Summary
Management of symptomatic carotid near occlusion especially in high-risk patients is different from outcome analysis of NASCET. We evaluated outcome in high-risk patients with symptomatic near occlusion.
For 48 patients with near occlusion out of 166 symptomatic high-risk patients who underwent carotid stenting, we assessed the procedural success defined as residual stenosis <30%, modified Rankin Scale (mRS) at one and six months following stenting, and the 13 cerebrovascular factors related to the outcome. Initial National Institutes of Health Stroke Scale (NIHSS) ≥4,1-3 and 0 were 13,14 and 21 patients each. We compared the outcome with patients who underwent CAS (n=118) due to symptomatic stenosis without near occlusion during the same period.
Our procedural success rate was 98%. A good outcome (mRS ≥2) was achieved in 44 patients (92%) at six months. There were five events (10%) within six months, i.e. three minor strokes, one major stroke caused by hemorrhage, and one death excluding two deaths not related to stroke. Hyperperfusion (n=4) was the most common cause of events leading to two minor strokes and a major stroke. Although initial NIHSS (P = .012) was related to poor outcome (mRS >2) compared to the CAS group, there was no statistical significance between two groups in the event rate of stroke, death or restenosis.
The outcome of carotid stenting in high-risk patients with symptomatic near occlusion did not reveal any difference compared with CAS. Poor outcome was related to the initial NIHSS (>4). Hyperperfusion tended to be more commonly related to an event occurring after stenting.
Key words: carotid arteries, stenosis or obstruction, stenting, outcome
Introduction
Hemodynamically, near occlusion of the internal carotid artery (ICA) is defined as an extreme atherosclerotic stenosis, usually at the ICA bulb, with minimal residual and slow flow through this segment and an extreme decrease of poststenotic perfusion pressure. As poststenotic perfusion is decreased, virtual luminal collapse occurs or there is marked luminal diameter decrease of an otherwise normal-appearing artery beyond carotid stenosis which is seen as the 'string sign' on an angiogram 1,2.
Because the arrival of the contrast medium into the distal ICA is considerably delayed if there is severe stenosis, a slower filling with reduction of the caliber of the ICA is clearly observed when compared with that of the external carotid artery (ECA). Severe intracranial stenosis or even occlusion may be suggested because of the absence of normal filling of the supraclinoid ICA due to the flow washout secondary to competitive flow from the collaterals 3. Despite this misleading arteriographic appearance, the ICA distal to the stenosis is frequently unaffected and successfully revascularized once the proximal stenosis is relieved 4.
Until recently, however, the management of a symptomatic near occlusion remained controversial and had not been studied properly. Many high-risk patients had been excluded from clinical trials, and the benefits of management have been assessed only in two large trials of low risk patients with presumably well compensated collaterals, specifically the North American Symptomatic CEA Trial (NASCET) and the European Carotid Surgery Trial (ECST). These studies found that CEA was less beneficial for symptomatic patients with ICA near occlusion than for patients who had severe stenosis not near occlusion 5-7. Therefore, the degree of potential benefit of this type of ICA stenosis is still unclear.
The short-term prognosis for patients with near occlusion was first reported in the NASCET trial. Patients with ICA near occlusion with or without a string-like lumen were at lower risk of stroke after medical treatment (11% to 18%), whereas endarterectomy led to a 4% reduction in stroke risk reduction compared with other patients in the severe category 8. However, patients at high risk for CEA have been excluded from most prospective trials due to concerns regarding possible complications and/or poor outcomes 9.
The recent development of endovascular devices and techniques such as the embolic protection device, endoluminal balloon, and metallic stent, has improved the results of carotid artery stenting, at least in surgically high-risk patients, as shown in the SAPPHIRE trials, although over 70% of those patients were asymptomatic 10. Because there have been no large clinical trials on percutaneous transluminal angioplasty (PTA) and stenting for ICA near occlusion especially in the high-risk patients, our study included high-risk patients with symptomatic ICA near occlusion of differing initial neurological status and evaluated the outcome after stenting from the viewpoint of the events associated with the procedure as well as the short-term clinical outcome.
