering therapy that involves this kind of persistent embryonic anastomosis, the therapeutic decision to sacrifice the PPTA in a specific pathological condition must take into account the particular variation and its potential branches and not only hemodynamic factors because the possibility of brain ischemia exists.
In our case the PPTA belongs to the superolateral-origin variation. An opacified tiny vessel coming from its superior wall definitely does not irrigate the brain parenchyma and most likely it is related to dural supply.
The surgical management aiming at complete and safe resection of a clival chordoma presenting with such a rare association of a PPTA in contact with or incorporated within the lesion should include preoperative endovascular occlusion of the involved vessel particularly when a transnasal approach is selected. Knowledge of the functional vascular anatomy of the PPTA and angiographic identification of the individual PPTA disposition are essential for the safety of such a procedure.
References
- 1.Quain R. The anatomy of the arteries of the human body. London: Taylor and Walton; 1844. [Google Scholar]
- 2.Saltzman GF. Patent primitive trigeminal artery studied by cerebral angiography. Acta Radiol (Stockh) 1959;51:329. doi: 10.3109/00016925909171103. [DOI] [PubMed] [Google Scholar]
- 3.Li MH, Li WB, Pan YP, et al. Persistent primitive trigeminal artery associated with aneurysm: report of two cases and review of the literature. Acta Radiol. 2004;45(6):664–668. doi: 10.1080/02841850410001196. [DOI] [PubMed] [Google Scholar]
- 4.Heckly A, Hamlat A, Carsin-Nicol B, et al. [Persistent primitive trigeminal artery associated with brain cavernoma. Case report] Neurochirurgie. 2004;50(4):492–495. doi: 10.1016/s0028-3770(04)98330-0. French. [DOI] [PubMed] [Google Scholar]
- 5.Nakai Y, Yasuda S, Hyodo A, et al. Infratentorial arteriovenous malformation associated with persistent primitive trigeminal artery--case report. Neurol Med Chir (Tokyo) 2000;40(11):572–574. doi: 10.2176/nmc.40.572. [DOI] [PubMed] [Google Scholar]
- 6.Komiyama M, Nakajima H, Nishikawa M, et al. High incidence of persistent primitive arteries in moyamoya and quasi-moyamoya diseases. Neurol Med Chir (Tokyo) 1999;39(6):416–422. doi: 10.2176/nmc.39.416. discussion 420-422. [DOI] [PubMed] [Google Scholar]
- 7.Murai Y, Kobayashi S, Tateyama K, et al. Persistent primitive trigeminal artery aneurysm associated with cerebellar hemangioblastoma. Case report. Neurol Med Chir (Tokyo) 2006;46(3):143–146. doi: 10.2176/nmc.46.143. [DOI] [PubMed] [Google Scholar]
- 8.Sato M, Kondo A, Otsuka S, et al. Trigeminal neuralgia: association with tentorial meningioma and persistent primitive trigeminal artery. Fukushima J Med Sci. 1995;41(1):87–93. [PubMed] [Google Scholar]
- 9.Boyko OB, Curnes JT, Blatter DD, et al. MRI of basilar artery hypoplasia associated with persistent primitive trigeminal artery. Neuroradiology. 1996;38(1):11–14. doi: 10.1007/BF00593207. [DOI] [PubMed] [Google Scholar]
- 10.Hattori T, Kobayashi H, Inoue S, et al. Persistent primitive trigeminal artery associated with absence of internal carotid artery. Surg Neurol. 1998;50(4):352–355. doi: 10.1016/s0090-3019(97)00491-6. [DOI] [PubMed] [Google Scholar]
- 11.Inoue T, Rhoton AL, Jr, Theele D, et al. Surgical approaches to the cavernous sinus: A microsurgical study. Neurosurgery. 1990;26:903–932. doi: 10.1097/00006123-199006000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Khodadad G. Persistent trigeminal artery in the fetus. Radiology. 1976;121:653–656. doi: 10.1148/121.3.653. [DOI] [PubMed] [Google Scholar]
- 13.Ohshiro S, Inoue T, Hamada Y, et al. Branches of the persistent primitive trigeminal artery-an autopsy case. Neurosurgery. 1993;32(1):144–148. doi: 10.1227/00006123-199301000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Salas E, Ziyal I, Sekhar L, et al. Persistent trigeminal artery: an anatomic study. Neurosurgery. 1998;43(3):557–561. doi: 10.1097/00006123-199809000-00082. [DOI] [PubMed] [Google Scholar]
- 15.Suttner N, Mura J, Tedeschi H, et al. Persistent trigeminal artery: a unique anatomic specimen--analysis and therapeutic implications. Neurosurgery. 2000;47(2):428–434. doi: 10.1097/00006123-200008000-00030. discussion 433-434. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson D, Shields CB. Persistent trigeminal artery: Its relationship to the normal branches of the cavernous carotid. J Neurosurg. 1974;39:244–248. doi: 10.3171/jns.1974.40.2.0244. [DOI] [PubMed] [Google Scholar]
- 17.Lasjaunias P, Berenstein A, TerBrugge K. Surgical neuroangiography. 2nd edition. Vol I. Heidelberg: Springer-Verlang; 2001. pp. 389–411. [Google Scholar]