Materials and Methods
From our institution's carotid stenting database of 166 patients treated during the last five years, 48 consecutive patients (age mean 69, M:F = 41:7) with near occlusion of the ICA stenosis, were enrolled in our study. All the patients were at high risk for CEA or had medical problems (Table 1). We excluded all patients who underwent stenting during revascularization as part of their acute stroke management or who had an asymptomatic near occlusion, a non-atheromatous vascular lesion such as Takayasu's arteritis, or who had dissection.
Table 1.
Criteria for high-risk patients
| Criteria | No of patients |
|---|---|
| Clinically significant cardiac disease | 11 |
| Congestive heart failure (NYHA functional class III/IV) | |
| Abnormal stress test | |
| Need for open-heart surgery | |
| Unstable angina (CCS class III/IV) | |
| Left ventricular ejection fraction ≤ 30% | |
| Planned coronary artery bypass graft or valve replacement | |
| Severe pulmonary disease | 2 |
| Chronic obstructive pulmonary disease manifested with FEV ≤ 30% | |
| Contralateral carotid occlusion or severe stenosis | 11 |
| Previous radical neck surgery or radiation therapy to the neck | 3 |
| Recurrent stenosis after endartererectomy | 0 |
| Age ≥ 75 yrs | 10 |
| Surgically inaccessible lesion at or above C2 or below the clavicle | 16 |
| Laryngeal palsy or laryngectomy | 0 |
| Symptom onset within 2 weeks | 20 |
| Neurologic symptoms change (≥ NIHSS 4) within 48 hours from onset | 13 |
| Arteriosclerosis obliterans | 4 |
| Cancer | 6 |
| History of major surgery within the past year | 4 |
| Renal problem (Creatinine level > 1.5 mg/dl) | 4 |
| * Number of patients | |
Near occlusion was defined as a definite delay in filling of the ICA to the head and visible collaterals 11. In all patients, it was accompanied by decreased diameter of the normal ICA beyond a stenosis, as compared with the contralateral ICA or ipsilateral ECA. Near occlusion, as evidenced by a tiny threadlike lumen (or "string sign") was observed in 8 patients, and a more normal-looking, although reduced, lumen (approaching near occlusion) was found in 40 patients 11.
The ECA/ICA ratio was 1.69 ± 0.73 SD in our patients with near occlusion and 1.28 ± 0.55 SD in our patients approaching near occlusion. We also determined the ratio between the ICA and the common carotid arteries (CCA) 12. Since abnormal poststenotic narrowing of the distal ICA was defined in the ECST as a severe carotid stenosis with an ICA:CCA ratio <0.42, the NASCET has shown that the lower limit of normal of the ICA:CCA ratio differs in men (0.40) and women (0.45), because of systematic sex differences in the normal carotid bifurcation anatomy 5,6,12,13. The ICA/CCA ratio was 0.31±0.11 SD in our patients with near occlusion and 0.40±0.12 SD in our patients approaching near occlusion.
Eleven out of 48 patients with near occlusion had contralateral >70% severe stenosis (n=6) or occlusion (n=5) (Table 1). Among them, two patients with bilateral near occlusion underwent bilateral stenting in a same session.
Carotid stenting procedure was the same as previously described and four patients with multichanneled near occlusion were included in a previous report 14-17. Procedural success was defined as having less than 30% stenosis after the procedure.
We compared the outcome with high risk patients who underwent CAS (n=118) due to symptomatic stenosis without carotid near occlusion during the same period. We obtained written informed consent from each patient and/or the patient's family, and our Institutional Review Board approved this study.
Patient Evaluation and Statistical Analysis
The time from the last ischemic event to admission to our center was a median time of 30 days. Thirty-one patients had had minor stroke, while 17 patients had experienced transient ischemic attack. Initial NIHSS were ≥4 in 13, 1-3 in 14, and 0 in 21 patients. The arteriographic criteria used to assess the degree of stenosis applied the method used for the NASCET.
The patients' pretreatment neurologic status was evaluated by neurologists using the National Institutes of Health Stroke Scale (NIHSS). We assessed the procedural success which was defined as residual stenosis <30%. The Modified Rankin Scale (mRS) was used to assess the clinical outcome after one month and again at six months, which represented short-term outcome (mRS at 1 month) and the mid-term outcome (mRS at six months), respectively.
Brain MRI (n=36) revealed border-zone (n=22), wedge-shaped pial (n=8), scattered focal or superficial perforator (n=3), deep perforator (n=1), and basal ganglia (n=2) lesion patterns of recent infarction on diffusion-weighted image (n=33) (Figure 1) and fluid-attenuated-inversion-recovery image (n=3). Twelve patients had no recent parenchymal lesion detected on MRI. Twenty-seven patients underwent brain perfusion analysis by MR perfusion (n=16) or by brain SPECT (n=11) after an acetazolamide (Diamox; Wyeth, Sydney, NSW, Australia) challenge. The perfusion results were divided into 3 types: (1) Patients with normal brain SPECT or normal mean transit time or normal blood volume and flow on MR perfusion were defined as being normal or having a mild perfusion change (n=0); (2) Patients with normal brain SPECT and decreased perfusion reservoir on acetazolamide challenge or increased mean transit time or time to peak with increased blood volume and normal blood flow on MR perfusion were defined as having a moderate perfusion deficit (n=10); and (3) Patients with decreased perfusion on brain SPECT with decreased or normal reservoir on the Diamox stress test or increased mean transit time and decreased blood flow on MR perfusion were defined as having a severe perfusion deficit (n=17) 24.
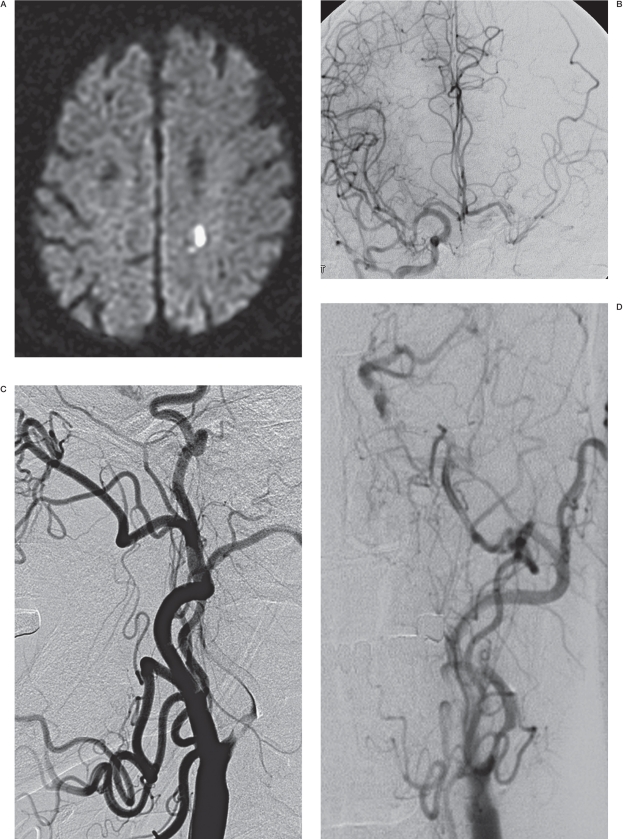
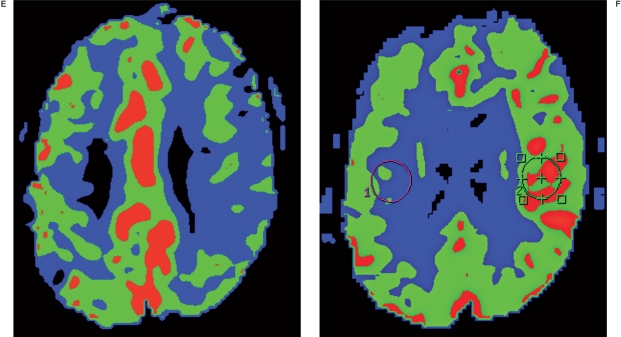
Figure 1.
A 77-year-old male presenting with aphasia (NIHSS = 3). A) Diffusion-weighted image showing an acute focal ischemic change in the left corona radiata. B) Right carotid angiogram showing incomplete cross filling through the anterior communicating artery. C, D) Left carotid arteriograms. C) Anteroposterior view, showing partial filling of the left middle cerebral arteries via collaterals through the external carotid artery. D) Lateral view, showing near occlusion of the left carotid bulb with a stasis of contrast agent in the left cervical internal carotid artery. Following successful stenting, this patient experienced a sudden deterioration of his neurological status, with no evidence of hemorrhage or further infarction on diffusion-weighted images (not shown). E) MR perfusion study showing decreased blood flow in the left MCA territory and revealing diffusion and perfusion mismatch corresponding to patient symptoms before stenting. F) MR perfusion performed immediately after stenting, showing hyperperfusion in the left brain. This patient recovered after strict blood pressure control and experienced no adverse events during the 16-month follow-up period.
With the outcome being a dependent variable, we treated 16 cerebrovascular atherosclerotic risk factors of age (≥70years), gender, hypertension, diabetes, cardiac disease, hyperlipidemia, family history, previous stroke history, smoking, alcohol, homocysteine (≥15 mol/L), high sensitivity C-reactive protein (≥0.6 mg/dL), body mass index (≥25), initial National Institutes of Health Stroke Scale (≥4), symptom mode (stroke vs. TIA) and symptom onset (>2 weeks) as independent variables. Continuous data were dichotomized at their median values and bivariate analyses were conducted between the outcome variables and the dependent variables. Fisher's exact tests were used and when a potential confounder was suspected, stratified analysis was conducted. Two-tailed p values were calculated, and p values greater than .05 were considered not statistically significant. Statistical computations were performed using SPSS for Windows (version 15.0; SPSS Inc., Chicago, Ill).
All patients excluding five patients who subsequently died and one patient with control who had been lost by the time of the six-month follow-up were clinically followed for six months. If a patient was not followed in an outpatient clinic, an experienced nurse telephoned the patients (37 out of 166 patients) to evaluate the possibility of any clinically relevant event and their mRS was transformed from a functional outcome including dependency, living situation, mobility, dressing, and toilet functions 18.
Follow-up study at six months for restenosis was completed in 40 patients by Doppler ultrasound examination (n=35), CTA (n=4) and cerebral angiography (n=1). Restenosis (>50%) was defined as a reduction in the luminal diameter using NASCET criteria on cerebral angiography or CTA, or using B mode ultrasound and/ or peak systolic velocity (>300 cm/sec or >fourfold the ICA/CCA ratio) on Doppler ultrasound 19.
Results
All patients except for one were successfully treated (residual stenosis is ≤30%). The procedural success rate was 98%. Good outcome (modified Rankin Score, mRS ≤2) was seen in 92% of our study patients at one and six months. Among 16 cerebrovascular atherosclerotic risk factors, initial NIHSS (P= .012) was associated with poor outcome (mRS >2) in one month and presence of family history (P= .086) was marginally associated with poor outcome at six months.
A poor outcome at six months included three patient deaths and a patient who had a major stroke event caused by hemorrhage (mRS = 4) (Table 2). There were five lesion-related events (10%) and overall seven events (15%) within six months, i.e. three minor strokes caused by hyperperfusion and/or reperfusion injury, a major stroke caused by hemorrhage, and three deaths related to hyperperfusion-related hemorrhage, chronic renal failure and lung cancer. The three deaths included a patient with contralateral occlusion who died of intracerebral and intraventricular hemorrhage in the ipsilateral frontal lobe four days following treatment and whose death was not related to the previous ischemic lesion, as seen on diffusion-weighted imaging, therefore suggesting hyperperfusion syndrome. The cause of death was regarded as being not related to the carotid lesion in two patients including a patient who died of complications from chronic renal failure two months following treatment, but this death was not related to the contrast agent, and a patient who died of an advanced lung cancer three month following stenting, which was detected two months after the carotid stenting.
Table 2.
Event rates and outcome.
| Events and Outcomes | Near occlusion (n = 48) |
Control (n = 118) |
P-value | |
|---|---|---|---|---|
| Event within 6 months | Minor stroke | 3 (3) | 4 (2) | 0.244(0.74) |
| Major stroke | 1 (1) | 3 (3) | ||
| Death | 3 (1) | 2 (1) | ||
| Hyperperfusion syndrome | 4 | 4 | 0.230 | |
| 1 month mRS | mRS≤2 | 44 | 98 | 0.223 |
| mRS >2 | 4 | 20 | ||
| 6 months mRS | mRS≤2 | 44 | 107 | 1.0 |
| mRS>2 | 4 | 10 | ||
| Restenosis | 3/40 | 7/92 | 1.0 | |
A patient with a major stroke event due to hemorrhage, presented with dysarthria as well as facial and arm weakness (initial NIHSS = 6) and a basal ganglia infarction associated with the diffusion-perfusion mismatch in the ipsilateral hemisphere, which led to hemorrhage one day after the uneventful stenting procedure.
Three minor strokes were regarded as being caused by hyperperfusion and/or reperfusion injury in two patients and having a thromboembolism in a patient who developed decreased vision in spite of good filling of ophthalmic artery. One patient developed aphasia and revealed delayed gadolinium enhancement of the cerebrovascular space on fluid attenuated inversion recovery as a hyperintense acute reperfusion marker 20. His symptom was improved next day after strict control of blood pressure in spite of the normal perfusion status on MR perfusion study. One patient discharged without any adverse events developed a mild transient weakness in the opposite extremities ten days following the procedure, due to a localized hemorrhage in the opposite basal ganglia, which was regarded as hyperperfusion syndrome because bilateral stenting was performed in this patient. None of these three patients revealed any neurological deficit at the one-year follow-up.
A 77-year-old male presenting with aphasia (NIHSS = 3) developed transient hyperperfusion immediately after stenting (Figure 1). In addition to both artery-to-artery embolisms (Figure 1A), hypoperfusion (Figure 1E) was related to the cause of symptoms representing a diffusion-perfusion mismatch as well as a symptom-diffusion mismatch. Subsequent hyperperfusion corresponding to the loss of autoregulation in the ipsilateral cerebral vessels required careful management of blood pressure after a stenting procedure, representing patients at high procedural risk as in our study. This patient recovered after strict blood pressure control and experienced no adverse events during a 16-month follow-up period. There was 8% asymptomatic restenosis rate (3/40).
Discussion
Near occlusion is not rare, as shown with 22.6% of the patients in ECST 7 and in 20.7% of the patients in NASCET 8 who had severe stenosis of the symptomatic ICA. Our series of high-risk patients who underwent carotid stenting for symptomatic ICA stenosis, revealed a 29% near occlusion rate. There have been no large clinical trials on PTA and stenting for ICA near occlusion except for a few recent reports of experience with stenting in small group of patients with ICA near occlusion 21,22. Although those studies included stable and even asymptomatic patients, our study obtained a 92% good outcome at six month even though 27% of our patients had an initial NIHSS (≥ 4) in symptomatic high-risk patients.
Our inclusion criteria differed greatly from those of NASCET because we included patients at much higher risk (Table 1), including those with perfusion deficits and/or recent infarcts. To date, these factors have not been evaluated in an RCT or even in a case-control study. Therefore, patients with near occlusion in NASCET, especially those with string signs, must have had compensated perfusion status by collaterals. NASCET only included patients with nondisabling stroke with persistence of symptoms or signs for more than 24 hours within the previous 120 days, in association with stenosis of 30% to 99% in the ipsilateral internal carotid artery, while excluding patients with a cerebral infarction on either side that deprived the patient of all useful function in the affected territory, as well as patients with a cardiac valvular or rhythm disorder likely to be associated with cardioembolic symptoms. In addition, patients were temporarily ineligible if they had uncontrolled hypertension, diabetes mellitus, or unstable angina pectoris; myocardial infarction within the previous six months; signs of progressive neurologic dysfunction; contralateral carotid endarterectomy within the previous four months; or a major surgical procedure within the previous 30 days. These patients could become eligible if the disorder causing their temporary ineligibility resolved within 120 days after their qualifying cerebrovascular event.
The benefits of surgery for near occlusion are still controversial. In addition, there have been few reports of surgical results in patients with symptomatic high-risk patients. Rothwell et al. 6 and Henderson et al. 5 reported that CEA had no benefit for reducing the poststenotic narrowing of ICA ( ICA/CCA ratio of <0.42) associated with poststenotic collaterals, but Morgenstern et al. 8 reported that surgery for near occlusion was indeed beneficial and resulted in a reduction in the stroke rate from 11.1 to 6.7% per year. Regarding surgery for ICA near occlusion, Greiner et al. 23 treated 53 patients with near occlusion by CEA. In this series, forty of the 53 (75.5%) patients were successfully revascularized, perioperative mortality was 1.9%, and perioperative morbidity was 7.5%.
The use of carotid stenting has been rapidly increasing since the introduction of embolic protection devices because endovascular therapy is less invasive than CEA 24. In a randomized study comparing CEA and stenting using a protective device for the treatment of high-risk patients with carotid stenosis, stenting had a better outcome than CEA 25. Few clinical results have recently been reported regarding carotid artery stenting experience for treating near occlusion in high-risk patients. Our clinical results showing 92% good outcome and 10% event rate in six months were fairly good in symptomatic high-risk patients from the viewpoints of periprocedural neurological morbidity, angiographic follow-up results, and stroke prevention, thus supporting the theory that carotid stenting may be considered as an alternative to CEA in high-risk patients with ICA near occlusion 21,22.
In our study, hyperperfusion was the most common and serious cause of adverse events. Although most of these events (3/4) developed immediately after the procedure and tended to be reversible after meticulous blood pressure control, a patient with contralateral ICA occlusion uneventfully discharged after the procedure developed a fatal hemorrhage leading to death four days after the stenting procedure. Therefore, there should be serious concerns regarding hyperperfusion syndrome in the patients who seem to experience loss of autoregulation due to decreased perfusion status in the ipsilateral brain hemisphere.
Conclusions
Carotid stenting leads to a favorable outcome in high-risk patients with symptomatic near occlusion and can be recommended based on its feasibility, low morbidity and mortality, and long-term patency of the stented ICA. The poor outcome is related to the initial NIHSS. However, there was no statistical significance between two groups in the event rate of stroke (minor and/or major stroke), death or resteno-sis at six months. Hyperperfusion is the most common cause of the events within six months after the stenting.
Acknowledgements and Funding
We acknowledge the assistance of Sun Moon Whang, B.S. and Eun Hye Kim, R.N. in the patient data collection as well as that of Yun Gyeong Jeong in the manuscript preparation. We thank Bonnie Hami, M.A. (USA) for her English editorial assistance. This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A080201).
References
- 1.Berman SS, Devine JJ, Erdoes LS, et al. Distinguishing carotid artery pseudo-occlusion with color-flow Doppler. Stroke. 1995;26(3):434–438. doi: 10.1161/01.str.26.3.434. [DOI] [PubMed] [Google Scholar]
- 2.Mehigan JT, Olcott CT. The carotid "string" sign. Differential diagnosis and management. Am J Surg. 1980;140(1):137–143. doi: 10.1016/0002-9610(80)90430-4. [DOI] [PubMed] [Google Scholar]
- 3.Suh DC, Kim JK, Choi CG, et al. Prognostic factors for neurologic outcome after endovascular revascularization of acute symptomatic occlusion of the internal carotid artery. Am J Neuroradiol. 2007;28(6):1167–1171. doi: 10.3174/ajnr.A0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pappas JN. The angiographic string sign. Radiology. 2002;222(1):237–238. doi: 10.1148/radiol.2221000366. [DOI] [PubMed] [Google Scholar]
- 5.Henderson RD, Eliasziw M, Fox AJ, et al. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke. 2000;31(1):128–132. doi: 10.1161/01.str.31.1.128. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Warlow CP. Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists' Collaborative Group. Stroke. 2000;31(3):622–630. doi: 10.1161/01.str.31.3.622. [DOI] [PubMed] [Google Scholar]
- 7.Group E. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351(9113):1379–1387. [PubMed] [Google Scholar]
- 8.Morgenstern LB, Fox AJ, Sharpe BL, et al. The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Neurology. 1997;48(4):911–915. doi: 10.1212/wnl.48.4.911. [DOI] [PubMed] [Google Scholar]
- 9.Wennberg DE, Lucas FL, Birkmeyer JD, et al. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA. 1998;279(16):1278–1281. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 10.Whitlow PL, Lylyk P, Londero H, et al. Carotid artery stenting protected with an emboli containment system. Stroke. 2002;33(5):1308–1314. doi: 10.1161/01.str.0000013947.17575.b3. [DOI] [PubMed] [Google Scholar]
- 11.Fox AJ, Eliasziw M, Rothwell PM, et al. Identification, prognosis, and management of patients with carotid artery near occlusion. Am J Neuroradiol. 2005;26(8):2086–2094. [PMC free article] [PubMed] [Google Scholar]
- 12.Rothwell PM, Gutnikov SA, Warlow CP. Reanalysis of the final results of the European Carotid Surgery Trial. Stroke. 2003;34(2):514–523. doi: 10.1161/01.str.0000054671.71777.c7. [DOI] [PubMed] [Google Scholar]
- 13.Schulz UG, Rothwell PM. Sex differences in carotid bifurcation anatomy and the distribution of atherosclerotic plaque. Stroke. 2001;32(7):1525–1531. doi: 10.1161/01.str.32.7.1525. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Choi BS, Choi JW, et al. Stent implantation of multichanneled pseudoocclusion of the internal carotid artery. J Vasc Interv Radiol. 2009;20(3):391–395. doi: 10.1016/j.jvir.2008.12.413. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Jung JH, Kim SM, et al. Simultaneous bilateral carotid stenting in high-risk patients. Am J Neuroradiol. 2010 doi: 10.3174/ajnr.A1970. doi: 10.3174/ajnr.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park ST, Kim JK, Yoon KH, et al. Atherosclerotic carotid stenoses of apical versus body lesions in high-risk carotid stenting patients. Am J Neuroradiol. 2010 doi: 10.3174/ajnr.A2000. ajnr.A2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pyun HW, Suh DC, Kim JK, et al. Concomitant multiple revascularizations in supra-aortic arteries: shortterm results in 50 patients. Am J Neuroradiol. 2007;28(10):1895–1901. doi: 10.3174/ajnr.A0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 19.Setacci C, Chisci E, Setacci F, et al. Grading carotid intrastent restenosis: a 6-year follow-up study. Stroke. 2008;39(4):1189–1196. doi: 10.1161/STROKEAHA.107.497487. [DOI] [PubMed] [Google Scholar]
- 20.Cho AH, Suh DC, Kim GE, et al. MRI evidence of reperfusion injury associated with neurological deficits after carotid revascularization procedures. Eur J Neurol. 2009;16(9):1066–1069. doi: 10.1111/j.1468-1331.2009.02650.x. [DOI] [PubMed] [Google Scholar]
- 21.Gil-Peralta A, Gonzalez A, Gonzalez-Marcos JR, et al. Internal carotid artery stenting in patients with symptomatic atheromatous pseudo-occlusion. Cerebrovasc Dis. 2004;17(Suppl. 1):105–112. doi: 10.1159/000074802. [DOI] [PubMed] [Google Scholar]
- 22.Terada T, Tsuura M, Matsumoto H, et al. Endovascular treatment for pseudo-occlusion of the internal carotid artery. Neurosurgery. 2006;59(2):301–309. doi: 10.1227/01.NEU.0000222650.09509.DE. discussion 309. [DOI] [PubMed] [Google Scholar]
- 23.Greiner C, Wassmann H, Palkovic S, et al. Revascularization procedures in internal carotid artery pseudoocclusion. Acta Neurochir (Wien) 2004;146(3):237–243. doi: 10.1007/s00701-004-0216-5. discussion 243. [DOI] [PubMed] [Google Scholar]
- 24.Cremonesi A, Manetti R, Setacci F, et al. Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke. 2003;34(8):1936–1941. doi: 10.1161/01.STR.0000081000.23561.61. [DOI] [PubMed] [Google Scholar]
- 25.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotidartery stenting versus endarterectomy in highrisk patients. N Engl J Med. 2004;351(15):1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]